Review - (2022) Volume 10, Issue 2
Review on Silver Nanoparticles in Dye Effluent Treatment
Vikram Mor1*, Gopal Arora2 and Mohinder Pal3
*Correspondence: Vikram Mor, Department of Environmental Science, Faculty of Science, Gurugram, Haryana, India, Email:
Abstract
The present condition of water resources demonstrated the predominance of effluent drainage-related pollution. Contaminated rivers pose significant economic and environmental concerns, requiring the creation of a realistic solution to deal with the repercussions. Physical and chemical treatment techniques for indigo effluent cleansing presently are in use time-consuming, expensive, or unsuccessful. Due to their better interface surface chemistry sensitivity, nanoparticles are emerged as a preferable alternative for degradation and decolorization. In this regard, the use of metal nanoparticles in treating wastewater has been widely studied. Efforts were undertaken to discover the kinematics and statistics optimisation of the treatment parameters in order to effectively remove dyes. In addition, the usage of gold Nano composites has been shown to be effective. In contrast, studies have revealed the mechanisms of gold Nanomaterials toxicity, at even small doses, and also their harmful biological problems when contained in treated water. As a consequence, the providence of Ag nanoparticles free in processed wastewaters, which could pollute underground water, aquatic environments and soil, is an important worry. The present state of information on the utilization of Ag nanoparticles and silver-based Nano composites in wastewater, as well as current findings on argent Nanomaterials toxicity decrease, is covered in this review.
Keywords
Adsorption, Dye, Photo-catalytic degradation, Silver nanoparticles, Wastewater treatment
Introduction
Water contamination is a serious issue that poses a danger to the living community. Water Natural resources have been polluted by harmful chemicals as a result of industrialization. Furthermore, water shortage has been a major issue for a long time, making it a major worry. Organic and inorganic dyes are amongst the major pollutants introduced into resources of water as effluents [1]. These colors are mostly made up of recognized carcinogens like halogenated and naphthalene. As a result, when these colors come into contact with biological systems like animals and people, they may always be converted into carcinogenic by microbial degradation.
Textile, papers, plastics, tires, concrete, and pharmaceuticals are among the industries that utilize dyes, with garment industry becoming the biggest consumer. It's upsetting to discover that around 10% of colors used in business are discharged into the atmosphere, presenting a massive environmental risk. Coloured water comes from the dispersion of colors into waterbodies, which is a visible difficult matter. These dispersed dye prevent most bulk of light from entering the afflicted water system, decreasing the quantity of oxygen leel Colorants may increase Biochemical Oxygen Demand of polluted water [2].
As a result, there are a variety of methods for removing dyes from water. Physical, chemical, and biological treatments techniques are classified according to the principle used eliminate the dyes. However, little is understood about how a material's bioactivity changes when the size of its constituent particles shrinks to nanoscale levels. There are a few studies in the literature that indicate promising findings when it comes to the action of various medicines and antibacterial compositions in nanoparticle form [3].
Dye removal mechanisms using silver nanoparticles
Silver compounds contains the silver ions that has been recognized for their antibacterial capabilities, which been utilized in a different diversity of applications ranging from cleaning medical equipment and domestic appliances to water purification. The effectiveness of metal nanoparticles in various applications is determined by the variables that influence their production. Metal precursors, solvents, and reducing agents are among them. Silver nitrate is by far the most frequently utilized metal salt of silver for silver nanoparticle production.
Ag NPs are useful in catalysis, bio sensing, and drug delivery. The bio-synthesis, use of silver and gold nanoparticles will be discussed. Hazardous reactants, high temperatures, and high pressures are used in traditional Ag NP production techniques. The use of ecologically friendly microbes and plant extract in the sustainable synthesis of the Silver NPs has lately gained popularity. The technique has many benefits, including simplicity, financial friendliness, cheap cost, mild reaction conditions, and the use of non-toxic substances. In these biological techniques for the production of NPs, extracts of live organisms serve as both reducing agents. Until date, Ag NPs have been effectively and quickly produced via the use of various Bacteria, Algae, Fungi, plants [4,5] shown in Figure 1.
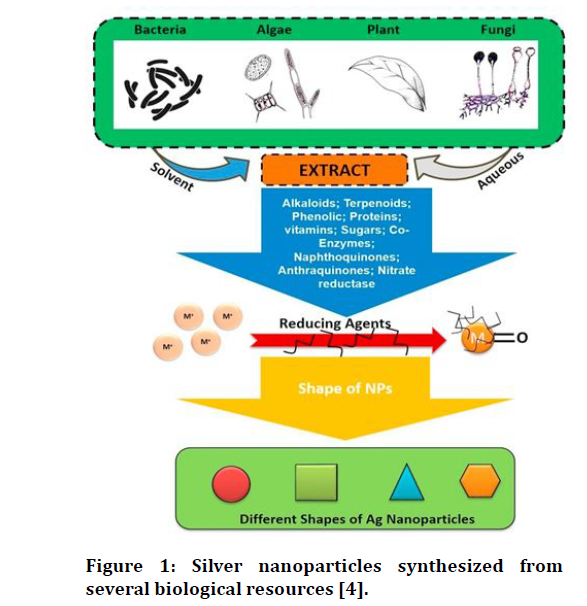
Figure 1. Silver nanoparticles synthesized from several biological resources [4].
Adsorption of silver nanoparticles
A greatest amount of methylene blue absorption by silver nanoparticle packed charcoal filters was 71.4 mg/g absorption. A straightforward green chemistry method has been developed to produce protein-covered AgNPs on the top of micro to build a rigid multipurpose composite material. This was given the title NSAgNP by them. The NSAgNP that was created has indeed been thoroughly investigated and assessed as a novel anti - fouling nanoparticle capable of removing dyes and dangerous microbes from waterbodies. Large surface area, low high porosity, and strong adsorption surface - to - volume catalytic activity improved not just dye absorption but also the effectiveness with which germs were destroyed and biofilm formation prevented. Multiplex dye adsorption, powerful antimicrobial, and antifouling performance of proposed bulk heterojunction nanocomposite may provide a superior option for water disinfection [6].
Photo catalytic degradation
In dye effluent treatment, photocatalysis is a key process. For the breakdown of different kinds of contaminants, photocatalytic oxidation procedures involve the illumination of semiconductor particles. The production of hydroxyl radicals, which are extremely reactive and nonselective oxidants towards organic molecules, is the basis for these activities. Because of its cheap energy consumption, strong oxidizing ability, moderate reaction conditions, and minimal secondary pollution, photocatalysis offers a number of important benefits in terms of pollutant breakdown. Doping of Ag nanoparticles in TiO2 has an impact on both anatase and rutile species.
In both instances, they see that the doping procedure causes an optical gap fluctuation of 1.6 eV even at very low silver percentages (Ag samples), quenches photoluminescence emission by approximately 50%, and raises optical absorbance in the visible region by around 20% illustrated in Figure 2.
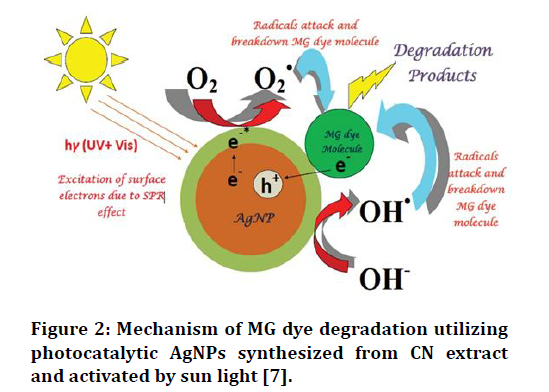
Figure 2.Mechanism of MG dye degradation utilizing photocatalytic AgNPs synthesized from CN extract and activated by sun light [7].
Adsorption and photocatalytic activity of silver nanocomposites in combination
Nevertheless, neither a documentation just on rapid m sorption (MB) and perhaps other dyes on solids CMSs, but just not hollowed or porosity of CMSs-based adsorbent, nor a research just on order to achieve plasmon photo-catalyst utilizing thermal CMSs adorned with nanomaterials available, to our knowledge. They initially demonstrated on a straightforward multi - step method for producing PbMoO4-coated carbon microspheres photocatalyst, and the enzymatic characteristics of CMSs for Dyes B deprivation of methylene blue light. Instead using PbMoO4 as a semiconductor, charcoal microspheres covered by Ag nanoparticles (AgNP-CMSs) were used in study. It's worth noting that the method employed in this research is simpler than the one utilized in the previous one, as well as the characteristics of AgNP differ from that of PbMoO4 nanoparticles. The AgNP-CMSs also exhibited a high sorption strength of approximately to 250 mg/g towards MB, having low membrane specific area, and the time required to reach adsorption isotherm was just about 1 minute. These AgNP-CMSs shown outstanding adsorb & catalyzed capabilities in the degradation of water RhB and MB beneath light waves [8].
Silver nanoparticle toxicity
Antimicrobial characteristic of silver nanoparticles (AgNPs) are increasingly being used in detergents, polymers, and textiles. Although the mechanism of AgNP poisoning have not been completely explored, silver is among the most poisonous trace metals known. There is dispute about whether toxicity is due to the impacts of soluble forms of Ag liberated from ENPs or if it is due to the impact of nanoparticles in general. The harmful effects of AgNPs on cell development, photosynthesis, and chlorophyll production in a marine diatom (Thalassiosira weissflogii) were shown to be due to release of dissolved silver. The AgNP toxicity to a freshwater alga (Chlamydomonas reinhardtii) photosystem II quantum yield was dependent on Ag release, perhaps locally at the algal/ AgNP interface. The AgNPs smaller than nm was much more toxic to nitrification than bigger AgNPs or dissolved Ag at comparable mass concentrations, indicating that toxicity was not entirely attributable to dissolved silver (Figure 3).
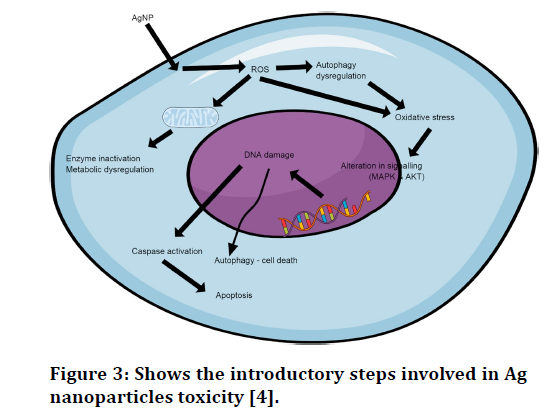
Figure 3.Shows the introductory steps involved in Ag nanoparticles toxicity [4].
Whenever Ag Nano Particles were released to the environment systems after the removal of dyes effluents, they are environmentally damaging because they impede nitrogen fixation, which really is critical in the hydrological cycle of different components. While silver nanoparticles' harm is mostly owing to its physicochemical characteristics, the exact mechanism of intracellular assimilation in snails has lately been studied. In the redox proteomics research on Shellfish, intracellular uptake through clathrin and caveolaemediated endocytosis was particularly reduced, as was the absorption of metal nanoparticles of 50 nm to 100 nm nm sizes. It is widely known how transcriptional control affects bacteria growth. Genomic sequence transcriptome monitoring may help them understand more about relevant process. As a consequence of expression of genes, peptide also plays an important role in a variety of biochemical pathways, such as speeding up metabolic events and transporting substances. As a consequence, variations in the enzyme may lead to variances in bacterial activity as well as biochemical disturbances. As a consequence, peptide synthesis efficiency should be tackled. Clearly, using transcriptome and proteome profiling to investigate at all of the possible pathways is a smart idea. Previous research has regrettably connected the cytotoxicity of Ag NPs to the oxidative reactions (ROS). Regardless of the fact that Ag NPs influence the production of a large number of anaerobic fermentation enzymes, the full transcriptome pattern of de-nitrification gut microbiome to Ag NPs remains unclear. As a consequence, a complete study of transcriptome and proteomic profiles is needed to investigate the effects of Ag NPs on de-nitrification and identify the underlying process [9].
Review of Literature
Ahmed and his colleagues investigated about the dyes are a common kind of organic contaminant that are used for the harmful impact at marine life in overall to the humans in specific. Several methods for removing colors from industrial and household effluents have evolved as science and technology has progressed. The more current techniques for removing colors from wastewaters were addressed in the study. Adsorbent, oxidation, flocculation-coagulation, membrane separation, and bioremediation have all been emphasized as wastewater treatment techniques. In addition, from 2010 to 2014, efforts were made to evaluate all known methods as well as newly published research. In addition, the performances and unique characteristics of these systems [2].
Wastewater reuse and dumping systems, and also the challenge of obtaining sufficient disinfecting while generating harmful byproducts, require new methods for efficient disinfecting and microbiological control, according to Q. Li and his colleagues. Photocatalysts manufacturing of oxidative stress that harm cell structures, malware contains the bacterial envelope, and disrupting electricity transmission. While some nanomaterials were used as antiviral drugs in consumer crops including such household cleansers, the possibility for disinfection or bacterial management at whole system water treatment has yet to be fully explored. This study looked at different nanoparticles' antimicrobial processes, analyzed their advantages, limitations, and utility for water disinfection and foulants management, and emphasized the need for another study into nanostructured materials for treating wastewater [10].
S. K. Das & his colleagues examined a novel generally pro absorbent composed of nano-silica with AgNPs for purifying water that is both cost14 effective and ecologically benign. Again for implementation of environmental nano, nutrient oxidation of metallic ions at air temp is utilized to produce well-dispersed AgNPs just on micro surface, termed NSAgNP. The coated peptides on AgNPs resulted in the creation of robust NSAgNP, which safeguarded the AgNPs against degradation and many other ions present in water. Both in solitary and multistep solutions, the NSAgNP exhibited excellent dye adsorption ability, and also adequate resilience to acidity or dye intensity fluctuations. AgNPs' antimicrobial activity delayed virus's initial adhesion to NSAgNP, significantly enhancing the nanomaterial's antifouling properties and reducing the biofilm. So according scanning electron and fluorescence microscopy studies, the electrostatic attraction of positive charges NSAgNP and negatively charged bacterial membrane produced irreversible damage the cell membrane. The strong removal of multiphase dyes, reusability, good sensitivity, adsorption capacity, and E. coli from modeled antifouling properties and contaminated water of NSAgNP will pave the framework for the growth of expense and ecologically responsible purification systems [6].
P. Barone looked into the visual & chemical compositions of Ag/TiO2 nano - composites in order to identify if those composite technologies could be utilized in Gratzel photoelectrochemical cells. Metal nano particles was doped in TiO2, across both crystalline forms anatase and rutile, to create nanocomposites. Photoluminescence and visually absorption experiments inside the Ultra violet range show a 50% reduction in photoluminescence emissions as well as a 20% rise in visual attenuation, while X-ray photoemission data suggest no chemical interaction between Ag and Ti. The transmittance, as estimated using Tauc's formula, fluctuates by around 1.6 eV.
M. B. Sumi examine the impact of sunlight on nanoparticles made from different extracts of both the Cocos nucifera (CN) rhizome, which were then evaluated for photocatalytic. The metal nanoparticles were created utilizing a therapeutic phytocomponent contained inside the rhizome of a Cocos nucifera. Huge use of the method necessitates the modification of process variables to enhance the conversion of electrostatic interaction to nanoparticles. The effect of process variables including such source salt concentration and synthesizing mix pH just on morphologically of silvery nanoparticles was determined. The optimum synthesis combination was found to be CN extraction and 5 mM iodide solutions at a ratio of 1:4. An acidic beginning pH of the synthetic liquid was found to enhance the formation of larger narrow size distribution silver nanoparticles. Energy from the sun was collected for the photoreduction of Neutral color green dye utilizing silver nanoparticles made to use a green synthesis method. The entire technique aims to use a readily available mineral wealth again for creation of silver nanoparticles and the deprivation of dyes with these granules, forming it ideal for handling of wastewater [7].
Discussion
The silver nanoparticles were created using M. tinctoria plant leaf extract at various pH levels. The production of silver nanoparticles was not seen in the acidic media. The size and amount of silver nanoparticles produced in an alkaline media are significantly influenced by the pH as measured by a UV-Vis spectrophotometer. SEM verified the spherical form of the particles with sizes ranging from 79 to 96 nm. XRD was used to determine the crystal nature of the material, and an EDX spectrum was used to predict the existence of elemental silver. Methylene blue dye was used to test the photo catalytic activity of green produced silver nanoparticles. The primary absorption peak at 660 nm progressively faded as the exposition duration increased, suggesting photo catalytic breakdown of the methylene blue dye.
Many antibacterial nanomaterials, including chitin, nAg, TiO2, and nano - tubes, show promise as alternative to synthetic antiseptics, which can generate harmful byproducts. Even though existing economic factors and undisclosed sentient health and the environment impacts preclude use of nanomaterials water disinfection in the nearish term, significant attention in dispersed treatment of water and repurpose systems, bolstered by worries about strained water supply systems, is expected to boost study in the area in the coming decades. The multifunctional AgNP-CMS materials made of solid CMSs that were neither hollow nor porous using a redox method at room temperature. The adherence to MB & photocatalyst activities toward RhB and MB were studied when AgNPCMSs were exposed to light waves. Given the low surface specific area and photocatalytic characteristics generated by visible light, the AgNP-CMSs was been shown to have rapid and rapid sorption capacity. Based on the review of AgNP-CMSs using current techniques, the origins of their rapid and high sorption and catalytic properties were investigated.
Conclusion
The research summarizes the significance of emerald nano - particle manufacturing that is used in wastewater purification. It has been demonstrated that using organic or ecologically responsible extract relevant to make silver nanoparticles efficiently removes various hues from municipal wastewater. Despite this, recent research shows that nanoparticles ejection into the environment is dangerous and harmful to marine and riparian living creatures at low quantities. In addition, use of silver ions in dye collection and treatment techniques requires extensive risk assessments and ecological health safety. As a consequence, it's critical to evaluate argent nanoparticle cytotoxicity or the risks of employing nanomaterials in dye wastewater treatment. One of the natural therapies for decreasing gold nanoparticle cytotoxicity and the impact on wastewater treatment plants has actually been found as the sulphides process. In vivo immune-toxicity studies, effect of nanomaterial shape on cytotoxicity, and comprehensive study on silver nanoparticle adsorbate on a variety of biological components are in the startup phase.
References
- Chequer FD, De Oliveira GR, Ferraz EA, et al. Textile dyes: Dyeing process and environmental impact. Eco-friendly textile dyeing and finishing 2013; 6:151-176.
- Ahmad A, Mohd-Setapar SH, Chuong CS, et al. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv 2015; 5:30801-18.
- Stoimenov PK, Klinger RL, Marchin GL, et al. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002; 18:6679-86.
- Marimuthu S, Antonisamy AJ, Malayandi S, et al. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J Photochem Photobiol 2020; 205:111823.
- Khodadadi B, Bordbar M, Yeganeh-Faal A, et al. Green synthesis of Ag nanoparticles/clinoptilolite using Vaccinium macrocarpon fruit extract and its excellent catalytic activity for reduction of organic dyes. J Alloys Compound 2017; 719:82-8.
- Das SK, Khan MM, Parandhaman T, et al. Nano-silica fabricated with silver nanoparticles: Antifouling adsorbent for efficient dye removal, effective water disinfection and biofouling control. Nanoscale 2013; 5:5549-5560.
- Sumi MB, Devadiga A, Shetty K V, et al. Solar photocatalytically active, engineered silver nanoparticle synthesis using aqueous extract of mesocarp of Cocos nucifera (Red Spicata Dwarf). J Exp Nanosci 2017; 12:14-32.
- Chen Q, Wu Q. Preparation of carbon microspheres decorated with silver nanoparticles and their ability to remove dyes from aqueous solution. J Hazardous Mater 2015; 283:193-201.
- Zheng X, Wang J, Chen Y, et al. Comprehensive analysis of transcriptional and proteomic profiling reveals silver nanoparticles-induced toxicity to bacterial denitrification. J Hazardous Mater 2018; 344:291-298.
- Li Q, Mahendra S, Lyon DY, et al. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res 2008; 42:4591-602.
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Author Info
Vikram Mor1*, Gopal Arora2 and Mohinder Pal3
1Department of Environmental Science, Faculty of Science, India2SBAS, Mathura, Uttar Pradesh, India
3RIMT University, Mandi Gobindgarh, Punjab, India
Received: 03-Feb-2022, Manuscript No. JRMDS-22-54073; , Pre QC No. JRMDS-22-54073(PQ); Editor assigned: 04-Feb-2022, Pre QC No. JRMDS-22-54073(PQ); Reviewed: 18-Feb-2022, QC No. JRMDS-22-54073; Revised: 21-Feb-2022, Manuscript No. JRMDS-22-54073 (R); Published: 28-Feb-2022
