Review - (2022) Volume 10, Issue 7
Quality of Life Assessment of Aortic Valve Neocuspidization for Autologous Pericardium: A Systematic Review
Komarov RN, Badalyan SS*, Lenkovets M, Tcheglov MI, Magomedova KA and IM Sechenov
*Correspondence: Badalyan SS, First Moscow State, Medical University, Moscow, Russian Federation, Email:
Abstract
In 2007, Ozaki et al. conducted the first aortic valve neocuspidation utilizing glutaraldehyde-treated autologous pericardium. Because long-term anticoagulation is not necessary, this procedure has become an option to tissue and mechanical valves. This approach has proven to be a viable alternative to bio prosthetic and mechanical valves, both of which have obvious drawbacks. Digital databases were searched from 2014 to 2022 for the phrases “autologous pericardium”, “aortic valve replacement”, and “aortic valve reconstruction”. Mortality, freedom from surgery, thromboembolic and endocarditis events, and echocardiography findings were all analysed in this study. Non-cardiac causes of death and reoperation were virtually nonexistent. Very few cases of thromboembolic or endocarditis have been reported in this study. A reduced average peak pressure gradient was seen in all investigations following surgery. Autologous pericardium-based aortic valve replacement is both safe and effective. Neocuspidization of the aortic valve offers a less invasive surgical option to biological and mechanical prostheses. Without the requirement for long-term oral anticoagulation, the short- and midterm outcomes are equivalent. Long-term follow-up data is necessary for this new strategy to be broadly implemented.
Keywords
Oral anticoagulation, Neocuspidization, Aortic valve replacement
Introduction
In North America and Europe, people over 75 year’s age are expected to have 3.4 percent of their Aortic Stenosis severe enough to require open-heart surgery [1]. As people live longer, the prevalence of valvular heart disease is expected to rise [2]. Aortic valve disease is fatal if left untreated, with a median survival time of less than two years for those with heart failure symptoms [3]. Aortic valve replacement is currently the usual therapy for aortic stenosis, either surgically or transcatheterally [4]. Young individuals face a difficult choice when it comes to valve replacement alternatives. An extensive study has demonstrated that mechanical prosthesis is functional and able to last for years [5]. An estimated 30 percent of all patients who have asymptomatic aortic stenosis are not surgically treated because of a perception of high operable risk, despite the fact that surgical aortic valve replacement has been shown to improve longterm outcomes [6]. However, because of the non-organic materials used, patients must take warfarin (a blood thinner) for the rest of their lives [7]. Haemorrhage, unpleasant effects, and medication interactions are all possible consequences of this. The biological tissue valve, which is often made from bovine or pig tissue, is now an option that reduces the requirement for warfarin [8]. Open cardiac surgery must be repeated or a valvein- valve trans catheter aortic valve replacement must be performed at a later stage in younger patients owing to deterioration [9].
Autologous pericardium-based aortic valve Neocuspidization with AVNeo is a promising treatment option for a variety of AV disorders [10]. Aortic stenosis therapy dates all the way back to 1672, when Rayger became the first person to document the osseous fusion of aortic valve cusps during an autopsy procedure [11]. As one of the oldest open-heart surgical methods, calcified as following aortic valve replacement has adverse outcomes despite its age [12]. Surgical replacement of damaged aortic valves has been the gold standard for the past half a century since the first successful procedures were reported in 1960 [13]. This material has been regularly utilized for valve replacements for many years now. Autologous and heterologous pericardia are currently accessible for valvuloplasty [14]. Heterologous or autologous pericardium can be utilised to replace the defective valve leaflets in most cases of aortic regurgitation. Duran et al. created the autologous pericardial aortic valve (APAV) in 1988, which was made from autologous pericardium and temporarily glutaraldehyde-treated [15].
The lack of antigenicity of this autologous tissue should theoretically minimize deterioration [16]. In addition, the procedure's effectiveness and safety have been confirmed in animal studies and short-term follow-up evaluations in various clinical investigations [17]. This procedure is biocompatible and does not necessitate the use of anticoagulant medication. Low transvalvular gradients may be achieved since no sewing ring is required, resulting in a large effective orifice area [18]. It has also been improved by a new approach that uses specific templates and a different size idea to make it more repeatable. Despite this, AVNeo is presently only used on a small number of adults, and is mostly used on children. The lack of data outside of Ozaki et al study contributes to the sluggish acceptance of AVNeo among adults. Since then, a number of new methods have come to light. When 88 patients between April 2007 and August 2009 had aortic valve Neocuspidization for aortic valve disease using autologous pericardium treated with glutaraldehyde, Ozaki et al. reported their first case study in 2011 [19]. Compared to aortic valve replacement, the natural expansion of the aortic root in systole with the maximum effective orifice area was obtained [20]. Few facilities have begun using the AVNeo method after the announcement of its favorable long-term outcomes. Greater failure risk of AVNeo compared to standard bio prosthetic valves is also a source of worry, as there has been no direct comparison between the two [21].
Methods
A comprehensive search was performed on PubMed, EMBASE, and Google Scholar. The search terms included “Ozaki technique” AND “Aortic Valve Neocuspidization” AND “AV Neocuspidization” AND “Autologous pericardium” AND “glutaraldehyde-treated autologous pericardium”. This research covered articles that were published from 2014 to 2022. Besides hand-searching several relevant high-impact journals, we also examined the reference lists of the included papers. The search was limited to English-language literature, and there was no restriction on the year of publication. We looked at everything, including Ozaki operation results. For this evaluation, we only considered complete, original papers. There was no room for case reports, commentaries, rerun studies, or conference abstracts. Two reviewers independently conducted the literature search. Aortic valve replacement in adults and the elderly is a complex procedure that requires careful consideration of the patient's medical history, treatment, comparisons, and outcomes. The initial author's name, publication date, research design, participants, population, techniques of aortic valve replacement, other concurrent treatments, primary outcomes, and conclusions were collected from the data.
Results
Study selection
From the 4323 articles primarily recognized in searching digital databases. After removing duplicate papers, 2567 articles remained. 1189 papers excluded after reviewing title and abstract which results in 761 papers. After removing papers based on references in addition to conferences, abstracts, comments and papers with incomplete data, 10 papers were selected for reviewing full-text after reviewing abstract and title as represented in the PRISMA flow diagram (Figure 1).
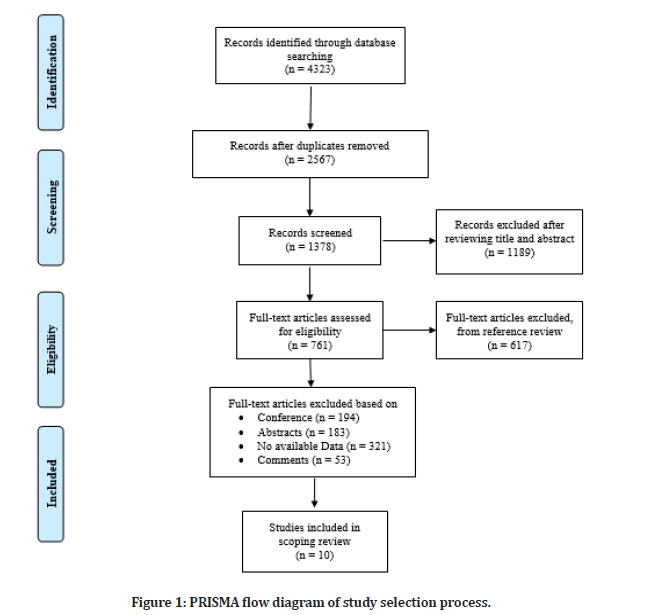
Figure 1: PRISMA flow diagram of study selection process.
Study characteristics
Seven out of 10 included studies were retrospective single-center series and remaining were prospective. This search found no randomized clinical trials. There was a wide range in patient sizes in each research. Characteristics of the included studies are presented in Table 1.
| Author (s), Year | Country | Study design | Sample Size | Mean age | Outcomes |
|---|---|---|---|---|---|
| Mourad et al. [22] | Australia | Prospective | 52 | 60 ± 14 | Five patients found with reoperation in 12 months mean follow-up with one mortality and no thromboembolic event. |
| Reuthebuch et al. [23] | Switzerland | Retrospective | 30 | 66.83±10.55 | No patient found with reoperation in 3 months mean follow-up with one mortality and two thromboembolic events. |
| Iida et al. [21] | Japan | Prospective | 144 | 77.5 ± 8.8 | Two patients found with reoperation in 30.4 ± 20.8 months mean follow-up with two mortalities and no thromboembolic event. |
| Vijayan et al. [24] | India | Retrospective | 20 | 25.5±14.2 | No patient found with reoperation in 6 months mean follow-up with no mortality and thromboembolic events. |
| Nguyen et al. [25] | Vietnam | Prospective | Nine | 47.4 | One patient found with reoperation in 28.5 ± 25.5 months mean follow-up with no mortality and one thromboembolic event. |
| Jovanovic et al. [26] | Balkan | Retrospective | 359 | 66.3 ± 11.3 | One patient found with reoperation with three mortalities and three thromboembolic events. |
| Ozaki et al. [19] | Japan | Retrospective | 416 | 71.2 ±12.0 | Total of four patients found with reoperation in 25.2±17.5 months mean follow-up with no mortality and thromboembolic event. |
| Ngo et al. [27] | Vietnam | Retrospective | 61 | 55.8 | Two patients found with reoperation in 18.5±5.7 months mean follow-up with two mortalities and no thromboembolic event. |
| Krane et al. [28] | Germany | Retrospective | 103 | 54±16.4 | Four patients found with reoperation in 426±270 days mean follow-up with two mortalities and one thromboembolic event. |
| Oliveira et al.[29] | Germany | Retrospective | 77 | 54.9 ±16.5 | One patient found with reoperation in 6 to 12 months mean follow-up with no mortality and thromboembolic event. |
Table 1: Study characteristics.
Synthesis of results
In Reuthebuch et al. [19] patients were operated on using the Ozaki procedure. Patients with just regurgitation included 12 patients (40 percent) while those with only aortic stenosis comprised 7 patients (23.33percent). Endocarditis was seen in one patient (3.33percent). One more patient died from aspiration pneumonia between surgical day 30 and the three-month follow-up. Within the first three months, no patient required reoperation or had another thromboembolic episode. After surgery, one patient experienced moderate aortic valve regurgitation. Valve endocarditis was revealed to be the cause, and the patient underwent a second procedure five months later to have a biological valve installed. Aortic valve stenosis was seen in none of the individuals at three months. One patient was found to have moderate aortic regurgitation [23].
In a prospective, single-center research, Mourad et al. used autologous pericardium in 16 individuals to perform AVNeo on 52 consecutive patients between September 2015 and March 2017. Aortic stenosis or endocarditis was the most common presenting symptom. The average person's age was 60.14. One stroke, two shortterm dialysis patients, and one death were among the early results. Traces of AR were found in four individuals (mean follow-up of 11.2–4.8 months) and the mean pressure gradient was 6.8–2.9 mmHg. Endocarditis necessitated the reoperation of five patients, and three patients died subsequently of noncardiac causes [22].
In Vijayan et al., aortic stenosis or regurgitation, or a combination of both, was treated Only one skilled surgeon was responsible for the entire procedure. A mechanical valve replacement was used as a comparison to see how well it worked. Pre-discharge and six months after surgery, a thorough echocardiographic examination was performed. Aortic valve pressure gradient, aortic valve orifice area, ejection percent, left ventricular diameters, and other postoperative echocardiographic parameters were assessed. There were various advantages to using an autologous pericardial aortic valve repair, including a smaller aortic valve pressure gradient and a larger aortic valve orifice area. Anticoagulation was not needed in any of the patients. During the follow-up period, there were no conversions to prosthetic valve replacement or reinterventions. Pericardial valve restoration using autologous tissue has various benefits over prosthetic valve replacement [24].
Surgical intervention was carried out on Krane, et al. [28]. After a median follow-up of 426 days, Patients with aortic stenosis (77.7percent) and aortic regurgitation (22.3percent), respectively, were the most common diagnoses among the study participants. There was a wide variety of ages in the population: 54.0 to 16.4 years (range, 13.8-78.5). A bicuspid valve was observed in 81 individuals (78.6 percent). (78.6 percent). Trileaflet aortic valve repair was achieved in all cases. neocommissions were established in 38 patients (36.9 percent). The average time to cross-clamp was 135 minutes and 20 seconds. The total reoperation rate was 96.1 percent, with four patients requiring reoperation. In 93.8 percent of patients, echocardiographic 6- to 12-month follow-up following surgery was available and showed no change in hemodynamic parameters compared with discharge. Comparing AVNeo with Trifecta Bio prosthesis, the mean pressure gradient was found to be substantially smaller in Trifecta. Within the first two years following surgery, AVNeo has a low reoperation rate. The hemodynamic performance is outstanding, and the optimum orifice area and mean pressure gradient stay steady during the first year [28].
In Ngo et al. autologous pericardium was used in the reconstruction of aortic valve leaflets. At 91.7±16.1 mm Hg, the peak and mean gradient pressure gradients were measured before surgery. One patient died in hospital owing to cardiac tamponade. Infectious endocarditis necessitated the reoperation of one patient six months after the original procedure. A mediastinal abscess claimed the life of another patient eight months following surgery. They had no aortic regurgitation or mild aortic regurgitation at the last follow-up visits for the surviving patients. With autologous pericardium, aortic valve repair was successful [27].
Iida et al. conducted AVNeo for aortic stenosis in 57 patients from December 2010 to June 2017. They were 77.58.8 years old on average. Preoperative echocardiography indicated an average peak pressure gradient of 89±32.9 mmHg that reduced to 22±10.7 mmHg one week after the surgery and to 19.2±9.7 mmHg 20 months after the procedure. There were no AVR conversions. There were two fatalities that were not connected to the heart. Two patients had reoperation owing to IE and recurrent AR. The mean followup duration was 30.4±20.8 months. Freedom from reoperation rates were 98.1 percent and 95.3 percent after 12 and 81 months of follow-up, respectively [29].
Nguyen, et al. was able to successfully treat nine patients with severe aortic valve disease using an upper ministernotomy. An endoscopic harvesting of the pericardium was conducted, followed by the performance of a ministernotomy and the completion of the Ozaki surgery in a manner identical to that of the traditional technique. In their study, no in-hospital or 30-day mortality was recorded, and no conversion to a complete sternotomy was necessary. On discharge, a transthoracic echocardiogram revealed five valves that were in good working order and three valves that had little regurgitation [25].
Jovanovic et al. analysed and assessed the operative outcomes and significant adverse events of various surgical methods. 8 (2 percent) of the patients had a stroke, 4 (1 percent) had a debilitating stroke, 1 (0.2 percent) had a myocardial infarction, and 13 had surgical site infection (3.2 percent). The three surgery groups had the same 30-day mortality rate and incidence of postoperative significant adverse events. Stroke and surgical site infection were more common in the fullsternotomy group, although not significantly so. Open Surgical Aortic Valve Replacement SAVR in a high-volume facility is linked to a low early mortality rate, while less intrusive techniques lead to shorter hospital stays and quicker postoperative recovery [26].
In Mitrev et al. [30] the SAVR cohorts' 6-year survival percentages were 89.9percent and 88.8percent, respectively. Neocusp degeneration led to an increase in mean gradient of 22.7 mm Hg during the course of the study. After xAVNeo and SAVR, early clinical results and 6-year survival rates were comparable. There were significant differences in the rate of structural valve decomposition and the absence of need for reoperation between the bovine pericardium (xAVNeo) technique and standard open-heart surgery [30].
Oliveira et al. [10] worked on 77 patients who underwent AVNeo following the Osaki surgery. Aortic stenosis and insufficiency were found in 84.4 percent and 15.6 percent of the patients, respectively. Freedom from reoperation was 97.4 percent at 1.76 years of follow-up. After the operation, two patients (2.6 percent) had moderate to severe aortic insufficiency. Both received a prosthetic AVR during the same hospital stay. Upon discharge, the mean aortic gradient was 9.34.2 mmHg, which reduced to 1.63.4 mmHg after six to 12 months. The Ozaki method resulted in outstanding early hemodynamic outcomes in terms of effective orifice area, pressure gradients, and prosthesis-patient mismatch [10].
Discussion
Replacement of the aortic valve dates back to the early days of cardiac surgery. Commissurotomy, free edge unrolling, annuloplasty, wedge resection, cusp suspension, free edge strengthening, supra-aortic crest augmentation, and other methods have been used to replace native valve cups in the aortic valve [31]. This form of conservative treatment is not always viable, especially in older individuals with calcified aorta. Simple cusp slicing or decalcification has not yielded satisfactory results. On the other hand, bioprosthetic valves appear to have a durability constraint, while mechanical prosthesis has a clear anticoagulation disadvantage [32]. Furthermore, neither prosthesis can achieve good hemodynamics when compared to a natural aortic valve. Aortic valve cusp tissue replacement has been attempted since the late sixties. Fascia lata, bovine pericardium, and dura mater have been utilized in a small number of patients. However, the findings are not good in the majority of circumstances [33]. Autologous pericardium that has been treated with glutaraldehyde has been employed in the aortic valve. With up to 16 years of follow-up on aortic valve restoration using human pericardium. An aortic valve repair with low thromboembolic events and mortality was demonstrated to be possible. Stentless aortic valve bioprosthesis was also equivalent in terms of performance [34].
An autologous pericardium-based aortic valve replacement is the first systematic study of its safety and effectiveness to our knowledge. Glutaraldehydetreated autologous pericardium was proven to be safe and effective for the replacement of an aortic valve in this investigation. AVR using autologous pericardium was shown to be safe, with a mortality rate of 1.75 percent. Heart disease accounted for 16 percent of all deaths, including leaflet dehiscence and the subsequent multiorgan failure syndrome, paravalvular/endocarditis abscess, fatal thoracic haemorrhage, and cardiac tamponade. Those complications were the result of heart disease. Most occurred within a year after release from the hospital. After patients had been released from the hospital, noncardiac causes of death such as pneumonia, cancer, and so on became more common. One-year mortality rates following aortic valve replacement with a mechanical vs. bio prosthetic valve are 7.74 percent for mechanical and 6.11 percent for bio prosthetic, a study of 66,453 patients found. There were three postoperative stroke-related thromboembolic events (0.21 percent). Most valve-related complications during aortic valve repair are caused by blood clots. Because of this, elderly people are more likely to suffer from post-surgical complications. Autologous pericardium valves were reported to have a lower rate of thromboembolic events than mechanical or bio prosthetic valves. For mechanical and biomechanical valve restoration, 9.8 percent and 7.96 percent of patients had thrombosis, respectively. Because endocarditis was the primary reason of reoperation, it had to be taken into consideration [35]. To prevent additional surgery, intravenous antibiotics might be used. Endocarditis and thromboembolic events are still more common in aortic valve reconstructions employing autologous pericardium, according to several investigations. Compared to using autologous pericardium for aortic valve replacement, the reoperation was more challenging [17].
Conventional aortic valve replacement is prone to problems because of the prosthetic valves used [36]. An anticoagulant is required for life in order to maintain the mechanical valve; nevertheless, bio prosthesis has significant rates of degradation and need an additional procedure. One research found that 85.0 1.2 percent of patients who had supra-annular porcine bio prostheses were free of reoperation after 18 years [37]. This study found that 1.12 percent of patients required a second procedure. Infective endocarditis was the most common cause. All of the studies we looked at had a freedom of operation rating of or higher than 94.1percent. A 98.9 percent success rate was seen after 76 months of followup. After one week or discharge, all trials in this review found an improvement in hemodynamic function. Before surgery, patients' preoperative peak pressure gradients varied from 66.0 28.2 to 92.0 31.2 mmHg; after surgery, they experienced peak pressure gradients that ranged from 10.6 3.3percent to 23.4 10.7percent. According to another study [38], patients having aortic valve reconstruction with autologous pericardium had significantly lower mean aortic pressures at six months' follow-up than those who underwent mechanical valve replacement. At 30 days, peak pressure gradients of 17.0 mmHg and 24.5 mmHg, respectively, were found in 154 patients undergoing stent less and stented bio prosthesis aortic valve replacement. In cardiac surgery, human autologous pericardium has been utilized. Patch repair and right ventricular outflow tract reconstruction are two examples of its application in congenital heart disease. In addition, leaflet extension in mitral valve repair has been accomplished using this technique as a backbone. Another study found that mitral valve repair might be improved by using glutaraldehyde-preserved autologous pericardium [39]. Autologous pericardium was found to have no calcification in 64 of the patients studied over a period of six months to nine years.
Experimentation with virtual Trifecta Bio prostheses showed that this technology was effective in a younger demographic [40]. A reduced mean pressure gradient and a larger effective orifice area were observed in AV Neo. At one-year follow-up, there was a 96.1 percent rate of reoperation-free survival. As an alternative to autologous tissue, Sheng et al. tested bovine pericardium. Early and midterm outcomes were equivalent to those of autologous tissue [41]. Because the glutaraldehyde solution handling and preparation process has been eliminated, the procedure time has been slashed by removing the drawback of poor-quality patients' own Autologous Pericardium. Hydroxy chromium was also applied to the bovine pericardium in order to enhance its durability in animal models [42].
Limitations
There are a number of key limitations to this review. Because the trials were not randomized, they had an inherent selection bias in their results. In addition, the sample sizes were modest and had a high degree of variability. Ozaki et al. [30] patients accounted for around 80percent of the sample, which may have been enlarged with other subgroups and objectives. There was a wide range in the average follow-up period, from 3 months to 1243 days. For aortic valve replacement, these characteristics may considerably alter results, although their long-term impact in the contemporary population is uncertain. The comparison of the pooled data was primarily confined to historical outcomes of conventional aortic valve replacements since just one research contained a control group.
Conclusion
In conclusion, aortic valve replacement with a glutaraldehyde-treated autologous pericardium can be safely performed, with excellent survival, a low rate of reoperation at short- to middle-term follow-up, and good hemodynamic improvement. Nevertheless, further studies with longer follow-up period are required to investigate the long-term freedom of operation and comparison with mechanical and bio prosthetic valve.
References
- Nitsche C, Scully PR, Patel KP, et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol 2021; 77:128-139.
- Wilson JB, Jackson LR, Ugowe FE, et ak. Racial and ethnic differences in treatment and outcomes of severe aortic stenosis: A review. Cardiovasc Interv 2020; 13:149-156.
- Boudoulas KD, Triposkiadis F, Boudoulas H. The aortic stenosis complex. Cardiology 2018; 140:194-198.
- Siontis GC, Overtchouk P, Cahill TJ, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur Heart J 2019; 40:3143-3153.
- Kolkailah AA, Doukky R, Pelletier MP, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis in people with low surgical risk. Cochrane Database Syst Rev 2019.
- Ribeiro HB, Lerakis S, Gilard M, et al. Transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis: The TOPAS-TAVI registry. J Am College Cardiol 2018; 71:1297-1308.
- Rogers T, Shults C, Torguson R, et al. Randomized trial of aspirin versus warfarin after transcatheter aortic valve replacement in low-risk patients. Cardiovasc Interv 2021; 14:e009983.
- Rahmani B, McGregor C, Byrne G, et al. A durable porcine pericardial surgical bioprosthetic heart valve: A proof of concept. J Cardiovasc Transl Res 2019; 12:331-337.
- Hirji SA, Percy ED, Zogg CK, et al. Comparison of in-hospital outcomes and readmissions for valve-in-valve transcatheter aortic valve replacement vs. reoperative surgical aortic valve replacement: a contemporary assessment of real-world outcomes. Eur Heart J 2020; 41:2747-55.
- Sá MP, Perazzo ÁM, Zhigalov K, et al. Aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium (Ozaki procedure): A promising surgical technique. Br J Cardiovas Surg 2019; 34:610-614.
- Freitas-Ferraz AB, Tirado-Conte G, Dagenais F, et al. Aortic stenosis and small aortic annulus: Clinical challenges and current therapeutic alternatives. Circulation 2019; 139:2685-2702.
- Bendary A, Ramzy A, Bendary M, et al. Transcatheter aortic valve replacement in patients with severe aortic stenosis and active cancer: A systematic review and meta-analysis. Open Heart 2020; 7:e001131.
- Kumar N, Kumar JE, Hussain N, et al. New or worsened mitral regurgitation after surgical aortic valve replacement: A systematic review. In Seminars in Cardiothoracic and Vascular Anesthesia 2021; 15:173-184.
- Kaneko S, Isoda S, Aoyama T, et al. Rapid anticalcification treatment for glutaraldehyde-fixed autologous tissue in cardiovascular surgery. J Cardiothoracic Surg 2022; 17:1-5.
- Arutyunyan V, Chernov I, Komarov R, et al. Immediate outcomes of aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium: A multicenter study. Bra J Cardiovas Surg 2020; 35:241-248.
- Sharma D, Iyer S, Subramaniam S, et al. Evaluation of antigenicity of components of tracheal allotransplant and effect of immunosuppressant regime in a rodent model. Indian J Plastic Surg 2020; 53:357-362.
- Tada N, Tanaka N, Abe K, et al. Transcatheter aortic valve implantation after aortic valve neocuspidization using autologous pericardium: A case report. Eur Heart J 2019; 3:105.
- Koechlin L, Schurr U, Miazza J, et al. Echocardiographic and clinical follow-up after aortic valve neocuspidization using autologous pericardium. World J Surg 2020; 44:3175-81.
- Ozaki S, Kawase I, Yamashita H, et al. Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease. Interactive Cardiovas Thoracic Surg 2011; 12:550-553.
- Madukauwa‐David ID, Midha PA, Sharma R, et al. Characterization of aortic root geometry in transcatheter aortic valve replacement patients. Catheter Cardiovasc Interv 2019; 93:134-140.
- Iida Y, Akiyama S, Shimura K, et al. Comparison of aortic annulus dimensions after aortic valve neocuspidization with those of normal aortic valve using transthoracic echocardiography. Eur J Cardio-Thoracic Surg 2018; 54:1081-1084.
- Mourad F, Shehada SE, Lubarski J, et al. Aortic valve construction using pericardial tissue: short-term single-centre outcomes. Interactiv Cardiovas Thorac Surg 2019; 28:183-190.
- Reuthebuch O, Koechlin L, Schurr U, et al. Aortic valve replacement using autologous pericardium: single centre experience with the Ozaki technique. Swiss Med Weekly 2018.
- Vijayan J, Lachma RN, Mohan Rao PS, et al. Autologous pericardial aortic valve reconstruction: Early results and comparison with mechanical valve replacement. Indian J Thorac Cardiovasc Surg 2020; 36:186-192.
- Nguyen DH, Vo AT, Le KM, et al. Minimally invasive Ozaki procedure in aortic valve disease: the preliminary results. Innovations 2018; 13:332-337.
- Jovanovic MM, Micovic SV, Peric MS, et al. Low-risk surgical aortic valve replacement in the era of transcatheter aortic valve implantation. Texas Heart Institute J 2022; 49:e207435.
- Ngo HT, Nguyen HC, Nguyen TT, et al. Reconstruction of aortic valve by autologous pericardium (Ozaki’s procedure): Single center experience in Vietnam. Asian Cardiovasc Thorac Ann 2021; 29:394-399.
- Krane M, Boehm J, Prinzing A, et al. Excellent hemodynamic performance after aortic valve neocuspidization using autologous pericardium. Annals Thoracic Surg 2021; 111:126-133.
- Iida Y, Fujii S, Akiyama S, et al. Early and mid-term results of isolated aortic valve neocuspidization in patients with aortic stenosis. Gen Thoracic Cardiovas Surg 2018; 66:648-652.
- Mitrev Z, Risteski P, Todorovska M, et al. Aortic valve neocuspidization using xenologous pericardium versus bioprosthetic valve replacement. Ann Cardiothorac Surg 2022; 113:1192-1199.
- Afifi A, Hosny H, Yacoub M. Rheumatic aortic valve disease—when and who to repair?. Ann Cardiothorac Surg 2019; 8:383.
- Sathananthan J, Sellers SL, Fraser R, et al. Impact of implant depth on hydrodynamic function with the ACURATE neo transcatheter heart valve following valve-in-valve transcatheter aortic valve replacement in Mitroflow bioprosthetic valves: An ex vivo bench study. EuroIntervention 2019; 15:78-87.
- Khorramirouz R, Go JL, Noble C, et al. In vivo response of acellular porcine pericardial for tissue engineered transcatheter aortic valves. Sci Report 2019; 9:1-1.
- Myers PO, Mokashi SA, Horgan E, et al. Outcomes after mechanical aortic valve replacement in children and young adults with congenital heart disease. J Thorac Cardiovasc Surg 2019; 157:329-340.
- Fukunaga N, Tamura N. Mitral valve repair with glutaraldehyde-treated autologous pericardium for active infective endocarditis. Gen Thorac Cardiovasc Surg 2022.
- Vemulapalli S, Carroll JD, Mack MJ, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. New Engl J Med 2019; 380:2541.
- Jamieson WE. Commentary: Impact of prosthesis size and prosthesis–patient mismatch on outcomes in younger female patients undergoing aortic valve replacement. In Seminars in Thoracic and Cardiovascular Surgery 2020; 32:229.
- Makkar RR, Yoon SH, Leon MB, et al. Association between transcatheter aortic valve replacement for bicuspid vs. tricuspid aortic stenosis and mortality or stroke. JAMA 2019; 321:2193.
- Kherallah RY, Koneru S, Krajcer Z, et al. Hemodynamic outcomes after valve-in-valve transcatheter aortic valve replacement: A single-center experience. Ann Cardiothorac Surg 2021; 10:630.
- Forrest JK, Ramlawi B, Deeb GM, et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol 2021; 6:50-57.
- Kolte D, Vlahakes GJ, Palacios IF, et al. Transcatheter versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol 2019; 74:1532-1540.
- Dangas GD, Tijssen JG, Wöhrle J, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. New Engl J Med 2020; 382:120-129.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Komarov RN, Badalyan SS*, Lenkovets M, Tcheglov MI, Magomedova KA and IM Sechenov
First Moscow State, Medical University, Moscow, Russian FederationReceived: 04-Jun-2022, Manuscript No. JRMDS-22-65855; , Pre QC No. JRMDS-22-65855 (PQ); Editor assigned: 06-Jun-2022, Pre QC No. JRMDS-22-65855 (PQ); Reviewed: 21-Jun-2022, QC No. JRMDS-22-65855; Revised: 28-Jun-2022, Manuscript No. JRMDS-22-65855 (R); Published: 05-Jul-2022
