Research - (2019) Volume 7, Issue 6
Preparation and Characterization Iron Oxide (Fe3O4) Magnetic Nano Particleg
Sahar IA Al-Baldawi1*, Anes IS Al-Sammarraie2 and Firas H Alwade3
*Correspondence: Sahar IA Al-Baldawi, Department of Research Materials, Ministry of Science and Technology, Baghdad, Iraq, Email:
Abstract
Introduction: Nanotechnology is enabling technology that deals with Nano-meter sized objects. It is expected that nanotechnology will be developed at several levels: Materials, devices and systems. The nanomaterial’s level is the most advanced at present, both in scientific knowledge and in commercial applications. A decade ago, nanoparticles were studied because of their size-dependent physical and chemical properties.
Aim: To consider magnetite nanoparticles, their applications, synthesis and characterization methods; to synthesis magnetite nanoparticles; to characterize magnetite nanoparticles by different techniques.
Materials and methods: The most conventional method for obtaining Fe3O4 or γ-Fe2O3 is by co-precipitation. This method consists of mixing ferric and ferrous ions in a 1:2 molar ratio in highly basic solutions at room temperature or at elevated temperature.
Results and discussion: Particle size is measured by zetasizer. The average distribution of magnetic nanoparticles is 11 ± 2 nm. Thus, we can know size of particle. Reveal size, shape and distribution on the solution are measured by transmission electron microscopy (TEM). The following distribution was calculated from the distribution of NPs the average 13 ± 2 nm. Thus, we see size and shape nanopartical. The ζ (Zeta) potential of magnetite nanoparticles was –24 ± 2 mV at pH 6.5 ± 0.1. The size distribution of nanoparticles was studied by the methods of dynamic light scattering.
Conclusion: The resulting nanoparticles were measured using dynamic scattering light and transmission electron microscopy. Average values respectively, amounted to 11 ± 2 nm and 13 ± 2 nm. Zeta has also been measured. Its average value was -24 ± 2 mV at pH=6.5 ± 0.1. What is caused by the presence of carboxylic groups of citric acid on surface of nanoparticles? Such a value of the zeta potential is sufficient for prevent the adhesion of nanoparticles.
Keywords
Nanotechnology, Nano-magntic, Nanoparticle, Nano medicine, Iron Oxide (Fe3O4)
Introduction
Nanotechnology is enabling technology that deals with nano-meter sized objects. It is expected that nanotechnology will be developed at several levels: Materials, devices and systems [1]. The nanomaterial’s level is the most advanced at present, both in scientific knowledge and in commercial applications. A decade ago, nanoparticles were studied because of their sizedependent physical and chemical properties [2]. Now they have entered a commercial exploration period [3,4]. Living organisms are built of cells that are typically 10 μm across. However, the cell parts are much smaller and are in the sub-micron size domain. Even smaller are the proteins with a typical size of just 5 nm, which is comparable with the dimensions of smallest artificial nanoparticles. This simple size comparison gives an idea of using nanoparticles as very small probes that would allow us to spy at the cellular machinery without introducing too much interference [5]. Understanding of biological processes on the Nano scale level is a strong driving force behind development of nanotechnology [6]. Out of plethora of size-dependent physical properties available to someone who is interested in the practical side of nanomaterial’s, optical [7] and magnetic effects are the most used for biological applications.
Magnetic nanoparticles have become a powerful and versatile diagnostic tool for biology and Nanomedicine [8]. Given their extremely small size and high magnetization, such nanoparticles can be introduced into a living organism, where they can provide a means of monitoring and influencing cellular processes [9]. Once suitably attached, nanoparticles can also be used to transport proteins, nucleic acids, and other biomolecules through microfluidic circuits. In recent years, a sustained effort has been made to design and develop bio detection systems which combine magnetic nanoparticles NPs. Thus, the aim of this work is synthesis and characterization of magnetite nanoparticles for different applications.
The following tasks were assigned:
To consider magnetite nanoparticles: Their applications, synthesis and characterization methods.
To synthesis magnetite nanoparticles.
To characterize magnetite nanoparticles by different techniques. Magnetic nanoparticles can find application in various fields.
Magnetic separation
The processes of isolation and separation of specific molecules are used in almost all areas of biosciences and biotechnology, and are the most documented and currently the most useful application of magnetic particles. Various magnetic particles have been developed as magnetic carriers in separation processes including purification and immunoassays [10], magnetic resonance image and cancer diagnosis. The applications of MRI have steadily increased over the past decade. MRI offers the advantage of high spatial resolution of contrast differences between tissues. Due to the unique function of this imaging modality, there is a need to develop effective contrast agents that will enhance and widen its diagnostic utility.
Paramagnetic ion chelates and ferromagnetic or super paramagnetic nanoparticles, with sizes that generally range between 3 and 10 nm, have been developed as MR contrast agents and used in clinical diagnosis particularly, in medicine for local heating of tissues, increasing contrast on MRI [11] and Magnetic–nanoparticle induced hyperthermia: Hyperthermia is a promising approach to cancer therapy, and various methods inducing hyperthermia, such as the use of hot water, capacitive heating, and induction heating among others, have been employed [12,13]. A major technical problem with the application of hyperthermic treatments is the difficulty in heating the local tumor region to the intended temperature without damaging n o r m a l tissue. Conventional hyperthermic systems are designed to heat tissue to approximately 42.5°C to 44.0°C. However, higher temperatures can kill a greater number of tumor cells, and in principle, tumor specific hyperthermia can kill all types of tumor cells. Disadvantages of their small particle size, nano particles have the potential to negatively affect the respiratory and digestive tracks and the skin or eye surface, thus exposes workers to hazards. Small production volumes and high cost remain the main barriers to the use of nanotechnology. The time for commercializing a product is long [14].
Materials and Methods
In the last decades, much research has been developed to the synthesis of iron oxide NPs, and many reports have described efficient synthesis approaches to produce the shape controlled, stable, biocompatible, and mono-dispersed iron oxide NPs. The most common methods including co-precipitation, thermal decomposition, hydrothermal synthesis [15], microemulsion, sonochemical synthesis, and sonochemical synthetic route [16] can all be directed to the synthesis of high quality of iron oxide NPs. In addition, these NPs can also be prepared by the other methods such as electrochemical synthesis [15], laser pyrolysis techniques [17], microorganism or bacterial synthesis (especially the Magnetotactic bacteria and iron reducing bacteria) [15,16], etc. The most conventional method for obtaining Fe3O4 or γ-Fe2O3 is by coprecipitation. This method consists of mixing ferric and ferrous ions in a 1:2 molar ratio in highly basic solutions at room temperature or at elevated temperature. The size and shape of the iron oxide NPs depends on the type of salt used (such as chlorides, sulfates, nitrates ,perchlorates, etc.), the ferric and ferrous ions ratio, the reaction temperature, the pH value, ionic strength of the media, and the other reaction parameters (e.g. stirring rate, dropping speed of basic solution). This method would critically affect the physical and chemical properties of the nano sized iron oxide particles. In addition, Fe3O4 NPs are not very stable under ambient conditions and are easily oxidized to Fe2O3 or dissolved in an acidic medium. In order to avoid the possible oxidation in the air, the synthesis of Fe3O4 NPs must be done in anaerobic conditions. Based on this point, Fe3O4 NPs can also be utilized to prepare the Fe2O3 NPs by oxidation or anneal treatment under oxygen atmosphere. And oxidation is not the important influence factor for Fe2O3 NPs due to its own chemical stability in alkaline or acidic environment. However, this method generates particles with a wide particle size distribution, which requires secondary size selection sometimes. A wide particle size distribution will result in a wide range of blocking temperatures (TB) due to TB depends on particle size and, therefore non-ideal magnetic behavior for many applications. Bomatı et al. reported a synthesis of mono dispersed, uniform, and narrow size distributional Fe3O4 NPs (the diameter of NPs was 8.5 ± 1.3 nm) by co-precipitation without surfactants, there action in an aqueous solution with a molar ratio of FeII/FeIII=0.5 and a pH=11–12, and the colloidal suspensions of the magnetite can be then directly oxidized by aeration to form colloidal suspensions of γ-Fe2O3 [17].
Sample synthesis
Magnetite nanoparticles were obtained by chemical precipitation from a mixed solution of biomedicine and trivalent iron salts. An initial solution of reagents was prepared by dissolving FeCl3·6H2O (1.3 g) and FeCl2·4H2O (0.478 g) in water (12 ml) under stirring with a magnetic stirrer at room temperature. A 0.1 M NaOH solution (100 ml) was placed into the reaction chamber. Magnetite nanoparticles were formed by the following chemical reaction.
For subsequent colloid stabilization, a 20-mg/ml solution (100 ml) of citric acid was preliminarily prepared. Nitrogen was bubbled through the chambers with the solution of the salts, stabilizing solution, and the reaction chamber containing sodium hydroxide for 10 min to remove dissolved oxygen. Then, the solution of iron salts was injected under nitrogen pressure for several seconds into the sodium hydroxide solution under rigorous stirring and the mixture was stirred for 30 min under nitrogen. The resulting black precipitate of magnetite nanoparticles was deposited with the use of a permanent magnet, and the supernatant was poured out by displacing it with nitrogen into a dump chamber. Then, a 20-mg/ml citric acid solution (25 ml) was added to the precipitate under pressure of nitrogen under stirring and stirred once again. The procedure was repeated four times (until the citric acid solution was consumed). Note that, due to the stabilizing effect of citric acid carboxylate ions, the particle size and colloid sedimentation rate decrease in the course of washing. In the last washing cycle, 5–10 ml of the liquid was kept and the system was intensely stirred. The resulting colloidal solution of magnetic nanoparticles was placed into a dialysis membrane, immersed in a vessel with water (1–1.5 L), and dialyzed for 4 days under gentle stirring. All processes of reagent mixing and washing were performed under nitrogen at a pressure regulated in the channels with a system of valves. The concentration of the resulting magnetic colloid determined by the solid residue method was 5 mg/Ml which illustrated in Figure 1.

Figure 1: Magnetic NPs instrument.
Advantage: It is safety and simplest method at laboratory and gives smaller size of nanoparticle less than 10 nm but disadvantage we need more time several week to prepare Fe3O4 or γ-Fe2O3.
Results and Discussion
Figure 2 reveals distribution of size particles measured by zetasizer. The average distribution of magnetic nanoparticles is 11±2 nm. Thus, we can know size of particle.
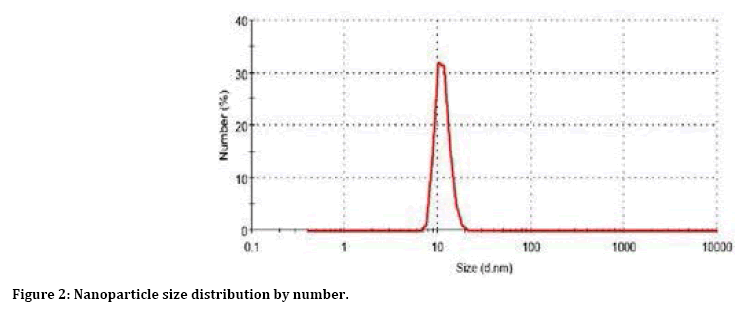
Figure 2: Nanoparticle size distribution by number.
Figure 3 reveal size and shape and distribution on the solution measured by transmission electron microscopy TEM. The following distribution was calculated from the distribution of NPs (Figure 3). The average 13 ± 2 nm. Thus, we see size and shape nanopartical.

Figure 3: Measurement of size by using TEM.
Figure 4 reveals distribution of magnetic nano particle measured by TEM. This distribution is community particle. The average distribution of these particles is 13±2 nm. Thus, we can use this test high efficiency. To comparison with other method the ferro fluid was formed by nanoparticles (2>d>10 ) nm associated in branched or dendritic structures [18] as shown in Figure 4.
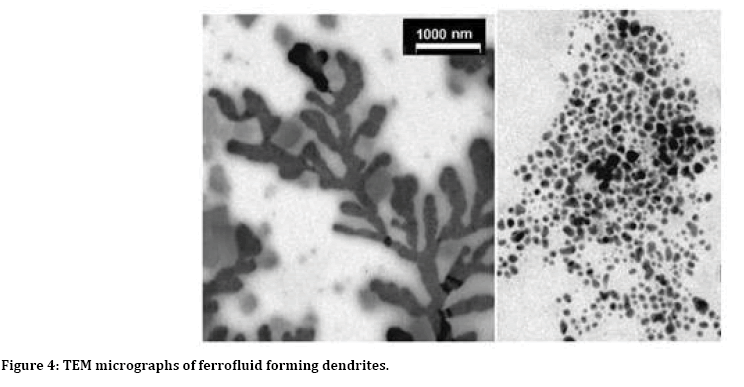
Figure 4: TEM micrographs of ferrofluid forming dendrites.
The zeta potential of magnetite nanoparticles was –24 ± 2 mV at pH 6.5 ± 0.1. The size distribution of nanoparticles was studied by the methods of dynamic light scattering (Figure 4) and TEM (Figure 3), which yielded average nanoparticle sizes of 13 ± 2, respectively. The nanoparticle size determined by dynamic light scattering was larger than that obtained from analysis of TEM images. This is explained by the fact that, in the former case the nanoparticle sizes are measured in an aqueous medium; therefore, the result obtained corresponds to the hydrodynamic particle radius (Figure 5).
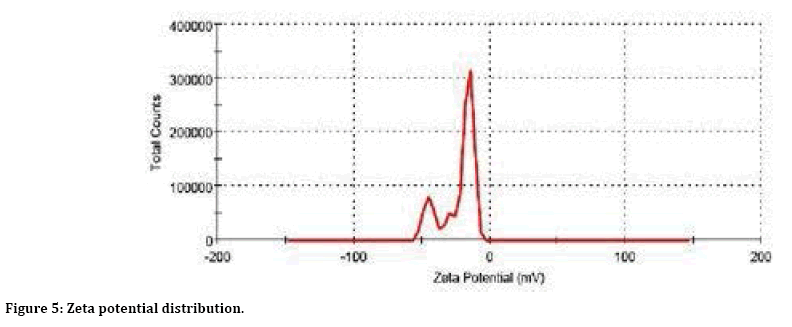
Figure 5: Zeta potential distribution.
Conclusion
Magnetic nanopartictes Fe3O4 have been prepared by chemical precipitation method combined with annealing under inert gas at 55°C. The resulting nanoparticles were measured using dynamic scattering light and transmission electron microscopy. Average values respectively, amounted to 11 ± 2 nm and 13 ± 2 nm. Zeta has also been measured. Its average value was -24 ± 2 mV at pH=6.5 ± 0.1. what is caused by the presence of carboxylic groups of citric acid on surface of nanoparticles. Such a value of the zeta potential is sufficient for prevent the adhesion of nanoparticles.
References
- Feynman R. There's plenty of room at the bottom. Science. 1991; 254:1300-1301.
- Murray CB, Kagan CR, Bawendi MG. Synthesis and characterisation of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu Rev Mater Sci 200; 30:545-610.
- Mazzola L. Commercializing nanotechnology. Nature Biotechnol 2003; 21:1137-1143.
- Paull R, Wolfe J, Hebert P, et al. Investing in nanotechnology. Nature Biotechnol 2003; 21:1134-1147.
- Taton TA. Nanostructures as tailored biological probes. Trends Biotechnol 2003; 20: 277-279.
- Whitesides GM. The 'right' size in Nanobiotechnology. Nature Biotechnol 2003; 21:1161-1165.
- Parak WJ, Gerion D, Pellegrino T, et al. Biological applications of colloidal nanocrystals. Nanotechnol 2003; 14:15-27.
- Pankhurst QA, Connolly J, Jones SK, et al. Applications of magnetic nanoparticles in biomedicine. J Phys Appl Phys 2003; 36.
- Dubertret B, Skourides P, Norris DJ, et al. In Vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002; 298: 17591762.
- Dunnill P, Lilly MD. Purification of enzymes using magnetic bioaffinity materials. Biotechnol Bioeng 1974; 16:987–990.
- Magin RL, Wright SM, Niesman MR, et al. Liposome delivery of NMR contrast agents for improved tissue imaging. Magn Reson Med 1986; 3:440–447.
- Cavaliere R, Ciocatto EC, Giovanella BC, et al. Selective heat sensitivity of cancer cells. Biochemical and clinical Studies. Cancer 1967; 20: 1351–1381.
- Ikeda N, Hayashida O, Kameda H, et al. Experimental study on thermal damage to dog normal brain. Int J Hyperthermia 1967; 10:553–561.
- Rabias I, Tsitrouli D, Karakosta E, et al. Rapid magnetic heating treatment by highly charged maghemite nanoparticles on Wistar rats exocranial glioma tumors at microliter volume. Biomicrofluidics 2010; 4:24111.
- Castaing R. In advances in electronics and electron physics. New York: Academic 1960; 317.
- Pascal C, Pascal JL, Favier F, et al. Electrochemical synthesis for the control of γ-Fe2O3 nanoparticle size. Morphology, microstructure, and magnetic behavior. Chem Mater 1996; 11:14.
- Bomati Miguel O, Mazeina L, Navrotsky A, et al. Calorimetric study of maghemite nanoparticles synthesized by laser-induced pyrolysis. Chem Mater 2008; 20:59.
- Alberto BD, Mohallema NDS, Novak MA, et al. Preparation of ferrofluid from cyclodextrin and magnetite, J Magnetism Magnetic Mater 2004; 272:2395–2397.
Author Info
Sahar IA Al-Baldawi1*, Anes IS Al-Sammarraie2 and Firas H Alwade3
1Department of Research Materials, Ministry of Science and Technology, Baghdad, Iraq2Department of Application of nuclear, Ministry of Science and Technology, Baghdad, Iraq
3Department of Basic Since, College of Dentistry, Mustansiriyah University, Baghdad, Iraq
Citation: Sahar IA Al-Baldawi, Anes IS Al-Sammarraie, Firas H Alwade, Preparation and Characterization Iron Oxide (Fe3O4) Magnetic Nano Particleg, J Res Med Dent Sci, 2019, 7(6): 107-112.
Received: 30-Oct-2019 Accepted: 18-Dec-2019
