Research - (2021) Volume 9, Issue 6
Role of Corticosteroids in Tubercular Pleural Effusion
Dharmic Suresh, Anand KR and Raja Amarnath G*
*Correspondence: Raja Amarnath G, Department of Pulmonary Medicine, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, India, Email:
Abstract
A prospective, randomized study was designed to determine the effect of corticosteroids, as adjuvant therapy in patients with Tubercular Pleural Effusion during treatment with ATT. The Present study focuses on to compare the efficiency of corticosteroids in Tubercular Pleural Effusion when given through various routes and to conclude the optimal route of drug administration. (Oral/Intra Pleural). The Present study includes to find if there is any correlation between the Pleural fluid ADA and USG findings at the end of treatment.
Keywords
Tuberculous pleurisy, corticosteroids, ATT, USG, Lymphoid tissue, Dexamethasone
Introduction
Tuberculous pleurisy is the second most common type of extrapulmonary tuberculosis [1] and is the most common cause of exudative pleural effusion in developing countries (30-60%). According to World Health Organization (WHO) the estimated prevalence of pleural effusion is 320 cases per 100,000 people in the third world countries.
Though India is the second-most populous country in the world, India has more new Tuberculosis (TB) cases annually than any other country. In 2011, out of the estimated global annual incidence of 9 million TB cases, 2.3 million were estimated to have occurred in India [2,3]. Hence it is important to consider the possibility of tuberculosis pleuritis in all patients with anundiagnosed pleural effusion in India [4]. Despite the availability of effective chemotherapy for Tuberculosis (TB), significant morbidity and mortality due to this disease continue to occur. While multiple factors complicate the therapy for TB, slow responses to effective antibiotics, especially far advanced disease, have long hampered clinical efforts [5,6]. As better anti-mycobacterial agents became available and clinical responses became more certain, the often-tardy pace of clinical improvement continued to frustrate both the patients and the physicians.
According to World Health Organization (WHO) recommendations, Tuberculous pleural effusion is treated like Pulmonary Tuberculosis as follows new cases: Category I regimen: 2 months with 4 anti tuberculosis therapy (ATT) drugs followed by 4 months with 2 anti-TB drugs. 2(HRZE)3+4(HR)3 Treatment failure/ Defaulters/ Relapse cases: Category II regimen: 2 months with 5 anti- TB drugs followed by 1month of 4 anti-TB drugs followed with5 months with 3 anti-TB drugs. 2(HRZES)3+ 1(H RZ E)3+ 5(H RE). The local signs and symptoms of TB Pleurisy are at most totally caused by the host's inflammatory response to the presence of the Mycobacterium tuberculosis bacillus [7,8]. Even in Pulmonary TB, the systemic wasting syndrome associated with progressive TB is also considered to be related to the host's inflammatory response, as mediated through excessive cytokine product ion [9]. Hence it 1s reasonable to postulate, therefore, that potent anti-inflammatory agents such as corticosteroids, known to suppress a myriad of inflammatory responses, may prove effective as adjunctive therapy in the management of TB Pleurisy.
Concerns regarding the potential adverse effects of steroids, especially m HIV positive patients exists. However, high quality evidence on the benefits and adverse effects associated with steroid use in TB pleural effusion is limited. There is currently insufficient data to support evidence-based recommendations regarding the use of adjunctive steroids in patients with Tuberculous pleurisy in current generation. Also, the studies on comparison of effect of steroids when administered through different routes are limited.
Randomised trials, which are effectively powered to evaluate both morbidity and mortality are needed. Similar considerations have prompted investigators to conduct a range of studies over the past 60 years in the efficacy of adjunctive corticosteroid usage in therapy for TB. A perusal of recent reviews of therapy for TB suggests that this older body of data, especially that addressing adjunctive use in extra-pulmonary forms like TB pleurisy, has been largely ignored. And not many studies compare the benefits of corticosteroids when administered through different routes. Early investigations on the effect of corticosteroid administration on M.tuberculosis infection were performed on animal models. Corticosteroid administration markedly enhanced the virulence of M. tuberculosis in these studies, m which no specific antituberculous agent was employed [10,11]. When antituberculosis agents were developed, repetitive studies suggested that the deleterious effects of corticosteroids on M. tuberculosis infection were, for the most part, abrogated when effective therapy. By the 1950s, anecdotal experience with human corticosteroid therapy for patients with TB, without anti-tuberculous therapy, was perilous [12,13], but co-administration of corticosteroids with anti-tuberculous agents could improve outcomes [14]. These data were suggestive enough to prompt the initiation of controlled studies investigating the utility of adjunctive corticosteroid therapy for Tuberculosis. In case of TB Pleurisy, studies of adjunctive steroids for the treatment of Tubercular Pleural Effusion show conflicting results. Nonrandomised studies done in the preHIV found that steroids led to more rapid resolution of the effusion and reduced likelihood of residual pleural thickening and pleural adhesions [15-20]. In contrast, a critical appraisal of published studies by Dooley et al. [20] demonstrated beneficial effects of steroids on acute symptoms but found no benefit for chronic end points such as fibrosis, irrespective of the dose. Hence this study was carried out.
Materials and Methods
The study on the "Role of corticosteroids m Tubercular Pleural effusion", was carried out in the department of Chest &TB, Sree Balaji Medical College and Hospital, Chennai, with an aim to determine the efficiency of corticosteroids in treatment of Tubercular pleural effusion when administered through different routes.
Study design
The present study was a Prospective Randomized Controlled Non-Blinded study.
Sample size
A total of 24 patients were enrolled into the study.
Inclusion criteria
• Patients in the age group between 15yrs- 70yrs.
• Patients diagnosed with Simple Tubercular Pleural Effusion, based on clinical, biochemical, radiological and pathological parameters.
• Patients with a minimal Pleural Effusion of at least 300ml or above in USG.
Exclusion criteria
• Cases of smear positive pulmonary tuberculosis.
• Patients with diabetes mellitus.
• Patients with chronic renal failure.
• Patients with chronic Hepatic failure.
• Patients with previous H/O pleural diseases.
• Cases of empyema/ loculated pleural effusion.
• Patients with acid peptic diseases.
• Patients on treatment with corticosteroids for any other disease.
• Patients with HIV serology positive.
• Uncontrolled hypertension.
Consent
This study was approved by the institutional Ethics Committee. An informed written consent was taken from all the patients enrolled in the study after a proper health education.
Treatment regimen
Patients were divided into 4 groups in total. All the groups were treated with Anti-Tubercular drugs as per RNTCP guidelines and therapeutic thoracentesis was done until near dryness.
GROUP l: This was considered the control group where patients (n= 6) were subjected only to Anti-Tubercular treatment as per RNTCP guidelines and therapeutic thoracentesis.
GROUP 2: In addition to ATT and therapeutic thoracentesis patients in group 2 (n=6) were given Oral corticosteroids in the form of Prednisolone 1mg/kg/day for first 2 weeks and slowly tapered to 0.5 mg/kg/day for next 2weeks followed by 0.25mg/kg/day for next 2 weeks followed by 0.1 0mg/kg/day for last 2 weeks.
GROUP 3: In this study group (n=6), in addition to ATT and therapeutic thoracentesis, 5mg Dexamethasone was injected into the pleural cavity during every sitting of therapeutic thoracentesis in all patients [21-24].
GROUP 4: In addition to ATT and therapeutic thoracentesis patients in group4 (n=6) were given Oral corticosteroids in the form of Prednisolone lmg/kg/day for first 2 weeks and slowly tapered to 0.5mg/kg/day for next 2days followed by 0.25mg/kg/day for next 2 weeks followed by 0.1 0mg/kg/day for last 2 weeks + 5mg Dexamethasone was injected into the pleural cavity during every sitting of therapeutic thoracentesis. All patients were treated in hospital for at least 10 days and their state of well-being was monitored on daily basis.
Patients symptoms, vital data, ESR, Weight, blood total count, Chest X-Ray, USG Chest were recorded at the time of admission, end of 2 months and at the end of 6 months and analyzed.
CXR: chest X-ray findings were categorized as
Massive Pleural effusion → Non homogenous opacity higher than lower border of anterior surface of 2nd rib.
Moderate Pleural effusion → Non homogenous opacity lower than lower border of anterior surface of 2nd rib but higher than lower border of anterior surface of 4t h rib.
Minimal Pleural effusion → Non homogenous.
CP angle blunting → CP angle >90 degrees.
No effusion/thickening Sharp CP angle.
The cases in the different treatment groups were segregated and the radiological clearance based on the CXR and USG were analysed periodically, thus giving the relation to the amount of overall radiological clearance among all the 4 groups. These observations are recorded and categorized as mentioned earlier. Close watch was kept for any complications of steroid therapy (Table 1).
| Quantification | Ultrasound visualization | Volume estimation (ml) |
|---|---|---|
| Minimal | Range, one probe | 100-500 |
| Moderate | Range, two probes | 500-1,500 |
| Large or massive | Range, three or more probes | >1,500 |
Table 1: Ultrasound quantification of pleural effusion.
Results
The study on the "Role of corticosteroids 1n Tubercular Pleural effusion", was carried out in the Chest & TB department of Sree Balaji Medical College and Hospital, Chennai. A total of 24 patients in 4 groups were enrolled into the study and various parameters were analysed. All the groups had equal number of subjects (n=6).
GROUP l: This was considered the control group where patients (n = 6) were subjected only to Anti Tubercular treatment as per RNTCP guidelines and therapeutic thoracentesis
GROUP 2: In addition to ATT and therapeutic thoracentesis patients in group 2 (n=6) were given Oral corticosteroids in the form of Prednisolone l mg/kg/day for first 2 weeks and slowly tapered to 0.5mg/kg/day for next 2weeks followed by 0.25mg/kg/day for next 2 weeks followed by 0.1 0mg/kg/day for last 2 weeks.GROUP 3: In this study group (n=6), in addition to ATT and therapeutic thoracentesis, 5mg of Dexamethasone was injected into the pleural cavity during each sitting of therapeutic thoracentesis in all patients.
GROUP 4: In addition to ATT and therapeutic thoracentesis patients in group4 (n=6) were given Oral corticosteroids in the form of Prednisolone 1mg/kg/day for first 2 weeks and slowly tapered to 0.5mg/kg/day for next 2days followed by 0.25mg/kg/day for next 2 weeks followed by 0.1 0mg/kg/day for last 2 weeks + 5mg Dexamethasone was injected into the pleural cavity during each sitting of therapeutic thoracentesis. Observations are analysed and results are tabulated below (Table 2 to Table 10, Figure 1 and Figure 2).
| Treatment Group | Frequency | Percent |
|---|---|---|
| Group l | 6 | 25 |
| Group 2 | 6 | 25 |
| Group3 | 6 | 25 |
| Group 4 | 6 | 25 |
| Total | 24 | 100 |
Table 2: Frequency.
| Age Group | Frequency | Percentage (%) |
|---|---|---|
| 15-30 | 9 | 37.5 |
| 31-45 | 7 | 29.1 |
| 46-60 | 6 | 25 |
| 61-75 | 2 | 8.3 |
Table 3: Age distribution.
| Sex | Frequency | Percent |
|---|---|---|
| Male | 12 | 50 |
| Female | 12 | 50 |
| Total | 24 | 100 |
Table 4: Sex distribution.
| Cough | 19 | 79.2 |
| Fever | 23 | 95.8 |
| Breathlessness | 17 | 70.8 |
| LOW/LOA | 22 | 91.7 |
| Chest Pain | 20 | 83.3 |
Table 5: Symptoms at baseline.
| Minimal pleural Effusion | 11 | 45 .8 |
| Moderate Pleural effusion | 4 | 16 .7 |
| Massive Pleural effusion | 9 | 37.5 |
| Total | 24 | 100 |
Table 6: CXR/USG at the beginning of treatment.
| Side | Frequency | Percent | |
|---|---|---|---|
| Bilateral | 1 | 4.2 | |
| Left | 11 | 45.8 | |
| Right | 12 | 50 | |
| Total | 24 | 100 |
Table 7: Side of lesion.
| Treatment groups | Symptoms | |||||
|---|---|---|---|---|---|---|
| Cough | Fever | Breathlessness | Loa/low | Chest Pain | ||
| Group 1 | N | 5 | 5 | 4 | 5 | 4 |
| % | 83.33 | 83.33 | 66.66 | 83.33 | 66.66 | |
| Group 2 | N | 5 | 6 | 5 | 5 | 5 |
| % | 83.33 | 100 | 83.33 | 83.33 | 83.33 | |
| Group3 | N | 5 | 6 | 5 | 6 | 5 |
| % | 83 .33 | 100 | 83.33 | 100 | 83 .33 | |
| Group 4 | N | 4 | 6 | 3 | 6 | 6 |
| % | 66.66 | 100 | 50 | 100 | 100 | |
| Total | N | 19 | 23 | 17 | 22 | 20 |
| % | 79.2 | 95.8 | 70.8 | 91.7 | 83.3 | |
Table 8: Frequency of symptoms in each group.
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Age | 24 | 17 | 72 | 38.54 | 16.1 |
| Pleural Fluid ADA (u/l) | 24 | 36 | 90 | 62.13 | 14.567 |
| Pleural fluid TC (cells/cu.mm) | 24 | 482 | 14800 | 4298 .54 | 3732.688 |
| Pulse (per min) | 24 | 60 | 130 | 101.5 | 15.517 |
| Respiratory Rate (per min) | 24 | 22 | 34 | 27.42 | 3.361 |
| Systolic pressure (mm/hg) | 24 | 90 | 150 | 117.5 | 15.393 |
| Diastolic pressure (mm/hg) | 24 | 60 | 90 | 75 | 9.78 |
| Weight (kgs) | 24 | 30 | 76 | 46.88 | 12.678 |
| ESR (mm) | 24 | 38 | 150 | 106 .1 7 | 24. 784 |
| Blood TC (cells/cu.mm) | 24 | 4600 | 23500 | 10081.25 | 5234.642 |
Table 9: Descriptive statistics.
| N | Mean | Std. Deviation | |||
|---|---|---|---|---|---|
| Pleural fluid ADA (u/l) | Group | 1 | 6 | 59.33 | 17.592 |
| Group | 2 | 6 | 56.83 | 9.888 | |
| Group | 3 | 6 | 60.83 | 15.791 | |
| Group | 4 | 6 | 71.5 | 13 .004 | |
| Total | 24 | 62.13 | 14.56 7 | ||
| Pleural fluid Tc (cell s/cu. Mm) | Group | 1 | 6 | 5797.5 | 2579.891 |
| Group | 2 | 6 | 1785.33 | 1249.999 | |
| Group | 3 | 6 | 3881.33 | 3870.04 | |
| Group | 4 | 6 | 5730 | 5287.022 | |
| Total | 24 | 4298.54 | 3732.688 | ||
| Pulse (per min) | Group | 1 | 6 | 90.67 | 19.906 |
| Group | 2 | 6 | 99 | 8.27 | |
| Group | 3 | 6 | 108 .33 | 11.553 | |
| Group | 4 | 6 | 108. 00 | 15.95 | |
| Total | 24 | 101.5 | 15.517 | ||
| Respiratory Rate (per min) | Group | 1 | 6 | 25.67 | 3.882 |
| Group | 2 | 6 | 26.67 | 2.066 | |
| Group | 3 | 6 | 2 | 3.033 | |
| Group | 4 | 6 | 2 | 3.882 | |
| Total | 24 | 27.42 | 3.361 | ||
| Systolic pressure (mm/hg) | Group | 1 | 6 | 110 | 16.733 |
| Group | 2 | 6 | 111.67 | 11.69 | |
| Group | 3 | 6 | 123.33 | 13.663 | |
| Group | 4 | 6 | 125.00 | 16.432 | |
| Total | 24 | 117.5 | 15.393 | ||
| Diastolic pressure (mm/hg) | Group | 1 | 6 | 71.67 | 11.69 |
| Group | 2 | 6 | 71.67 | 9.832 | |
| Group | 3 | 6 | 80 | 8.944 | |
| Group | 4 | 6 | 76.67 | 8.165 | |
| Total | 24 | 75 | 9.78 | ||
| Weight | Group | 1 | 6 | 45 | 18.111 |
| Group | 2 | 6 | 47 | 9.757 | |
| Group | 3 | 6 | 46.83 | 10.572 | |
| Group | 4 | 6 | 48.67 | 14 . 010 | |
| Total | 24 | 46.88 | 12 . 678 | ||
| ESR (mm) | Group | 1 | 6 | 109.33 | 23.619 |
| Group | 2 | 6 | 112.33 | 16.99 | |
| Group | 3 | 6 | 99.67 | 36.031 | |
| Group | 4 | 6 | 103.33 | 23 .721 | |
| Total | 24 | 106.17 | 24.784 | ||
Table 10: Descriptive statistics for each group at baseline.
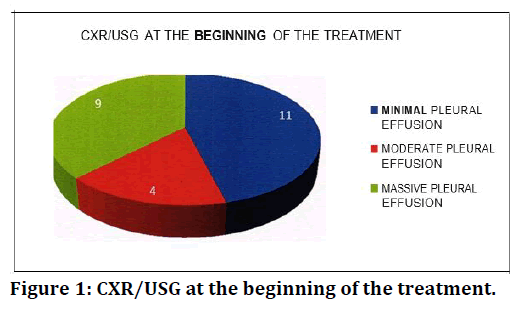
Figure 1. CXR/USG at the beginning of the treatment.
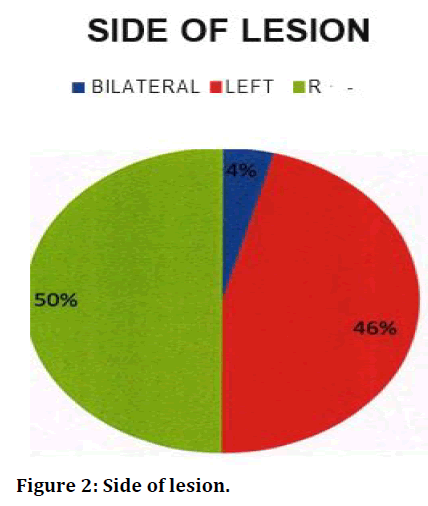
Figure 2. Side of lesion.
Discussion
Despite the use of effective anti-tuberculous treatment regimens, the disease still poses serious risks to patients. In addition to the untoward consequences of overwhelming infection, the inflammatory reaction to Mycobacterium tuberculosis can result significant tissue damage. Healing, particularly by fibrous scarring, can render organs severely damaged and dysfunctional. Therefore, the long term disabling effects of treated tuberculosis remain an important challenge. Suppression of unwanted inflammatory events arising in response to the infection with M. tuberculosis seems logical.
Tuberculous effusions may resolve and heal without any long-term sequelae. However, in some instances, the healing of effusions arises with a legacy of pleural fibrosis, possibly due to disordered fibrin turnover, whereby an imbalance between fibrin deposition and fibrinolysis occurs. Pleural fibrosis can result in clinically relevant pleural thickening and impairment of lung function. Therefore, the intent of treatment is to foreshorten the acute phase of the disease and to restore the integrity of the pleura preventing lasting fibrosis and thickening [2-31].
Hence this study was done in the view to analyse the use of corticosteroids in TPE as adjuvant therapy if at all any, 1n this modern ATT era and to compare the efficacy of the same when given through different routes. And our results are very much promising.
This study had equal number of male and female subjects, but many studies suggest male predominance in Tubercular Pleural Effusion. Tubercular pleural effusion is usually unilateral and the volume of fluid accumulated is often minimal to moderate size 90. Consistent with this study 62.5% subjects had minimal or moderate pleural effusion in our research. In our study only 1 of our patients (4%) had bilateral effusion and the rest 96% had unilateral effusion. Out of the 24 patients 12 had right sided effusion and 11 patients had a left sided effusion. In comparison to l ner studies [32] pleural effusion was more common in right side (55%) than on the left (32%); In both right and left side effusion were of equal distribution.
In this study the cell count in the pleural fluid ranged from 482 to 14800 cells/mm3 with a mean of 4298.54; Lymphocytic predominance was found in all but 2 patients (92%) [33-37] demonstrated similar lymphocytic predominance in 1991 and 1973, respectively. Pleural fluid ADA levels in the study ranged from 36- 90 with a mean of 62.13. ADA activity is highest in the lymphoid tissue. Its activity is 10 to 20 times higher in T lymphocytes than B lymphocytes [34] stated that the high ADA activity in tubercular effusion is because ADA is being locally synthesized by T lymphocytes within the pleural cavity and thus it 1s a reflection of a local cellular immune response. This view was also shared [33,36].Tom Patterson also showed that high ADA activity of more than 50.0 IU/L in tubercular effusion. The most common symptom encountered by our study group was fever (95.8%) followed by LOW/LOA (91.7%), Chest pain (83.5%), cough (79.2%). These findings are compatible with the studies done earlier.
One of the important observations this study was faster resolution of symptoms in subjects who were treated with oral corticosteroids. Though there was no significant difference in the resolution of symptoms among the groups at the end of the 6 months of treatment, subjects in group 2 fared better at the end of 6 months and showed faster resolution of symptoms compared to other groups at the end of 2 months, followed by Group 4 and 3 (Group 2 > Group 4=Group 3> Group 1). Similar outcome was obtained by several researchers even during pre- devised ATT era, notable being works. Similar conclusions were made in a review in 2007 which was a meta-analysis on several studies. Also certain studies on use of intra pleural corticosteroids showed superior results over control groups but contradictorily in our study though group 3 fared better than the control group, it was not as good as Group 2 where oral corticosteroids were only given.
In our study we observed weight was maximum group 2 followed by group 1 at the end of two months, but at the end of treatment the mean weight gain in Group 1, 2, 4 were same. The weight gain in Group 3 was poor when compared to other groups. But there was no significant variation in weight gain among each group at the end of two months (p=.498) or at the end of 6 months (p=0.333). Hence the weight gain can also be attributed to increased appetite caused directly by the steroid rather than decrease in disease toll. Also, there were no significant Variation in ESR among the 4 groups of 2 months and at the end of 6 months, though group 2 fared better than other groups. When compared with other studies: Showed significant decrease in ESR in their studies [38,39].
In our study resolution of opacity in CXR was termed when there is radiological clearance from minimal pleural effusion to CP angle blunting or from CP angle blunting to no evidence of RPE [since all patients were discharged with minimal pleural effusion after repeated thoracentesis]. At the end of two months Group 2 did not have any patients (0%) with minimal pleural effusion while Group 4 had 1 (16%). They showed better resolution of capacity compared to Group 1 and group 3 who still had 50% patients each, with Minimal Pleural effusion. But no statistical significance could be achieved (p=0.249). But at the end of 6 months only Group 2 showed better resolution of the effusion compared to other groups but with negative significance with 5out of 6 patients showing no evidence of pleural thickening/ effusion [0.374]. CXR findings in this study mirrored the findings of Galarza et al where recipients of corticosteroids, though had better resolution of radiological opacity did not have any significant variation. But certain studies observed contrasting results.
The most striking finding in this study was the development of Residual pleural thicke in significant number of subjects in control group comlJ<lfed to the study groups, at the end of the treatment. Here, Group 2 and Group 4 fared better than other groups.5 subjects (83.3%) in Group 2 and 4 Subjects (66.66%) in group 4 did not show any evidence of Pleural thickening/effusion while 3 subjects (50%) developed significant thickening in the control Group.(p=0.049),while Group 3 had 4 patients with mild thickening.
On comparison with earlier studies: Menon et al had arrived at similar conclusion, but they did not measure exact pleural thickening since they were only using CXR and also they were performed in the 1960s when the treatment of tuberculosis had not been fully developed. But other major researchers 20'n '74-&o suggested otherwise and summarized that the most salutary effects appeared to be on resolution of acute symptoms (usually defined as pain, fever, and dyspnea) but corticosteroids possess no clinically significant efficacy for the prevention of the chronic endpoint of fibrosis (with consequent restrictive lung disease). They also confirmed diminution of radiographically determined persistent scarrmg m corticosteroid-treated patients, but clinical correlations were lacking. This contrasting difference between Indian and Western studies could be due to the late presentation of the patients and hence late detection of cases leading to a greater number of patients developing pleural thickness.
It has been estimated that up to 50° of tuberculous pleural effusions develop pleural thickening and such development can cause restrictive lung impairment. While it is simple and logical to conceive that corticosteroid improve the clinical outcomes in TB by suppressing the host-mediated inflammation, direct evidence for such an effect in humans has proved elusive. A critical appraisal of published studies demonstrated beneficial effects of steroids on acute symptoms but found no benefit for chronic endpoints such as fibrosis, irrespective of the dose [29]. Even the most recent reviews 11,12 stated, on balance, the evidence does not support the use of steroids in the treatment of pleural tuberculosis. Collectively, the trials confirm an initial benefit in rapid resolution of effusion and symptom control. However, no lasting advantage was found, none for the development of fibrosis and pleural thickening.
But our study showed that corticosteroids when given as adjuvant therapy in treatment of Tubercular Pleural Effusion along with ATT as per RNTCP guidelines has significant effect not only in improvement of acute symptoms but also in preventing complications and chronic endpoints like Pleural Thickening.
Thwaites et al have put forward an interesting hypothesis to explain the clinical benefit of corticosteroids in TBM. They observed that treatment-limiting adverse events that necessitated a change in ATT were less common in the corticosteroid arm, and such changes in ATT were independently associated with death on multivariable analysis. Based on these finding s, they proposed that "dexamethasone may improve outcomes by reducing the frequency of adverse events that necessitate: a change in the antituberculosis drug dose or regimen, severe clinical hepatitis, in particular." This hypothesis needs verification in future studies to ·find its association with TPE also. This suggests that the absorption of systemic corticosteroids by the pleura through systemic blood supply might be a major positive factor in favour of oral corticosteroids, whereas the locally instilled corticosteroids might not get absorbed properly into the systemic circulation as the exudative fluid hinders proper drainage as well as prevents direct absorption through the capillaries due to increased oncotic pressure as a result of increased pleural fluid protein.
Also, this might be due to insufficient dose of intra pleural Dexamethasone, which would be a major factor considering the drug interaction with Rifampicin. Though Hydrocortisone equivalent dosage was given, there are no sufficient data regarding dosage of intra pleural Dexamethasone. Another important finding in this study was the positive correlation between Pleural fluid ADA and pleural thickening in USG at the end of treatment more than half of the subjects with Pleural fluid ADA - rn-.de' than 50 1/U developed Pleural thickening, but analysis cannot be made since subjects received 4 different treatment which could have altered the course of the fibrosis process. Among the groups Group 4 had all the patients in Category II (100%), while Group2 and 3 had 5 patients each (83%) while Group 1 had 4 patients (66%) in category II.
Here subjects with ADA higher than 50 1/U were more prone to develop pleural thickening than subjects with ADA lesser than the cut off level. Within the groups, Group 2 and Group 4 fared better with a smaller number of patients developing pleural thickening [1 out of 5(20%) and 2 out of 6(33%)] respectively, in spite of having more patients in Category I I [5 out of 6(83%) and 6 out of 6(100%)] respectively.
Whereas in Group 1 and Group 3 significant number of patients developed pleural thickening, 4 out of 4(100%) p=0.050 and 4 out of 5(80%) p=0.050] respectively, in [4 out of 6(66%) and 5 out of 6(83%)] patients falling under Category I I respectively. In comparison with other studies: Extremely limited data are available regarding this study. Uskul B et al in 2005 found significant correlation between Pleural fluid ADA and pleural thickening in a study on 21 TPE patients and have suggested that immunological mechanism might also play a role in the development of pleural thickening. Especially statistically significant difference between two groups in ADA2 levels suggesting that activated T lymphocytes play an effective role in the development of pleural thickening as found by another study 106 that the inflammatory changes caused by M. tuberculosis in the pleural cavity effectively stimulate the production of ADA2. Hence based on the inference of these studies and our study results we suggest that high levels of ADA in more individuals might have played a role in increased incidence of Pleural thickening in Group 3 and Group 4 than Group 2 in spite all 3 groups were subjected to corticosteroid therapy in one form or the other
There had been some limitations in this study. First being the small number of the study population. Secondly the detection of pleural thickening was done by ultrasonography of thorax and there could have been some bias or inter-observer variations among the radiologists, and we did not assess the patients with pleural thickening beyond six months. We did not include any tool for objective assessment of improvement in symptoms. Third, PFT was not done to assess if there was any restrictive pattern in people with Residual Pleural Thickening. Also, Randomization within groups based on severity of the effusion would've yielded even more better results [40,41].
Conclusion
We conclude that the most effective route to administer Corticosteroids will be through Oral route as it gave better results over Intra pleural as well as Oral and intra pleural combined. Though the level of pleural fluid ADA positivity for TB 1s set at 40 1/U we suggest administering of corticosteroid in patients with a pleural fluid ADA of more than 50 1/U would be beneficial as that group of people are more prone to develop residual pleural thickening. We believe our findings are useful 1n practicing evidence-based medicine and provide preliminary benchmark data for further larger prospective studies in our country with a huge burden of tuberculosis.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The encouragement and support from Bharath Institute of Higher Education and Research, Chennai is gratefully acknowledged. For provided the laboratory facilities to carry out the research work.
References
- Light RW. Update on tuberculous pleural effusion. Respirology 2010; 15:451-458.
- Afghani B, Lieberman JM. Paradoxical enlargement or development of intracranial tuberculomas during therapy: Case report and review. Clin Infect Dis 1994; 19:1092-1099.
- https://tbcindia.gov.in/index1.php?lang=1&level=1&sublinkid=4160&lid=2807
- Maher D, Chaulet P, Spinaci S, et al. Treatment of tuberculosis: Guidelines for national programmes. Treatment of tuberculosis: guidelines for national programmes. Second Edn 1997; 1-77.
- Dannenberg AM. Pathogenesis and immunology: Basic aspects. In: Schlossberg D. Tuberculosis. 3rd Edn. New York: Springer-Verlag, 1994; 17-39.
- Rook GAW, Taverne J, Leveton C, et al The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor m the pathogenesis of tuberculosis. Immunology 1987; 62:229-234.
- Hart PD' A, Rees RJW. Enhancing effect of cortisone on tuberculosis in the mouse. Lancet 1950; 2:391-395.
- Youmans GP, Youmans AS. The effect of hormonal preparations on the survival of mice injected intravenously with virulent, attenuated, and avirulent mycobacteria. Am Rev Tuberc 1954; 69:790-796.
- Ilavsky J, Foley EJ. Studies on the effect of cortisone with chemotherapeutic agents on tuberculous peritonitis in mice. Antibiot Chemother 1954; 4:1068-1074.
- Johnson JR, Davey WN. Cortisone, corticotropin, and antimicrobial therapy in tuberculosis in animals and man: a review. Am Rev Tuberc 1954; 70:623-636.
- LeMaistre CA, Tompsett R, Muschenheim C, et al. Effects of adrenocorticotropic hormone and cortisone in patients with tuberculosis. J Clin Invest 1951; 30:445 - 56.
- Favez G, Aguet F, Sourdat P, et al. The treatment of certain forms of tuberculosis with a combination of prednisone (or hydrocortisone) and antibiotics. Dis Chest 1957; 32:70-78 .
- Jesiotr M. Treatment of pulmonary tuberculosis with ACTH and cortisone in addition to specific anti¬ tuberculosis therapy. Dis Chest 1958; 33:180-192.
- Des Prez RM, Organick A. Corticotropin and corticosteroid therapy in tuberculosis. Arch Intern Med 1958; 101:1129-1142.
- Shubin H, Lambert RE, Heiken CA, et al. Steroid therapy and tuberculosis. JAMA 1959; 170:1885-1890.
- Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis 2009; 49:1350-7.
- Kruijshaar ME, Abubakar Increase in extra pulmonary tuberculosis England and Wales1999- 2006. Thorax 2009; 64:1090-5.
- Meno.n NK. Steroid therapy m tuberculous effusion. Tubercle 1964; 45:17-20.
- Singh D, Yesikar SS. Role of intrapleural corticosteroids in tuberculous pleural effusion: a clinicotherapeutic trial of 50 cases. J Indian Med Assoc 1965; 45:306-309.
- Dooley DP, Carpenter JI, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: A critical reappraisal of the literature. Clin Infect Dis 1997; 25:872-87.
- Anthony Seaton. The pleura. Crofton and doughlas 's text book of respiratory diseases. 2000; 12:152-1181.
- P.S.Shankar. Text book of chest medicine. 3rd Edn 1990; 225-253.
- Shankar PS. Text book of pulmonary tuberculosis. 2nd Edn 1990.
- Bannister LH. Respiratory system. Gray's anatomy, 38th Edn 2000; l662-1676.
- Ganong WF. Review of medical physiology. 21st Edn 2003; 649-700.
- Light RW. Disorders of the pleura, Mediastinum, diaphragm and chest wall. Harrison's, principles of internal medicine. 16th Edn 2004; 2:l565-1569.
- Fraser RG, Pare JAP, Pare PD, et al. The pleura diagnosisof diseases of the chest. 3rd Edn 1991; 4:2713-2740.
- Haslett C, Chilvers ER, Corris PA. Disease of the pleura, diaphragm and chest wall. Principles and practice of medicine. Davidson's 22nd Edn 2002; 569-575.
- Vives M, Porcel JM, de Vera MV, et al. A study of light's criteria and possible modifications for distinguishing exudative from transudative pleural effusions. Chest 1996; 109:1503-7.
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: The diagnostic separation of transudates and exudates. Annals Internal Med 1972; 77:507-13.
- Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res 2004; 120:316.
- Awaad AA, El‐Meligy RM, Zain GM, et al. Experimental and clinical antihypertensive activity of Matricaria chamomilla extracts and their angiotensin‐converting enzyme inhibitory activity. Phytotherapy Res 2018; 32:1564-73.
- Chopra RK, Goldman R, Sinatra ST, et al. Relative bioavailability of coenzyme Q10 formulations in human subjects. Int J Vitamin Nutrition Res 1998; 68:109-13.
- Irwin M, Patterson T, Smith TL, et al. Reduction of immune function in life stress and depression. Biol Psychiatr 1990; 27:22-30.
- Light RW. Pleural effusions. Med Clin 2011; 95:1055–1070.
- Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation 200; 112:3608–3616.
- Reddy AL, Raj GS, Badusha M, et al. Analytical study of clinical and etiological profile of patients presenting with pleural effusions to a tertiary hospital. J Evolution Med Dent Sci 2015; 4:15305-13.
- Moudgil H, Sridhar G, Leitch AG. Reactivation disease: The commonest form of TB pleural effusion in Edinburgh,1980-1991.Respir Med 1994; 88:301-304.
- Mathur KS, Mathur JS, Sapru RP. Treatment of tuberculous pleural effusion with local instillation of hydrocortisone. Diseas Chest 1965; 47:83-7.
- Menon NK. Steroid therapy tuberculous effusion. Tubercle 1964; 45:17-27.
- Uskul B, Turker H, Ulman C, et al. The relation of the pleural thickening in tuberculosis pleurisy with the activity adenosine deaminase. Monaldi Arch Chest Dis 2005; 12:101-107.
Author Info
Dharmic Suresh, Anand KR and Raja Amarnath G*
Department of Pulmonary Medicine, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaCitation: Dharmic Suresh, Anand KR, Raja Amarnath G,Role of Corticosteroids in Tubercular Pleural Effusion , J Res Med Dent Sci, 2021, 9(6): 107-116
Received: 08-May-2021 Accepted: 14-Jun-2021
