Research - (2022) Volume 10, Issue 9
Potentials Compounds from Marine Invertebrates against Gram Negative Multi Drug Resistant (MDR) Pathogens
MD Nadeem Bari1*, Kuppannan Gobianand2, Malarvizhi Arthanari2, MD Rizwan Ansari1 and Imran Mohammad1
*Correspondence: MD Nadeem Bari, Department of Basic Medical Sciences, College of Medicine, Prince Sattam Bin Abdulaziz University, Saudi Arabia, Email:
Abstract
Globally, the occurrences of multi-drug resistant gram-negative bacteria have been created an imperative crisis. These bacteria have the restricted treatment preference resulting serious human health threats. Therefore, there is a need for searching an alternative measure should be taken against multidrug resistant bacteria immediately. Consequently, in this review, we focused on the marine antibacterial compounds which are documented from marine invertebrates like sponges, corals, bryozoans and tunicates with potent activity against these drug resistant bacteria were investigated. Marine network is a known as well as significant ecological niche for the discovery of promising bioactive compounds (peptides, macrocyles, terpenes, alkaloids, quinines etc.,) along with strong antibacterial potency against these resistant bacteria. This triggered us to review the marine bioactive compounds with excellent potency to fight against multi drug resistant bacteria.
Keywords
Marine Invertebrates, Multi drug resistance, Sponges, Corals, Tunicates, Bryozoans
Introduction
The antibiotics are an amazing invention in field of modern medicine which rules the entire world by saving the human lives. Unfortunately, antibiotic resistant strains are reported, owing to the extreme use of antibiotics resulting multi drug resistant [1]. The earlier report says, in 2050, 300 million deaths will be arising due to the infection caused by multidrug resistant organisms [2]. Presently, many of the bacterial strains were developed resistant to an antibiotic which leads to high morbidity and mortality [3]. Particularly, the occurrences of multidrug drug resistant gram-negative bacteria are becoming a world crisis [4-6]. Multidrug resistant refers; it is a reaction of organism to antimicrobial agents can occur through a variety of mechanisms like mutation in chromosomes, alteration in the target site, gene transfer [7]. The resistant strains are gaining much attention as well as the research focus for the development of new antibiotics or bioactive compounds which will fight against multi drug resistant organisms since of their “priority status” [8]. In these circumstances, the progress or establishment of novel antimicrobial agents with evident bioactivity with scientific importance is needed [9,10] to fight against multi drug resistant gram-negative bacteria. Consequently, in this review emphasize the antibacterial compounds from marine invertebrates against gram negative bacteria which are resistant to multiple drug of choice.
Marine ecosystems are varied and prosperous environment that contains huge range of different life structure with diverse chemical and physical states represents the significance of the invention of extensive choice of natural bioactive products by means of distinctive quality [11,12]. This marine product showed a wide variety of biological properties which has the probable relevance in the field of pharmaceutical and healthcare settings. The marine products obtained from marine invertebrates (sponges, soft corals, bryozoans and tunicates) are attracted owing to their structural as well as bioactive diversity which is not found in the terrestrial surroundings [13-15]. Till date, more than 40,000 marine compounds were isolated and identified with potent biological properties and many of them are patented and it has been approved [16]. Moreover, marine compounds with excellent biological activity from marine invertebrates have proved that these are precious source for the drug development and discovery. Importantly, many of the approved commercial drugs are from marine invertebrates particularly from sponges. So far, eight marine drugs were approved by European Medicines Agency and FDA (food and drug administration) [17]. Hence, in this review, we emphasized the antibacterial compounds produced by marine invertebrates (sponges, corals, bryozoans and tunicates) against multi-drug resistant gram-negative bacteria such as Acinetobacter baumanii, Enterobacteriaceae, Neisseria gonorrhoeae, Helicobacter pylori, Pseudomonas aeruginosa, Campylobacter sp., Salmonella sp., Shigella sp. are urgently needed the new antibiotics to deal with them.
Antibacterial compounds from marine invertebrates
Sponges
Sponges, belonging to Phylum porifera are important sessile marine invertebrates, survive in the harsh marine environmental conditions which provide the way for the finding of novel biological materials with different biological performance like anti-inflammatory, antimicrobial, anticancer, etc., owing to the carbon moiety present in the structure make them perfect candidate for the drug discovery [18]. Still, more than 8000 sponge species were identified [19] and approximately, 5000 natural marine compounds were discovered [20,21]. The compounds obtained from sponges have various chemical structures including terpenes, alkaloids, peptides, sterols and nucleosides leads to various biological activities [22]. With this background, Kubota et al. (2016) [23] isolated a compound hyrtinadine D from marine sponge Hyrtios erecta exhibited antimicrobial actions against E. coli and Gram-positive bacterium at 16 μg/ml MIC concentrations. In contrast, the compounds namely Hyrtiosenolides A and B identified in red sea sponge Hyrtios species showed minimum inhibitory effect against E. coli when tested at 100 μg/ml concentration [24]. The compounds agelasidine C and D have been isolated from marine sponge genus Agelas clathrodes had the antibacterial activity against Proteus vulgaris and Klebsiella pneumoniae [25]. Kobayashi, et al. [26] were isolated the Ageliferin and bromoageferin from marine sponge A. conifera and investigated the antimicrobial activity against bacteria and found that, the compounds have the potency to inhibit E. coli with the concentrations at 10 μg/disk.
Walker and his co-workers [27] identified the compound sceptrin from marine sponge A. sceptrum and investigated for its antimicrobial activity human pathogens such as Pseudomonas aeruginosa. Consequently, a study from Konuklugil [28] group reported the antimicrobial activity of thirty-three methanolic extracts which were obtained from the turkey sea marine sponges against multi-drug resistant gram-negative pathogens P. aeruginosa and Proteus vulgaris and identified the most effective compounds based on the collection area. This recommends that, the secondary metabolites production can be affected by surrounding environment. The antimicrobial compounds were identified from Mediterranean Sea sponge Axinella verrucosa in Syria. The antibacterial activity of methanolic extract was tested against Acinetobacter septicus, P. vulgaris and P. aeruginosa using disc diffusion method. The spectroscopic analyses of the extracted compounds were identified as hymenialdisine, 10E hymenialdisine and spongiacidine and these compounds were revealed the synergistic actions between the compounds [29]. In the same way, a study reported the extract (ethyl acetate) of marine sponge, A. damicorins from the Monasir was tested against the P. aeruginosa and other human pathogens. The same group investigated the antibacterial activity of marine sponge Agelas oroides (Demospongiae) against the multi drug resistant pathogens and revealed the broad spectrum activity. These studies suggest that, the extracts of both sponges have complex mixture of structurally diverse brominated pyrrole alkaloids [30].
Besides to Demospongiae, another group of sponge is known for their wide production of biological active compounds. As a result, a calcispongia known as Clathrina clathrus was collected in Mediterranean Sea located in France were evaluated for their antimicrobial compounds. The antimicrobial compounds were extracted using methanol and the NMR study has shown the existence of clathridimine, a novel alkaloid two aminoimidazole, clathridine along with preclathridine and clathridine-zinc complex. Here, the compound clathridimine exhibited the antibacterial activity against E. coli [31]. Alam, et al. [32] evaluated the antibacterial activity of Siphonocholin marine steroid recognized in red marine sponge, Siphonochalina siphonella against multidrug resistant P. aeruginosa and A. baumannii biofilm formation. The marine compound was able to decrease the virulence function resulting inhibition of biofilm formation at 64 and 256 μg/ml for P. aeruginosa and A. baumanii respectively. Constantly, bioactive compounds such as sipholenone A, sipholenol A, neviotine A and sipholenol L have been isolated and identified from the red sea sponge S. siphonella was evaluated for their antibacterial property against P. aeruginosa and E. coli [33].
Seven new cyclic peptides callyaerins were isolated from ethyl alcoholic fraction of red sea sponge Callyspongia aerizusa and examined for their diverse biological activity such as antibacterial, cytotoxic and antifungal activity. Callyaerins E has the antibacterial property against E. coli [34]. The peptide obtained from marine sponge has the distinct structures with specific amino acid which is uncommon or not present in other sources [35]. Several researches say the marine sponge peptide are safe, economical and has the broad range of biological properties [36,37].
Likewise, alkaloid-based compound is another group of metabolites which has been broadly documented in marine sponges particularly, bromopyyrole alkaloidsbased compounds only found in the marine sources. Consequently, El-Hawary and his co-workers (2019) [38] evaluated the antibacterial of two brominated indole alkaloids compounds from red sea sponge Callyspongia siphonella against P. aeruginosa. Moreover, a sponge A. ingens collected from the southeast Sulawesi marine park produced a novel compound 3-alkylpiperdine alkaloid that showed a specific antibacterial activity against E. coli by inhibiting amyloid beta 42 productions which is induced by aftin 5 at 26 μM concentration. Esposito and his team (2019) [39] isolated eleven alkaloid compounds have been recognized from the marine sponge A. ingens in the same location. Out of eleven alkaloids, compound Halicyamine showed antibacterial activity against E. coli at 100 μg/disc. In the same way, the bioactive bis- indole alkaloids, spongosoritins A–D and spongocarbamides A were identified from marine sponge Spongosorites sp and evaluated for the antibacterial activities against multi drug resistant gram-negative bacteria such as E. coli, K. Pneumonia and Salmonella sp and displayed a strong activity against all the tested organisms [40]. Also, the same group of compound Myrindole A was recognised in the marine sponge, Myrmekioderma sp and displayed the antibacterial activity against E. coli at 37.5 μM [41]. A compound, Zamamidine D collected from a marine sponge, Okinawan Amphimedon sp showed potent antibacterial against E. coli [42].
Similarly, a dimeric compound strongylophorine was isolated from Philippine marine sponge Strongylophora (Petrosia) exhibited anti Salmonella typhi activity [43]. The antimicrobial compounds 9-diynoic acid, 11E-tetradecadiene-5, C14 acetylenic acid 7E were isolated from Peninsula marine sponge of Oceanapia which contains CH=CH-C unit. These compounds displayed inhibitory effect against both gram positive and gram-negative bacteria [44]. The glycolipid compound Caminosides BD was identified from the Marine Sponge (Caminus sphaeroconia) showed antibacterial activity against E. coli [45] and the antibacterial activity of nitrogen heterocyclic marine sponge compound cribrostatin was tested against multidrug resistant Neisseria gonorrhoeae [46]. Four alkaloid toxins were identified from Arenosclera brasiliensis (marine sponge) evaluated against antibiotic resistant bacteria P. aeruginosa [47]. Urban et al. (1999) [48] studied the antibacterial activity of Axinellamines which is isolated from the marine sponge Axinella sp in Australia against Helicobacter pylori. The penaresidin compounds were isolated from Penares sponges evaluated the antibacterial potency and exhibited the good activity against E. coli [49]. The compounds identified in marine sponges are summarized in Table 1 and Figure 1.
| Compound Name | Sponge Name | Target Organism | References |
|---|---|---|---|
| Hyrtinadine D | Hyrtios erecta | E. coli | Kubota, et al. [23] |
| Hyrtiosenolides A and B | Hyrtios sp | E. coli | Youssef, et al. [24] |
| Agelasidine C and D | Agelas clathrodes | Proteus vulgaris and Klebsiella pneumoniae | Medeiros, et al. [25] |
| Ageliferin and bromoageferin | A. conifera | E. coli | Kobayashi, et al. [26] |
| Sceptrin | A. sceptrum | Pseudomonas aeruginosa | Walker, et al. [27] |
| Methanolic extracts | the turkey sea marine sponges | P. aeruginosa and Proteus vulgaris | Konuklugil, et al. [28] |
| Hymenialdisine, 10E hymenialdisine and spongiacidine | Axinella verrucosa | Acinetobacter septicus, Proteus vulgaris and Pseudomonas aeruginosa | Yassin, et al. [29] |
| Ethyl acetate extract | A. damicorins | P. aeruginosa and other human pathogens | Ines, et al. [30] |
| Brominated pyrrole alkaloids | Agelas oroides | Multi drug resistant pathogens | Ines, et al. [31] |
| New 2 aminoimidazole alkaloid and clathridine | Clathrina clathrus | E. coli | Roué, et al. [32] |
| Siphonocholin | Siphonochalina siphonella | Acinetobacter baumannii and P. aeruginosa | Alam, et al. [33] |
| Sipholenone A, sipholenol A, neviotine A and sipholenol L | Siphonochalina siphonella | E. coli and P. aeruginosa | Al-Massarani, et al. [34] |
| New cyclic peptides callyaerins E | Callyspongia aerizusa | E. coli | Ibrahim, et al. [35] |
| Brominated indole alkaloids | Callyspongia siphonella | P. aeruginosa | El-Hawary, et al. [36] |
| 3-alkylpiperdine alkaloid | A. ingens | E. coli | Dewi, et al. [37] |
| Halicyamine | A. ingens | E. coli | Esposito, et al. [38] |
| Strongylophorine | Strongylophora | Salmonella typhi | Balbin, et al. [39] |
| 11E-tetradecadiene-5, 9-diynoic acid, C14 acetylenic acid 7E | Oceanapia | Gram negative organism | Matsunaga, et al. [40] |
| Caminosides BD | Caminus sphaeroconia | E. coli | Linington, et al. [41] |
| Cribrostatin | Marine sponge | Neisseria gonorrhoeae | Pettit, et al. [42] |
| Alkaloid toxins | Arenosclera brasiliensis | P. aeruginosa | Torres, et al. [43] |
| Axinellamines | Axinella | Helicobacter pylori. | Urban, et al. [44] |
| Zamamidine D | Okinawan Amphimedon | E. coli | Kubota, et al. [45] |
| Myrindole A | Myrmekioderma | E. coli | Moosmann, et al. [46] |
Table 1: Antibacterial compounds isolated from marine sponges.
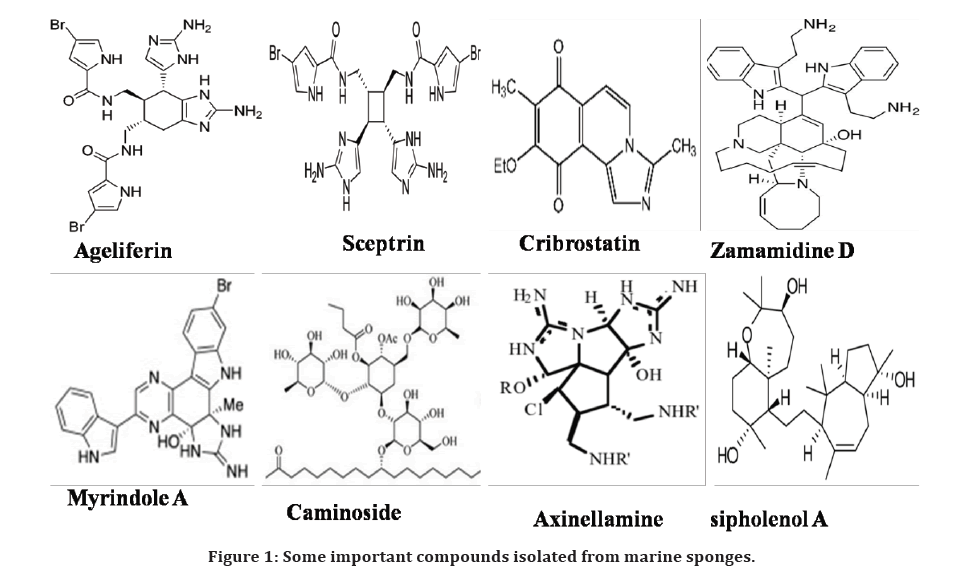
Figure 1. Some important compounds isolated from marine sponges.
Corals
Corals are one of the most important marine resources for the production of secondary metabolites such as steroids, terpenes, alkaloids and prostaglandins which provides lead for drug discovery. Keeping this in mind, bioactivity of two new cerebrosides, ceramide, sarcoehrenosides A and B, and three cerebrosides 3, 5, and 6 isolated from the Taiwan Donhsha Islands octocoral Sarcophyton ehrenbergi was investigated for their antibacterial activity Salmonella Enteritidis and Shigella sonnei [50]. Krishna and his team (2004) [51] isolated the compounds namely; two new sphingosines from Pseudopterogorgia australiensis, in Indian Ocean and their structures were analysed using spectral analysis. Compounds 2S,3S,4R- 2-((2'R)-2'-hydroxy nonadecanoyl amino) nonadecane- 1,3,4-triol, (2S,3R,4E)-2-(heptadecanoylamino) octadec- 4-ene-1,3-diol and 2-(docosanoyl amino nonadecane- 1,3-diol exhibited medium antibacterial activity against P. aeruginosa, P. vulgaris and E. coli,. The antibacterial activity of Two sphingolipids, (2S,3S,4R)-1,3,4- triacetoxy-2-l((R)-20–acetoxy octadecanoyl) amino] octadecane and (2S,3S,4R)-1,3,4-trihydroxy- 2-[((R)- 20-hydroxytetradecanoyl) amino] tricosane which were isolated from Sinularia leptoclados evaluated against human pathogens and exhibited kind antibacterial effect against Gram-negative bacterias [52]. A soft coral Sarcophyton troheliophorum isolated from red sea produced the secondary metabolite compounds Sarcotrocheliol acetate and Sarcotrocheliol exhibited the potent antibacterial activity against Acinetobacter sp at 4.34 μM MIC concentration [53]. Antibacterial activity of steroids compounds was exhibited the potent activity against E. coli [54].
A Diterpene compound isolated from Sarcophyton sp in Borneo showed the potent antibacterial activity against Vibrio sp [55]. Novel Cembranoid Diterpene compound Sarcophytol B from the sea soft coral Sarcophyton sp in China was displayed the antibacterial activity against Vibrio sp [56]. S. trocheliophorum from red sea produced the novel compounds trocheliane (tetracyclic biscembrane hydrocarbon) and sarcotrocheldiol A and B and diterpene cembrene-C that was evaluated for their antibacterial activity against multi drug resistant A. baumanii [57]. Furthermore, the antibacterial activity of xeniumbellal and penta hydroxygorgosterol isolated from the soft coral Xenia umbellate was assessed against A. baumannii and showed the potent antibacterial activity at 0.22 and 0.28 mM MIC concentration [58].
The diterpenoids compounds namely 9-deoxyxeniolide-A and B which were isolated from the Philippines sea soft coral Xenia sp exhibited the antibacterial activity [59]. Similarly, another study reported the seven novel diterpenoids which are xenicane type compounds like xeniolides I–K and novaxenicins A–D. The compounds were separated X. novae-britanniae in southern Kenya and displayed the antibacterial activity against E. coli at 1.25g/ml [60]. The antibacterial activity of ethyl acetate extract of J. Juncea collected from Indian Ocean was evaluated against E. coli and exhibited the activity which shows the gorgonian is the main basis for interesting compounds [61].
A group of novel diterpenoids, gemmacolides N–S with known already existing compounds isolated in the genus gorgonian Dichotella gemmacea in China and their biological activity was evaluated. The compounds juncenolide D, and juncins R, S, U and gemmacolides N, O, Q displayed the activity against E. coli [62]. Similarly, the same group has isolated the six novel briarane diterpenoids namely gemmacolides T-Y with already existing compounds from the same soft coral sea gorgonian D. gemmacea in China and those were exhibited the antibacterial activity against E. coli [63]. Likewise, the antibacterial activity of novel gemmacolides AZ–BF (briarane diterpenoids) those were isolated from gorgonian Dichotella gemmacea in china and evaluated for their bioactivity. The compound dichotelllides O was active against E. coli [64]. These Briarane diterpenoids are the interesting group of oxidized molecules consists of bicyclic carbon structure with γ-lactone ring combined with the 10-membered ring which is mainly isolated from gorgonians that are reported for wide varieties of bioactivities [65,66]. The compounds isolated from marine corals are summarized in Table 2 and Figure 2.
| Compound Name | Coral Name | Target Organism | References |
|---|---|---|---|
| Cerebrosides and ceramide | Sarcophyton ehrenbergi | Salmonella Enteritidis and Shigella sonnei | Cheng, et al. [50] |
| Sphingolipids | Sinularia leptoclados | Gram negative bacteria | Bala, et al. [51] |
| Sphingosines | Pseudopterogorgia australiensis | E. coli, Proteus vulgaris, and P. aeruginosa | Krishna, et al. [52] |
| Xeniolide I | X. novae-britanniae | E. coli | Bishara, et al. [53] |
| Sarcotrocheliol acetate and Sarcotrocheliol | Sarcophyton troheliophorum | Acinetobacter sp | Al-Footy, et al. [54] |
| 11 α -acetoxy-cholesta-24-en-3β,5α,6β-triol | Sarcophyton sp | E. coli | Wang, et al. [55] |
| Cembradiene | Sarcophyton sp | Vibrio sp | Kamada, et al. [56] |
| Sarcophytol B | Sarcophyton sp | Vibrio sp | Cao et al. [57] |
| Trocheliane (tetracyclic biscembrane hydrocarbon) and sarcotrocheldiol A and B and diterpene cembrene-C | S. trocheliophorum | A. baumanii | Zubair, et al. [58] |
| Xeniumbellal and penta hydroxygorgosterol | Xenia umbellate | A. baumanii | Ayyad, et al. [59] |
| Gemmacolides N, O, Q | Dichotella gemmacea | E. coli | Li, et al. [60] |
| Gemmacolides T-Y | D. gemmacea | E. coli | Li, et al. [61] |
| Dichotelllides O | D. gemmacea | E. coli | Li, et al. [62] |
Table 2: Antibacterial compounds isolated from marine corals.
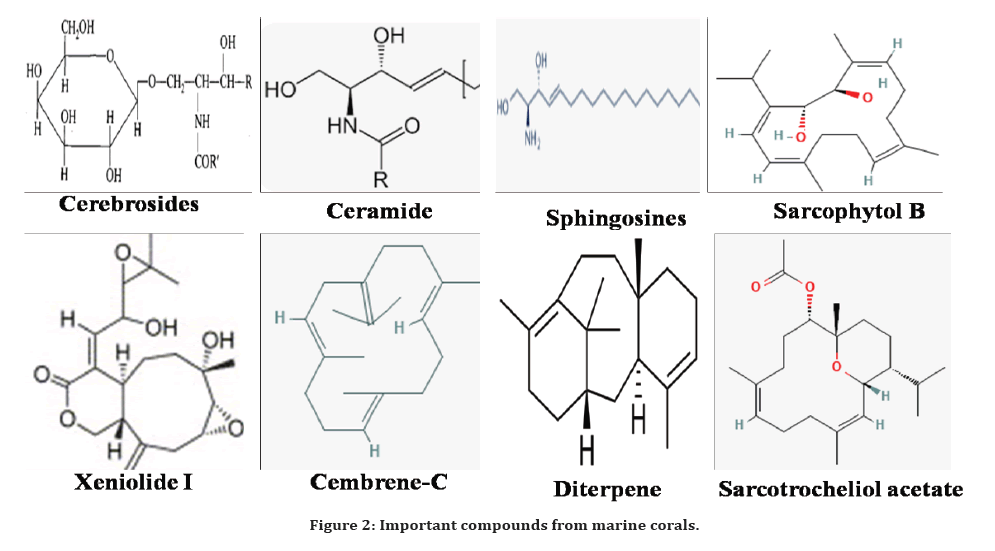
Figure 2. Important compounds from marine corals.
Tunicates
In Didemnidae family, an ascidian called Polysyncraton lithostrotum produced the compound namenamicin consisted of enediyne warhead and S-methyl group with sugar was evaluated for its bioactivity and the compound showed the excellent bioactivity against K. pneumonia at 0.25 μg/mL which suggesting that, this compound is more potent than the penicillin G [67]. A novel poly unsaturated amino alcoholic compound Crucigasterins was isolated from a tunicate Pseudodistoma Crucigaster and exhibited the strong antibacterial activity against gram negative organism E. coli at 100 g/ml [68]. Other amino alcoholic compounds Pseudoaminols A–G from tunicate Pseudistoma sp obtained in Korean coast and their structure was similar to the above compound crucigasterins. The isolated compound has the glycine based amino alcohol along with the carboxymethyl group and showed the important activity against E. coli and S. typhimurium [69]. The other antibacterial amino alcoholic compound distaminolyne A isolated from P. opacum exhibited the activity against E. coli at 64 μg/ ml [70]. Two spiroketals compounds Didemnaketals F and G were obtained from an Ascidian Didemnum sp in red sea and displayed the antibacterial activity against E. coli [71]. Spiroketal molecule is an important molecule occurred in natural products particularly, in case of marine ecosystem, Didemnum sp have been the significant source of spiroketal molecules [72].
Styelin, an antimicrobial peptide was isolated from a tunicate Styela clava showed a broad-spectrum activity against variety of pathogens such as gram positive and gram-negative pathogens (P. aeruginosa, S. typhimurium and E. coli) [73,74]. Saude and his team (2014) [75] was isolated a antimicrobial peptide clavanin consists of approximately 18 to 23 aminoacide and phenylalanine residues in their structure and exhibited the antibacterial activity against multi-drug resistant E. coli, K. Pneumonia and P. aeruginosa. Similarly, from the Halocynthia Papillosa in solitary sea, two cationic peptides namely papillosin and halocyntin were evaluated against a library of gram-negative bacteria. The authors explored the mechanism of action of two peptides wherein the protein interaction indicating the affinity with membrane lipids [76]. The compound homodimer dicynthaurin containing two cysteine helical peptides has been isolated from H. aurantium which is similar to marine ascidian peptide heteromer halocidin [77] and showed an antibacterial activity against multi drug resistant E. coli and P. aeruginosa at 140 μg/ml [78]. Similarly, turgencins a novel peptide was recognized from a tunicate Synoicum turgens in Arctic region and displayed activity against E. coli at 0.8 μM [79]. Consequently, the peptide like compounds halocyamines A and B consist L- DOPA and a 6- bromoindole DOPA were identified from H. roretzi in Japan and evaluated their biological activity against P. aeruginosa [80].
From the Micronesian Eudistorma sp, Eudistomins W and X were isolated and tested for their biological efficacy against various pathogens. The Eudistomins X was active against E. coli at 5-10 μg/disk [81]. The antibacterial alkaloid compounds Didemnolines A–D has been recognised in the Didemnum sp collected from islands of Mariana which were evaluated for biological activity and displayed the efficacy against E. coli [82] and also an alkaloid compound Ascididemin was displayed an activity against E. coli at 2.6 μM [83]. The compounds isolated from marine tunicates are summarized in Table 3 and Figure 3.
| Compound Name | Tunicate Name | Target Organism | References |
|---|---|---|---|
| Namenamicin | Polysyncraton lithostrotum | K. pneumonia | McDonald, et al. [67] |
| Crucigasterins | Pseudodistoma Crucigaster | E. coli | Jares, et al. [68] |
| Pseudoaminols A–G | Pseudistoma sp | E. coli and S. typhimurium | Won, et al. [69] |
| Distaminolyne A | P. opacum | E. coli | Wang, et al. [70] |
| Didemnaketals F and G | Didemnum sp | E. coli | Shaala, et al. [71] |
| Styelin | Styela Clava | P. aeruginosa, E. coli and S. typhimurium | Lee, et al. [72] |
| Papillosin and halocyntin | Halocynthia Papillosa | E. coli | Galinier, et al. [73] |
| Dicynthaurin | H. aurantium | E. coli and P. aeruginosa | Jang, et al. [74] |
| Halocyamines A and B | H. roretzi | P. aeruginosa | Azumi, et al. [75] |
| Turgencins | Synoicum turgens | E. coli | Hansen, et al. [76] |
| Eudistomins X | Eudistorma sp | E. coli | Schupp, et al. [77] |
| Didemnolines A–D | Didemnum sp | E. coli | Schumacher, et al. [78] |
| Ascididemin | Cystodytes | E. coli | Lopez, et al. [79] |
Table 3: Antibacterial compounds isolated from marine tunicates.
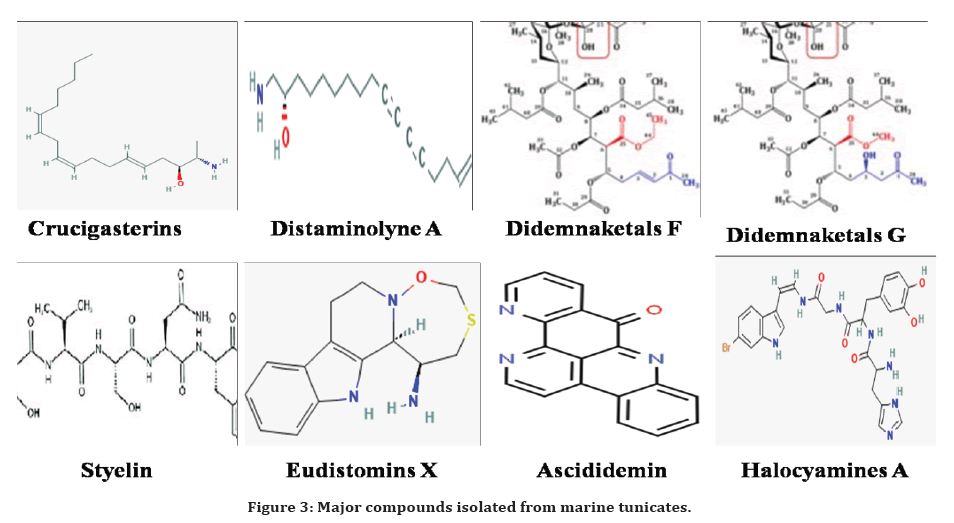
Figure 3. Major compounds isolated from marine tunicates.
Bryozoans
A novel steroid compounds ponasterone and ponasterone were identified from the bryozoans Alcyonidium gelatinosum in Arctic region exhibited the strong activty against E. coli and P. aeruginosa [84]. Another group identified six new alkaloids compounds pterocellins A-F from the bryozoans Pterocella vesiculosa and evaluated for their bioactivity. The result suggested that, a compound Pterocellins A and B inhibits the growth of two human pathogens such as E. coli and P. aeruginosa [85,86].
Conclusion
The marine antibacterial compounds identified in marine invertebrates (sponges, corals, tunicates and bryozoans) tested against gram negative multidrug resistant bacteria are concise in table 1, 2 and 3. It is suggested that, these marine compounds are distinct, lavish and effectively fight against the drug resistant bacteria. Most of the antibacterial compounds were isolated from marine sponges and corals. These marine compounds were effective against gram negative organisms and also have other biological properties which are not discussed here. These compounds may be entered into the clinical trials resulting discovery of distinct and novel compound with antibacterial potency to fight against drug resistant bacteria.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al- Kharj, Saudi Arabia for its support for this research work.
Conflict of Interest
The authors declare no conflicts of interest relevant to this article.
References
- Ventola CL. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm Ther 2015; 40:277.
- ONeill J. Antimicrobial resistance: Tacking a crisis for the health and wealth of nations. Rev Antimicrob 2014; 20:1-16.
- de Kraker ME, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050?. PLoS Med 2016; 13:e1002184.
- Pop-Vicas A, Opal SM. The clinical impact of multidrug-resistant gram-negative bacilli in the management of septic shock. Virulence 2014; 5:206–212.
- Martens E, Demain AL. The antibiotic resistance crisis, with a focus on the United States. J Antibiot 2017; 70:520-526.
- Exner M, Bhattacharya S, Christiansen B, et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg Infect Control 2017; 12:1-10.
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010; 74:417–433.
- Organization WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed? WHO, Geneva, 2017.
- Aminov RI. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front Microbiol 2010; 1:1–7.
- Moellering RC. Discovering new antimicrobial agents. Int J Antimicrob Agents 2011; 37:2–9.
- Puglisi MP, Sneed JM, Ritson WR, et al. Marine chemical ecology in benthic environments. Nat Prod Rep 2019; 36:410–429.
- Pietra F. Biodiversity and natural product diversity. 1st Edn. Pergamon Press: Oxford, UK, 2002; 21:19-47.
- Rohde S, Nietzer S, Schupp PJ. Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE 2015; 10:e0132236.
- McClintock JB, Amsler CD, Baker BJ. Overview of the chemical ecology of benthic marine invertebrates along the western Antarctic Peninsula. Integr Comp Biol 2010; 50:967–980.
- Eskander R, Al-Sofyani AA, El-Sherbiny MMO, et al. Chemical defense of soft coral sinularia polydactyla from the red sea against marine biofilm-forming bacteria. J Ocean Univ 2018; 17:1451–1457.
- Horta A, Alves C, Pinteus S, et al. The marine origin of drugs. In Phycotoxins. 2nd Edn. John Wiley & Sons: London, UK, 2015.
- Carroll AR, Copp BR, David RA, et al. Marine natural products. Nat Prod Rep 2019; 36:122-173.
- Jiménez C. Marine natural products in medicinal chemistry. ACS Med Chem Lett 2018; 9:959–961.
- Rohde S, Schupp PJ. Allocation of chemical and structural defenses in the sponge Melophlus sarasinorum. J Exp Mar Biol Ecol 2011; 399:76–83.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid Based Complement Altern Med 2013.
- Jha RK, Zi-rong X. Biomedical compounds from marine organisms. Mar Drugs 2004; 2:123–146.
- Laport M, Santos O, Muricy G. Marine sponges: Potential sources of new antimicrobial drugs. Curr Pharm Biotechnol 2009; 10:86-105.
- Kubota T, Nakamura K, Sakai K, et al. Hyrtinadines C and D, New Azepinoindole-type alkaloids from a marine sponge Hyrtios sp. Che Pharm Bull 2016; 64:975–978.
- Youssef DT, Singab AN, Van Soest RW, et al. Hyrtiosenolides A and B, two new sesquiterpene gamma-methoxybutenolides and a new sterol from a Red Sea sponge Hyrtios species. J Nat Prod 2004; 67:1736–1739. ‘
- Medeiros MA, Lourenço A, Tavares MR, et al. (–)-Agelasidine A from Agelas clathrodes. Z Natur 2006; 61:472-476.
- Kobayashi J, Tsuda M. Ageliferins, potent actomyosin ATPase activators from the okinawan marine sponge Agelas sp. Tetrahedron 1990; 46:5579–5586.
- Walker RP, Faulkner DJ. Sceptrin, an antimicrobial agent from the sponge Agelas sceptrum. J Am Chem Soc 1981; 103:6772–6773.
- Di Cesare Mannelli L, Palma Esposito F, Sangiovanni E, et al. Pharmacological activities of extracts and compounds isolated from mediterranean sponge sources. Pharm 2021; 14:1329.
- Yassin M, Ammar I, Shabbar A. Screening a mediterranean sponge axinella verrucosa for antibacterial activity in comparison to some antibiotics. J Pharmacogn Phytochem 2013; 1:66–75.
- Ines T, Amina B, Khaled S, et al. Screening of antimicrobial activity of marine sponge extracts collected from Tunisian coast. Proc West Pharmacol Soc 2007; 50:152–155.
- Roué M, Domart-Coulon I, Ereskovsky A, et al. Cellular localization of clathridimine, an antimicrobial 2-aminoimidazole alkaloid produced by the Mediterranean calcareous sponge Clathrina clathrus. J Nat Prod 2010; 73:1277–1282.
- Alam P, Alqahtani AS, Husain FM, et al. Siphonocholin isolated from red sea sponge Siphonochalina siphonella attenuates quorum sensing controlled virulence and biofilm formation. Saudi Pharm J 2020; 28:1383–1391.
- Al-Massarani SM, El-Gamal AA, Al-Said MS, et al. In vitro cytotoxic, antibacterial and antiviral activities of triterpenes from the red sea sponge, Siphonochalina siphonella. Trop J Pharm Res 2015; 14:33–40.
- Ibrahim SRM, Min CC, Teuscher F, et al. Callyaerins A-F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg Med Chem 2010; 18:4947–4956.
- Yeung BK, Nakao Y, Kinnel RB, et al. The kapakahines, cyclic peptides from the marine sponge Cribrochalina olemda. J Organic Chem 1996; 61:7168-7173.
- Zhan KX, Jiao WH, Yang F, et al. Reniochalistatins A–E, cyclic peptides from the marine sponge Reniochalina stalagmitis. J Natur Products 2014; 77:2678-2684.
- Wang X, Yu H, Xing R, et al. Characterization, preparation and purification of marine bioactive peptides. BioMed Res Int 2017.
- El-Hawary SS, Sayed AM, Mohammed R, et al. Bioactive brominated oxindole alkaloids from the red sea sponge Callyspongia siphonella. Mar Drugs 2019; 17:465.
- Esposito G, Linh HM, Arlette L, et al. A collection of bioactive nitrogen containing molecules from the marine sponge Acanthostrongylophora ingens. Mar Drugs 2019; 17:472.
- Park JS, Cho E, Hwang JY, et al. Bioactive bis (indole) alkaloids from a Spongosorites sp. sponge. Mar Drugs 2021; 19:3.
- Moosmann P, Taniguchi T, Furihata K, et al. Myrindole A, an antimicrobial bis-indole from a marine sponge Myrmekioderma sp. Org Lett 2021; 23:3477-3480.
- Kubota T, Nakamura K, Kurimoto SI, et al. Zamamidine D, a manzamine alkaloid from an okinawan Amphimedon sp. marine sponge. J Nat Prod 2017; 80:1196-1199.
- Balbin-Oliveros M, Edrada RA, Proksch P, et al. A new meroditerpenoid dimer from an undescribed Philippine marine sponge of the genus Strongylophora. J Nat Prod 1998; 61:948–952.
- Matsunaga S, Okada Y, Fusetani N, et al. An antimicrobial C14 acetylenic acid from a marine sponge Oceanapia species. J Nat Prod 2000; 63:690–691.
- Linington RG, Robertson M, Gauthier A, et al. Caminosides BD, antimicrobial glycolipids isolated from the marine sponge Caminus sphaeroconia. J Nat Prod 2006; 69:173-177.
- Pettit RK, Fakoury BR, Knight JC, et al. Antibacterial activity of the marine sponge constituent cribrostatin 6. J Med Microbiol 2004; 53:61-65.
- Torres YR, Berlink RG, Nascimento GG, et al. Antibacterial activity against resistant bacteria and cytotoxicity of four alkaloid toxins isolated from the marine sponge Arenosclera brasiliensis. Toxicon 2002; 40:885-891.
- Urban S, De Almeida Leone P, Carroll AR, et al. Axinellamines A-D, novel imidazo-azolo-imidazole alkaloids from the Australian marine sponge Axinella sp. J Org Chem 1999; 64:731-735.
- Ohshita K, Ishiyama H, Takahashi Y, et al. Synthesis of penaresidin derivatives and its biological activity. Bioorg Med Chem 2007; 15:4910-4919.
- Cheng, SY, Wen ZH, Chiou SF, et al. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J Nat Prod 2009; 72:465–468.
- Krishna N, Muralidhar P, Kumar MM, et al. New Sphingosines from a gorgonian, Pseudopterogorgia australiensis Ridley, of the Indian Ocean. J Nat Prod 2004; 67:1423–1425.
- Bala SRG, Venkata RD, Bheemasankara RC, et al. Isolation and structural determination of new sphingolipids and pharmacological activity of africanene and other metabolites from Sinularia leptoclados. Chem Pharm Bull 1999; 47:1214–1220.
- Al-Footy K, Alarif W, Asiri F, et al S. Rare pyrane-based cembranoids from the Red Sea soft coral Sarcophyton troheliophorum as potential antimicrobial-antitumor agents. Med Chem Res 2014; 24:505–512.
- Wang Z, Tang H, Wang P, et al. Bioactive polyoxygenated steroids from the South China sea soft coral, Sarcophyton sp. Mar Drugs 2013; 11:775–787.
- Kamada T, Zanil II, Phan C, et al. A new cembrane, from soft coral genus Sarcophyton in Borneo. Nat Prod Commun 2018; 13:123–124.
- Cao F, Zhou J, Xu K, et al. New cembranoid diterpene from the South China sea soft coral Sarcophyton sp. Nat Prod Commun 2013; 8:1675–1678.
- Zubair M, Alarif WM, Al-Footy KO, et al. New antimicrobial biscembrane hydrocarbon and cembranoid diterpenes from the soft coral Sarcophyton trocheliophorum. Turk J Chem 2016; 40:385–392.
- Ayyad SEN, Alarif WM, Al-Footy KO, et al. Isolation, antimicrobial and antitumor activities of a new polyhydroxysteroid and a new diterpenoid from the soft coral Xenia umbellata. Z Nat 2016; 72:27–34.
- Vervoort HC, Fenical W. Antibacterial diterpenoids from an undescribed soft-coral of the genus Xenia. Nat Prod Lett 1995; 6:49–55.
- Bishara A, Rudi A, Goldberg I, et al. Novaxenicins A-D and xeniolides I-K,seven new diterpenes from the soft coral Xenia novaebrittanniae. Tetrahedron 2006; 62:12092–12097.
- Sastry VG, Sastry AVS, Kumar KE, et al. Chemical examination and biological evaluation of some marine coelenterates of the Indian Ocean. Int J Chem Sci 2008; 6:1291-1298.
- Li C, La MP, Sun P, et al. Bioactive (3Z,5E)-11,20-epoxybriara-3,5-dien-7,18-olide diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Mar Drugs 2011; 9:1403-1418.
- Li C, La MP, Tang H, et al. Bioactive briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Bioorg Med Chem Lett 2012; 22:4368-4372.
- Li C, La MP, Tang H, et al. Chemistry and bioactivity of briaranes from the South China Sea gorgonian Dichotella gemmacea. Mar Drugs 2016; 14:201.
- Blunt JW, Copp BR, Keyzers RA, et al. Marine natural products. Nat Prod Rep 2015; 32:116–211.
- Sung PJ, Su JH, Wang WH, et al. Survey of briarane-type diterpenoids-Part IV. Heterocycles 2011; 83:1241–1258.
- McDonald LA, Capson TL, Krishnamurthy G, et al. Namenamicin, a new enediyne antitumor antibiotic from the marine Ascidian polysyncraton lithostrotum. J Am Chem Soc 1996; 118:10898–10899.
- Jares-Erijman EA, Bapat CP, Lithgow-Bertelloni A, et al. Crucigasterins, new polyunsaturated amino alcohols from the mediterranean tunicate pseudodistoma crucigaster. J Org Chem 1993; 58:5732-5737.
- Won TH, You M, Lee SH, et al. Amino Alcohols from the Ascidian pseudodistoma Sp. Mar Drugs 2014; 12:3754-3769.
- Wang J, Pearce AN, Chan STS, et al. Biologically active acetylenic amino alcohol and N-Hydroxylated 1,2,3,4-Tetrahydro-Carboline constituents of the New Zealand Ascidian pseudodistoma opacum. J Nat Prod 2016; 79:607-610.
- Shaala LA, Youssef DTA, Ibrahim SRM, et al. Didemnaketals F and G, new bioactive spiroketals from a red sea ascidian Didemnum species. Mar. Drugs. 2014; 12: 5021–5034.
- Zang FM, Zhang SY, Tu YQ. Recent progress in the isolation, bioactivity, biosynthesis, and total synthesis of natural spiroketals. Nat Prod Rep 2018; 35:75-104.
- Lehrer RI, Tincu JA, Taylor SW, et al. Natural peptide antibiotics from tunicates: Structures, functions and potential uses. Integr Comp Biol 2003; 43:313-322.
- Lee IH, Cho Y, Lehrer RI. Styelins, broad-spectrum antimicrobial peptides from the solitary tunicate, styela clava. Comp Biochem Physiol 1997; 118:515-521.
- Saude AC, Ombredane AS, Silva ON, et al. Clavanin bacterial sepsis control using a novel methacrylate nanocarrier. Int J Nanomed 2014; 9:5055-5069.
- Galinier R, Roger E, Sautiere PE, et al. Halocyntin and papillosin, two new antimicrobial peptides isolated from hemocytes of the solitary tunicate, Halocynthia papillosa. J Pept Sci 2009; 15:48–55.
- Lee IH, Lee YS, Kim CH, et al. Dicynthaurin: An antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. Biochim Biophys Acta 2001; 1527:141–148.
- Jang WS, Kim KN, Lee YS, et al. Halocidin: A new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett 2002; 521:81-86.
- Hansen IK, Isaksson J, Poth AG, et al. Isolation and characterization of antimicrobial peptides with unusual disulfide connectivity from the colonial ascidian Synoicum turgens. Mar Drugs 2020; 18: 51.
- Azumi K, Yoshimizu M, Suzuki S, et al. Inhibitory effect of halocyamine, an antimicrobial substance from ascidian hemocytes, on the growth of fish viruses and marine bacteria. Experientia 1990; 46:1066-1068.
- Schupp P, Poehner T, Edrada R, et al. Eudistomins W and X, two new β-carbolines from the micronesian tunicate Eudistoma sp. J Nat Prod 2003; 66:272-275.
- Schumacher RW, Davidson BS. Didemnolines A-D, New N9-Substituted b-Carbolines from the marine ascidian Didemnum Sp. Tetrahedron 1995; 51:10125-10130.
- Lopez-Legentil S, Turon X, Schupp P. Chemical and physical defenses against predators in cystodytes (Ascidiacea). J Exp Mar Biol Ecol 2006; 332:27–36.
- Kine H, Johan I, Eirin G, et al. Ponasterone A and F, ecdysteroids from the arctic bryozoan Alcyonidium gelatinosum. Molecules 2018; 23:1481-1490.
- Yao B, Prinsep MR, Nicholson BK, et al. The pterocellins, novel bioactive alkaloids from the marine bryozoan Pterocella vesiculosa. J Nat Prod 2003; 66:1074–1077.
- Prinsep MR. Further pterocellins from the New Zealand marine bryozoan Pterocella vesiculosa. J Nat Prod 2008;71:134–136.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar , Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar , Cross Ref
Indexed at, Google Scholar,Cross Ref
Indexed at, Google Scholar,Cross Ref
Indexed at, Google Scholar,Cross Ref
Indexed at, Google Scholar,Cross Ref
Indexed at, Google Scholar,Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar ,Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
MD Nadeem Bari1*, Kuppannan Gobianand2, Malarvizhi Arthanari2, MD Rizwan Ansari1 and Imran Mohammad1
1Department of Basic Medical Sciences, College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia2Department of Microbiology, Vivekanandha College of Arts and Sciences for Women, Namakkal, Tamil Nadu, India
Received: 09-Aug-2022, Manuscript No. jrmds-22-71560; , Pre QC No. jrmds-22-71560(PQ); Editor assigned: 11-Aug-2022, Pre QC No. jrmds-22-71560(PQ); Reviewed: 26-Aug-2022, QC No. jrmds-22-71560(Q); Revised: 31-Aug-2022, Manuscript No. jrmds-22-71560(R); Published: 07-Sep-2022
