Research - (2021) Volume 9, Issue 7
Sabrin Sabah Rashid* and Hussein A Jawad
*Correspondence: Sabrin Sabah Rashid, Institute of Laser for Postgraduate Studies, University of Baghdad, Iraq, Email:
Abstract
Background: Cleaning and preparing root canals is one of the most important conditions for effective root canal therapy. In the field of endodontics accept many advancements, such as hand- and engine-determined instruments and altered irrigating solutions. Objective: The aim of this study was to investigate the effect of Er.Cr:YSGG photon-induced photoacoustic streaming (PIPS) technique at short pulse duration (60 μs) with different powers setting (0.25 W, 0.5 W, 0.75 W, 1 W, 1.25 W) alone and with irrigant on smear layer removal of apical third. Materials and methods: Thirty-six extracted single-rooted mandibular premolar was used. The roots length was uniform to 14 mm from the anatomic apex and instrumented using the rotary system to size 40. Er.Cr: YSGG pulse laser (waterlase iplus Biolase, CA, USA) 2780 nm used at short pulse duration (60 μs) delivered by MD/iplus Glass Tips (MZ6)600 μm in diameter, length=6 mm, calibration factor=1.00. The samples was divided into three groups of the following irrigation methods: (a) conventional irrigation with open-ended needles, (b) Er.Cr:YSGG induced photoacoustic streaming with 17% EDTA at (0.25W, 0.5W, 0.75W, 1W, 1.25W) (c) Er.Cr:YSGG induced photoacoustic streaming with 5.25% NaOCl at (0.25W, 0.5W, 0.75W, 1W, 1.25W). Then, the roots were painted with nail varnish externally, and 2% methylene blue dye inject into root canal. The tooth was split horizontally at the apical third. The dye penetration measured by using analytical software (measure picture CAD-KAS Kessler Germany). ANOVA test was used to analyse repeated measure mean between tested concentration and control. Data expressed as mean ± SE. LSD test was used to calculate the significant differences between tested mean. Results: The mean values of the percentage of dye penetration area was ranging from (20.56 ± 0.36 control, Er.Cr: YSGG laser with 17% EDTA at 0.25W=70.60 ± 0.33, 0.5W=89.62 ± 0.27, 0.75W=79.42 ± 0.35, 1W=73.19 ± 0.16, 1.25W=28.05 ± 0.04. Er,Cr:YSGG laser with 5.25% NaOCl at 0.25W=53.19 ± 0.1, 0.5W=66.55 ± 0.2, 0.75W=69.77 ± 0.2, 1W=25.94 ± 0.11, 1.25W=19.01 ± 0.05). The Percentage of dye penetration in Er,Cr:YSGG laser with 17% EDTA was significantly high at 0.5 W power while for Er.Cr:YSGG laser with 5.25 % NaOCl at 0.75 W laser group which also showed a distinct Percentage of dye penetration. Conclusion: We concluded that the use of Er.Cr:YSGG pulse laser (5 Hz , air and water off) at short pulse duration (60 μs) with 17% EDTA using PIPS technique for activation of irrigant in endodontic treatment is effective in smear layer removal at 0.5 W . While the PIPS proved an acceptable result when the laser is used alone assisted by 5.25% NaOCl for both function disinfection and shock wave generation at 0.75W.
Keywords
Er.Cr:YSGG laser, PIPS, Smear layer, Irrigation
Introduction
One of the primary criteria for effective root canal care is precise cleaning and shape of root canals. The presence of microorganisms in root canals, as well as those that invade the dentinal tubules, abnormal canal shape, apical deltas, and thin isthmia are all factors that lead to ineffective endodontic therapy [1]. As a result, chemical disinfection through irrigating solutions is required in addition to mechanical instrumentation. Many advancements in endodontics have occurred, such as hand- and enginedriven instruments and altered irrigating solutions [2]. On the root canal walls, the instrumentation creates an amorphous smear layer of inorganic and organic material. The thickness of the smear layer is normally 1–2 m, but it can reach as far into the dentinal tubules as 40 m. It prevents antimicrobial agents from entering the infected dentinal tubule [3-5]. Irrigation is an integral aspect of effective root canal therapy since it performs many mechanical, chemical, and (micro) biological functions. Irrigation is also the best way to access parts of the root canal wall that are not touched or washed by mechanical instrumentation. Most of the endodontic irrigation research has focused on the impact of irrigation on the smear layer [6]. A ideal chemical irrigant can have a physical flush for debris removal as well as serve as a tissue solvent, lubricant, and bactericidal element [7]. Since no one solution holds all the desired properties, safe and efficient irrigation necessitates the use of two or more solutions [8]. When combined with water, sodium hypochlorite detaches into Na+ and hypochlorite ion (OCl-), making it a common organic solvent. Hypochlorous acid has an antibacterial effect and helps in the removal of the stain sheet's organic portion. It can be present in concentrations ranging from 0.5 to 6. [9]. Chemical irrigant Ethylene Diamine Tetra-acetic Acid (EDTA) has been a favoured irrigant for the removal of smear layer across time. A chelating agent helps in the removal of the smear layer's inorganic components while also acting as an irrigation aid [10]. The use of lasers to agitate or activate root canal irrigant is a relatively new phenomenon in endodontics. The mechanism of contact between dental hard tissue (enamel, dentine) and the Erbium laser family is explosive thermomechanical ablation or water-mediated ablation, which occurs at wavelengths between 2.7 and 3 m and results in the expulsion of mineral particles while preserving their mineral structure [11]. The effect of laser radiation on eliminating the smear layer could be more effective than other traditional agitation methods [12]. Ayca Yilmaz et al. used confocal laser scanning microscopy to investigate the performance of various final irrigation approaches on sealer penetration in 2020. They discovered that these study groups had higher penetration rates than the control group, with a peak penetration depth of 652 μm [13]. Minimally invasive endodontic therapy using the ErCr: YSGG laser of 7.96 J/Cm2 fluence for smear layer removal and disinfection will improve the condition of patients with multiple apical diagnoses in a single visit root canal procedure [14]. In terms of debris and smear layer removal, using irrigant activators improves debris and smear layer removal, especially in the middle and apical thirds, and no activation technique was able to completely eliminate debris and smear layer from root canals [15]. The current study investigated the effects of different power setting (0.25W, 0.5W, 0.75W, 1W, 1.25W) of Er.Cr:YSGG laser at short pulse duration (60 μs) for removal of smear layer in apical third using single-rooted human mandibular premolars with irrigant (17% EDTA) and without irrigation assisted by 5.25% NaOCl as disinfection and shock generation for PIPS technique.
Materials and Methods
Thirty-six single-rooted completely formed, straight human teeth extracted for orthodontic or periodontics purposes. The teeth were examined clinically and radiographically to ensure that there is no root fracture, open apex, and/or root resorption. Then, they were stored in a plastic container containing 0.1% thymol solution until the performing of the experiment.
The roots length was uniform to 14mm from the anatomic apex. The exact apical foramen location and canals patency were recognized by using a stainless-steel K-file #10 (Dentsply, Maillefer, Ballaigues, Switzerland) as in Figure 1 and the correct working length was standard by deducting 1mm from the length previously determined.
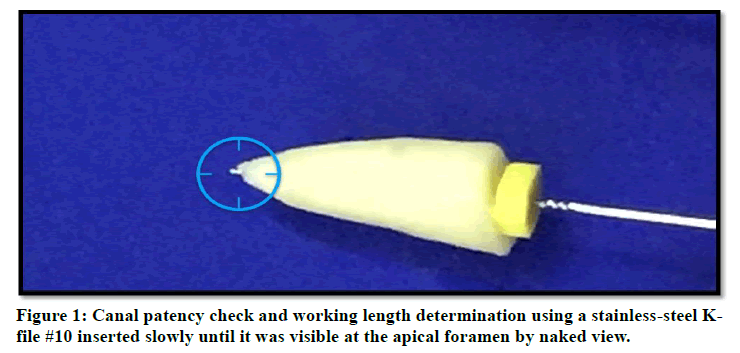
Figure 1: Canal patency check and working length determination using a stainless steel K file #10 inserted slowly until it was visible at the apical foramen by naked view.
The canals were instrumented mechanically by Rotary Protaper Gold NiTi endodontic files (SX, S1, S2, F1, F2, F3, F4) (Fanta, China) up to size F4 (size 40, 0.06 taper) as seen in Figure 2. The canal irrigated with 2.0 ml of 5.25% NaOCl (Chloraxid Extra, PPH Cerkamed, and Stalwa Wola, Poland) at each instrument change. Er.Cr:YSGG pulse laser (waterlase iplus Biolase, CA, USA) 2780 nm used at short pulse duration (60us) delivered by MD/iplus Glass Tips (MZ6) 600 μm in diameter, length=6mm, calibration factor=1.00.
Figure 2: Root canal instrumentation by Protaper GOLD NiTi files.
The specimens were divided into three groups:
Group 1: Control group (n=6)
The samples irrigated with 5 ml 17% EDTA (Disodium edetate, PPH Cerkamed, Stalwa Wola, Poland) for 1 min by Side-vented irrigation needle which was placed into the root canal within 2 mm of the working length. Then delivering 5 ml of distilled water and with paper point F4 (Diadent, Korea) the canal was dried.
Group 2: 17% EDTA+ Er, Cr: YSGG laser 2780nm (n=15 each power have three sample)
Each sample irrigated as the following procedure with different power (0.25W, 0.5W, 0.75W, 1W, 1.25W) used:
The samples irrigate with 5mL EDTA 17% and agitated with Er:Cr:YSGG pulsed laser, 2780 nm for 1 minute. The delivery was by MD/iplus Glass Tips (MZ6). setting was Power=0.25 W or 0.5W or 0.75W or 1W or 1.25W , repetition rate: 5 Hz, pulse Duration: 60 μs. Water and air was off. The fibre tip inserted just into canal orifice as seen in Figure 3. The procedure followed by delivering the final rinse (5 ml of distilled water) and with paper point F4 the canal was dried. Each fibre tip was used for only one canal, after that discarded.

Figure 3: D/iplus Glass Tips (MZ6) inserted just into canal orifice.
Group 3: 5.25%NaOCl+ Er, Cr: YSGG laser 2780nm (n=15 each power have 3 sample)
Each sample irrigated as the following procedure with different power (0.25W, 0.5W, 0.75W, 1W, 1.25W) used:
Er:Cr:YSGG pulsed laser, 2780 nm used for 1 minute with 5mL NaOCl 5.25% for shock wave generation and disinfection .The delivery was by MD/iplus Glass Tips (MZ6).setting was Power=0.25 W or 0.5W or 0.75W or 1W or 1.25W , repetition rate: 5 Hz, pulse duration: 60 μs. Water and air was off. The fibre tip inserted just into canal orifice. The procedure followed by delivering the final rinse (5 ml of distilled water) and with paper point F4 the canal was dried. Each fibre tip was used for only one canal, after that discarded.
Permeability test experiment
The test was prepared to assess the dye penetration area in apical third of root canal. The wax was used to seal root apex. Two layers of nail paint and left to dry covered the surface of roots. Then 2% methylene blue dye (India) introduced into the canal by hypodermal syringe with needle gauge 23, by placing the needle 2 mm inside the canal, then k-file # 20 (dentsply, Maillefer, Ballaigues, Switzerland) inserted and withdrawn one time to confirm that the dye was reached to the apical root third.
After that, the dye left inside the canal for 20 min. at room temperature (27-29°c). When time had been passed, they were washed comprehensively below running tap water to clean external root surface and with absorbent paper cone the root canal was dried constantly until the cone appears white [16,17].
Root sectioning for permeability test
The tooth was split at the fourth millimetres from root apex with the aid of diamond disc (China) just below the guiding line representing the apical third as seen in Figure 4. Pictures was taken by Professional Digital SLR camera (Nikon D7100, Nikon Corporation, Thailand) for each sample with 40X magnification.

Figure 4: The tooth was split vertically at the fourth millimetres from root apex with the aid of diamond disc just below the guiding line representing the apical third.
Radicular dentin permeability measurements and evaluation
The images were opened with measure pictures V 1.0 software (CAD-KAS Kassler Computer software GbR, Germany), for computing of radicular dentin Permeability. The dye penetration area and the total root section area were calculated then subtract the root canal hole area from both earlier mentioned areas to acquire the actual area of dye penetration and root section. First, calibration was done by numerical scale to convert pixel unit into millimetre (Figure 5).
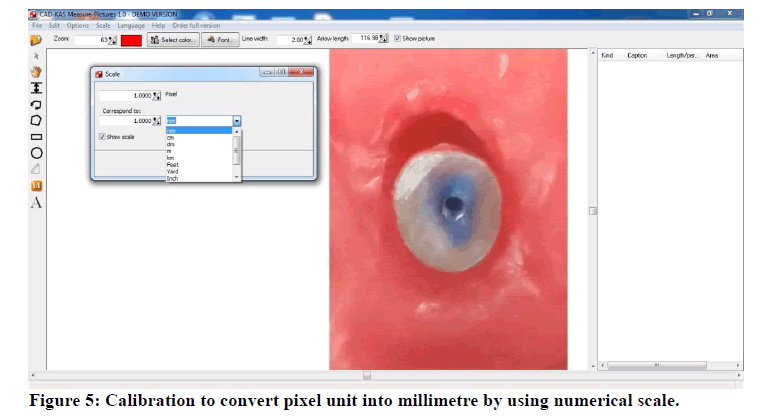
Figure 5: Calibration to convert pixel unit into millimetre by using numerical scale.
Consequently, and after completion of measuring areas (Figure 6), the dye-penetrated area was divided by the root third area resulting in dye penetration in root third, and multiplied by 100% resulting in the percentage of dye penetration in root third see the following equation:
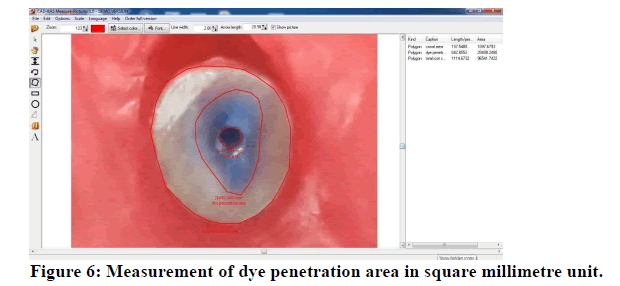
Figure 6: Measurement of dye penetration area in square millimetre unit.
Dye Penetration in Root Section=(Net dye penetration area /Net total root third area) × 100.
Statistical analysis: ANOVA test was used to analyse repeated measure between tested concentration and control. Data expressed as mean ± SE. LSD test was done to analyse the significant differences between tested mean, the letters (A, B, and C) represented the levels of significant, highly significant start from the letter (A) and decreasing with the last one. Similar letters mean there are no significant differences between tested mean. Values of p>0.05 were reflected statically non-significant while p ≤ 0.05 and <0.01, 0.001 were reflected significantly different, highly significantly different correspondingly. Estimate of correlation coefficient between difference parameters in this study. The statistical analysis was done by SPSS (v 20).
Results
Permeability test
As it was stated in methodology, roots were sectioned transversely at apical area of the root.
After that the dye penetration area and the entire root section area were measured, then subtract both of them from the root canal area to acquire the net dye penetration area and root section area. Subsequently the dye penetration area was then multiplied by 100% and divided by the root third area, resultant in the percentage of dye penetration in apical root third [18].
Dye Penetration in Root Section=(Net Dye Penetration Area /Net Total Root Third Area) × 100.
The Data of permeability of root canal dentin stated as percentage of dye penetrating area at apical third of root canal are showed in Table 1.
Table 1: Dye Penetration of three groups (A) Control (B) 17%EDTA+ Er:Cr.YSGG (C)5.25%NaOCl + Er:Cr.YSGG.
| (A)Control | Power | (B) 17%EDTA+Er:Cr.YSGG | (C) 5.25%NaOCl+Er:Cr.YSGG |
|---|---|---|---|
| 20.56 ± 0.36 | 0.25W | 70.71098 | 53.2715 |
| 69.99 | 53.3 | ||
| 71.1 | 52.99 | ||
| 20.56 ± 0.36 | 0.5W | 89.76463 | 66.55742 |
| 89.1 | 66.9 | ||
| 90 | 66.2 | ||
| 20.56 ± 0.36 | 0.75W | 79.27453 | 69.40433 |
| 78.9 | 70.1 | ||
| 80.1 | 69.8 | ||
| 20.56 ± 0.36 | 1W | 73.46328 | 25.7323 |
| 73.2 | 25.98 | ||
| 72.9 | 26.1 | ||
| 20.56 ± 0.36 | 1.25W | 28.08477 | 18.93397 |
| 27.98 | 19.1 | ||
| 28.1 | 18.99 |
The table shown that the high percentage of dye penetration area seen in activated laser group with 17%EDTA at 0.5W while for Erbium laser group assisted by 5.25% NaOCl at 0.75W.
The summary of descriptive and statistical test for the percentage of dye penetrating area between control and experimental groups are shown in Table 2. Among groups as seen in a table below the highest mean percentage were presented in activate Erbium laser group with 7% EDTA at 0.5W while for Erbium laser group assisted by 5.25% NaOCl at 0.75W, and the lowest mean rank percentage were appeared in Erbium laser group with 5.25% NaOCl at 1.25W with highly significant difference among groups.
Table 2: Descriptive and statistical test of permeability among groups.
| Mean ± SE | (E) 17%EDTA+ Er:Cr.YSGG | (F) 5.25%NaOCl+Er:Cr.YSGG | p value Between E & F Groups |
|---|---|---|---|
| Control | 20.56 ± 0.36 | ------- | ----- |
| 0.25 W | C 70.60 ± 0.33 | C 53.19 ± 0.1 | 0.001 |
| 0.5W | A 89.62 ± 0.27 | B 66.55 ± 0.2 | 0.001 |
| 0.75 W | B 79.42 ± 0.35 | A 69.77 ± 0.2 | 0.05 |
| 1 W | C 73.19 ± 0.16 | D 25.94 ± 0.11 | 0.0001 |
| 1.25 W | D 28.05 ± 0.04 | E 19.01 ± 0.05 | 0.05 |
| p value within the group | 0.01 | 0.001 | ------ |
| p value between tested groups and control | 0.0001 | NON |
In Figure 7 the mean values of the percentage of dye penetration area among five power in 17%EDTA+ Er:Cr.YSGG were shown the high percentage with 0.5 W and the lowest with control group. The dye penetration area decreases as power increase due to multiphoton absorption and molecular dissociation.
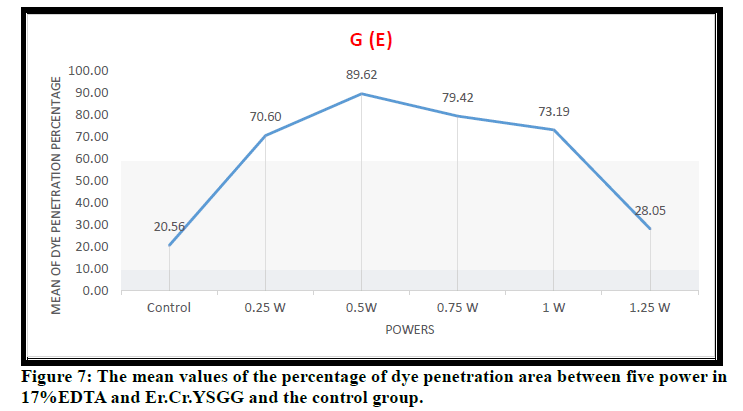
Figure 7: The mean values of the percentage of dye penetration area between five power in 17%EDTA and Er.Cr.YSGG and the control group.
In Figure 8 the mean values of the percentage of dye penetration area between five power in 5.25 % NaOCl and Er: Cr.YSGG were shown the high percentage with 0.75W and the lowest with 1.25W. The dye penetration area decreases as power increase due to multiphoton absorption and molecular dissociation.
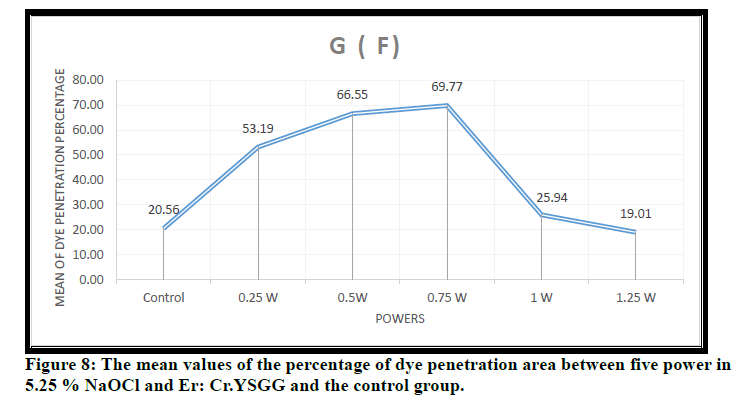
Figure 8: The mean values of the percentage of dye penetration area between five power in 5.25 % NaOCl and Er: Cr.YSGG and the control group.
In Figure 9 the mean values of the percentage of dye penetration area among five power in both17%EDTA+ Er:Cr.YSGG and 5.25 % NaOCl + Er:Cr.YSGG were shown that the high percentage of dye penetration area was seen in 17%EDTA+ Er:Cr.YSGG at 0.5 W and for 5.25 % NaOCl + Er:Cr.YSGG was seen at 0.75W. The reason was the irradiation influence of the Er, Cr: YSGG laser at sub ablative power settings is enhanced by the existence of EDTA. This leads to significantly better percentage of dye penetration area than all group because when the specimens cleaned with EDTA, the delivering and transforming of the laser energy to thermal energy directly into the dentin matrix that existing a low mineral constitute because of the effect of EDTA [19].
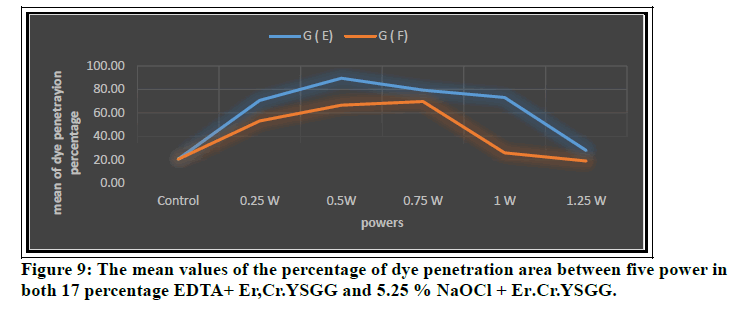
Figure 9: The mean values of the percentage of dye penetration area between five power in both 17 percentage EDTA+ Er,Cr.YSGG and 5.25 % NaOCl + Er.Cr.YSGG.
Discussion
The present study investigate the effect of Er.Cr:YSGG photon-induced photoacoustic streaming (PIPS) technique at short pulse duration (60 μs) with different powers setting (0.25W, 0.5W, 0.75W, 1W, 1.25W) on smear layer removal of apical third with and without irrigant . The results of the present study revealed that Er:Cr.YSGG laser -activated irrigant (17% EDTA) with PIPS tip had best effect in removing the smear layer from the apical third of the root canals at 0.5W, while when Er:Cr.YSGG laser used alone assisted by 5.25% NaOCl for shock wave generation and disinfection the best result seen at 0.75W. The reason was the irradiation influence of the Er.Cr:YSGG laser at sub ablative power settings is enhanced by the existence of EDTA . This leads to significantly better percentage of dye penetration area than all group because when the specimens cleaned with EDTA, the delivering and transforming of the laser energy to thermal energy directly into the dentin matrix that existing a low mineral constitute because of the effect of EDTA (19). The creation of bubbles incorporates the process of laser-activated irrigation [20]. As erbium laser energy is absorbed by water, it induces evaporation [21, 22]. In front of the laser beams, the vapor bubble begins to expand and create void. As a result, the PIPS optic tip was placed only in the coronal portion of the root canals, essentially removing the apical smear layer.
At low power, each impulse in the PIPS technique interacts with the water molecules, causing expansion and successive shock waves that result in the creation of a strong streaming fluid [23]. Photon-induced photoacoustic streaming tips were used at sub ablative levels with precise settings and a novel tip structure. To produce peak output pulses, this technique employs low energy levels and fast microsecond pulse rates (60 μs). The intense photoacoustic shock wave it prompts helps three-dimensional movement of the irrigation solutions [24]. The PIPS technique's subablative parameters provide a photomechanical effect, which happens as light energy is pulsed in a fluid, rather than a thermal effect [25] which occur when power increase that result in multiphoton absorption and molecular dissociation. This explains our result that when power increase dye penetration area will decrease.
Standard laser applications need typical preparing for at least size 30, with the laser tip reaching the root apical third. The PIPS tip, on the other hand, does not require penetrating the canal end and is just put in the root canal's coronal reservoir. As a result, this procedure permits for minimally invasive cleaning of the root canal [24].
Conclusion
We concluded that the use of Er.Cr:YSGG pulse laser (5 Hz, air and water off) at short pulse duration (60 μs) with 17% EDTA using PIPS technique for activation of irrigant in endodontic treatment is efficient in smear layer removal at 0.5 W. While the PIPS proved an acceptable result when the laser is used alone assisted by 5.25% NaOCl for both function disinfection and shock wave generation at 0.75W.
Acknowledegements
I would like to thank Institute of Laser for Postgraduate Studies, University of Baghdad for financial support.
Conflict
I declare there is no conflict of interest.
References
- Aksel H, Serper A. Concentration and time-dependent effect of initial sodium hypochlorite on the ability of QMix and ethylenediaminetetraacetic acid to remove smear layer. J Conserv Dent 2017; 20:185189.
- Mittal A, Dadu S, Yendrembam B, et al. Comparison of new irrigating solutions on smear layer removal and calcium ions chelation from the root canal: An in vitro study. Endodontology. 2018; 30:55–61.
- Calt S, Serper A. Smear layer removal by EGTA. J Endod 2000; 26:459-61.
- Palaniswamy U, Kaushik M, Surender LR, et al. A SEM evaluation of smear layer removal using two rotary instrument systems with EDTA and vinegar as a root canal irrigant. J Res Dent 2016; 4:17–21.
- Berastegui E, Molinos E, Ortega J. To comparison of standard and new chelating solutions in endodontics. J Dent Sci 2017; 2:000131.
- Dioguardi M, Gioia GD, Illuzzi G, et al. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur J Dent 2018; 12:459–466.
- Kamble AB, Abraham S, Kakde DD, et al. Scanning electron microscopic evaluation of efficacy of 17% Ethylenediaminetetraacetic acid and chitosan for smear layer removal with ultrasonic: An in vitro study. Contemp Clin Dent 2017; 8:621–6.
- Topbas C, Adiguzel O. Endodontic irrigation solutions: A review. Int Dent Res 2017; 7:54-61.
- Schmidt TF, Teixeira CS, Felippe MCS, et al. Effect of ultrasonic activation of irrigants on smear layer removal. J Endod 2015; 41:1359-1363.
- Dasilva Beraldo Â, Silva RV, da Gama Antunes AN, et al. Scanning electron microscopic evaluation of smear layer removal using isolated or interweaving EDTA with sodium hypochlorite. Iran Endod J 2017; 12:55-9.
- Brugnera A, Zanin F, Barbin EL, et al. Effects of Er: YAG and Nd: YAG laser irradiation on radicular dentine permeability using different irrigating solutions. Lasers Surg Med 2003; 33:256-9.
- Grinkeviciute P, Povilaityte G, Siudikiene J. The effect of laser irradiation in smear layer removal–a review of the literature. Eur Int J Sci Technol 2019; 8:2019.
- Yilmaz A, Yalcin TY, Helvacioglu-Yigit D. Effectiveness of various final irrigation techniques on sealer penetration in curved roots: A confocal laser scanning microscopy study. Bio Med Res Int 2020; 2020.
- Shaheed Ali Abbas. Endodontic therapy using Er,Cr:YSGG laser: Clinical and confocal laser microscope study. Dissertation University of Baghdad, 2020.
- Khaleefah Fatimah Jamal. SEM study evaluating the effect of different endodontic irrigant activators on smear layer and debris removal from root canal dentin. Dissertation University of Baghdad, 2020.
- Esteves-Oliveira M, de Guglielmi CA, Ramalho KM, et al. Comparison of dentin root canal permeability and morphology after irradiation with Nd: YAG, Er: YAG, and diode lasers. Lasers Med Sci 2010; 25:755-60.
- Zmener O, Pameijer CH, Serrano SA, et al. Significance of moist root canal dentin with the use of methacrylate-based endodontic sealers: an in vitro coronal dye leakage study. J Endodont 2008; 34:76-79.
- Cheung GS, Stock CJ. In vitro cleaning ability of root canal irrigants with and without endosonics. Int Endodont J 1993; 26:334-43.
- Hülsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: Mode of action and indications for their use. Int Endodont J 2003; 36:810-30.
- Meire MA, Poelman D, De Moor RJ. Optical properties of root canal irrigants in the 300–3,000-nm wavelength region. Lasers Med Sci 2014; 29:1557-62.
- Brugnera A, Zanin F, Barbin EL, et al. Effects of Er:YAG and Nd:YAG laser irradiation on radicular dentine permeability using different irrigating solutions. Lasers Surg Med 2013; 33:256–259.
- Kivanc BH, Ulusoy OI, Gorgul G. Effects of Er:YAG laser andNd:YAG laser treatment on the root canal dentin of human teeth: A SEM study. Lasers Med Sci 2008; 23:247–252.
- DiVito E, Peters OA, Olivi G. Effectiveness of the erbium: YAG laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med Sci 2012; 27:273–280.
- DiVito E, Lloyd A. ER:YAG laser for 3-dimensional debridement of canal systems: use of photon-induced photoacoustic streaming. Dent Today 2012; 31:122 4–7.
- DiVito E, Peters OA, Olivi G. Effectiveness of the erbium:YAG laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med Sci 2012; 27:273–80.
Author Info
Sabrin Sabah Rashid* and Hussein A Jawad
Institute of Laser for Postgraduate Studies, University of Baghdad, IraqCitation: Sabreen Sabah Rasheed, Hussien A Jawad,Radicular Dentine Permeability when Using Er.Cr:YSGG Laser with PIPS Technique, J Res Med Dent Sci, 2021, 9(7): 228-234
Received: 13-Mar-2021 Accepted: 12-Jul-2021
