Research Article - (2022) Volume 10, Issue 8
Role of Er.Cr: YSGG Laser and Fluoride in Caries Resistance
Ahmed Adnan Hadi* and Basima Mohammed Ali
*Correspondence: Ahmed Adnan Hadi, Department of Physics, Institute of Laser for Postgraduate Studies, University of Baghdad, Iraq, Email:
Abstract
The purpose of this study was to compare the effectiveness of using the Er.Cr: YSGG laser with and without Acidulated Phosphate Fluoride (APF) to improve tooth resistance to caries. Sixty enamel sample obtained from first premolar teeth (4 mm × 4 mm) were randomly divided into six groups (n=10): G1: control group (no treatment), G2: Acidulated Phosphate Fluoride (APF) only, G3: Er, Cr: YSGG laser at 0.25 W, 20 Hz, 1% water and 10% air, G4: Er, Cr: YSGG laser at 0.50 W, 20 Hz, 1% water and 10% air, G5: Er, Cr: YSGG laser at 0.25 W, 20 Hz, 1% water and 10% air and acidulated phosphate fluoride, G6: Er, Cr: YSGG laser at 0.5 W, 20 Hz, 1% water and 10% air and acidulated phosphate fluoride. The samples were subjected to pH cycling for 10 days after being irradiated and treated. The samples were tested for digital vickers micro hardness on (9.8 N) at the enamel surface on baseline, after laser irradiation and after ph cycling. Selected sample were further evaluated by scanning electron microscopy (n=1) for each group after surface laser irradiated. Date were statistically analysis (α=0.05).
Results: There was a statistically significant decline in micro hardness values between baseline and post PH-cycling assessments in the control, laser, and fluoride+laser groups (P<0.05). However, as compared to the baseline, the fluoride and laser+fluoride groups showed a considerable increase in micro hardness values.
Conclusion: When compared to the control group, acidulated phosphate fluoride application with laser irradiation of 0.5 w, 20 Hz, 1% water, and 10% air enhanced enamel surface micro hardness and avoided enamel demineralization.
Keywords: Fluoride, Dental caries, Demineralization, Er.Cr: YSGG laser, Enamel micro hardness
Introduction
Tooth enamel is a major tissue that covers the crown of the tooth and is readily visible. With 96 percent inorganic substance, 4 percent organic material, and 4 percent water, enamel is the most mineralized tissue in the human body. It's an extremely hard, white to off-white, highly mineralized substance that acts as a barrier to protect the tooth, but acids from food and drink can damage it [1,2]. Dental caries is still one of the most prevalent infectious disorders in children and adolescents. There is widespread agreement that prevention is preferable to cure. This is also true in terms of caries prevention. For caries prevention, several techniques have been proposed. Fluoride therapy occupies a unique position in caries prevention [3]. Fluoride application was a proven way of caries prevention. Fluoride strengthens the enamel surface by forming fluorapatite crystals, which protect it from demineralization. Fluoride also possesses cariostatic properties and promotes enamel remineralization caries prevention can be ensured by using fluoride in conjunction with a strategy to enhance its absorption and uptake. A novel strategy recommended for this goal is laser therapy combined with the use of fluoride chemicals [4,5].
Another substance generated from milk's casein protein that has been recommended for caries prevention is Casein Phosphopeptide Amorphous Calcium Phosphate (CPP-ACP) [6]. Many wavelengths, including CO2, Nd:YAG, Argon, and Er: YAG, have been used to study the effects of laser irradiation with fluoride application, and have shown a considerable synergism in lowering enamel demineralization and enhancing fluoride retention Several studies have shown the benefits of laser irradiation for preventative dentistry, as well as some conflicting results for the Er, Cr: YSGG and CO2 lasers [7,8]. For dental prophylaxis, the erbium laser is often employed. It ablates the hard tooth structure while causing the least amount of stress to the pulp and surrounding structures. The Er, Cr: YSGG laser of 2780 nm, cavity preparation, caries removal, operations, endodontics, and other procedures are all common uses in dentistry. It is also commercially available, which provides a substantial benefit to practitioners. Because of its increased absorption by water and OH- contents of hydroxyapatite, this wavelength has also been examined for caries prevention as a result, at the ablation threshold, Er, Cr: YSGG laser irradiation increases surface temperatures of up to 800°C [9-12]. Higher absorption of laser energy in the enamel surface due to increased surface temperature during irradiation may produce changes in the crystallographic structure of enamel, depending on the fluence utilized.
Several theories have been proposed to explain how laser irradiation improves enamel hardness. The following mechanisms have been proposed:
- Decreasing enamel permeability by melting and recrystallizing enamel crystals;
- Decreasing enamel solubility by the formation of less soluble products such as tetracalcium diphosphate monoxide; and
- Reducing enamel solubility through minute structural changes, such as decreasing the enamel's water and carbonate content while increasing its hydroxyl ion content and pyrophosphate production [13,14].
Fluoride compounds combined with laser irradiation may result in the production of a stronger enamel structure while also minimizing the negative effects of laser irradiation. The physical, chemical, and kinetic changes that occur after laser irradiation appear to increase fluoride penetration depth and substantively in the enamel structure [15]. Previous research has looked into using fluoride and a laser at the same time to improve enamel resistance. However, the findings on this subject are debatable. Furthermore; a research, laser irradiation increased fluoride uptake by the tooth structure. The goal of this in vitro study was to estimated how Er.Cr:YSGG laser combined or not with fluoride, affected tooth enamel surface to acid resistance after artificial caries production [16].
Materials and Methods
Sample preparation
In this study, 30 maxillaries first premolars and third molars were taken from Iraqi patients aged 14 to 28 years old who were undergoing orthodontic treatment or remove impaction. All soft tissues were removed, and the teeth were polished with fluoride free, ultra-fine pumice and put the sample in ultrasonic cleaner. Each teeth sectioning mesiodistally in two parts. A reflected optical microscope was used to examine the teeth and look for any abnormalities on the surface (to be discarded). To avoid tooth drying, the teeth were dipped in 0.1 percent thymol as disinfectant, then immersed in distilled water and stored in the refrigerator at 4°C. Before laser irradiation, the teeth were coated with acid-resistant varnish, leaving two 4 × 4 mm broad windows on enamel surface buccally and lingually [17].
Study groups
The 60 samples obtained were randomly assigned to one of six groups (N=10), each with a different surface treatment.
- Group (G1): Control group, teeth did not receive any treatment.
- Group (G2): Teeth treated with acidulated phosphate fluoride 1.23% only.
- Group (G3): Teeth treated with Er.Cr: YSGG laser only at 0.25 W, 20 Hz, 1% water, 10% air.
- Group (G4): Teeth treated with Er.Cr: YSGG laser only at 0.5 w 20 Hz, 1% water, 10% air.
- Group (G5): Teeth treated with Er.Cr: YSGG laser at 0.25 W 20 Hz, 1% water, 10% air and acidulated phosphate fluoride 1.23%.
- Group (G6): Teeth treated with Er.Cr: YSGG laser at 0.5 W 20 Hz, 1% water, 10% air and acidulated phosphate fluoride 1.23%.
Laser irradiation
An Er, Cr: YSGG laser (Waterlase iPlus; Biolase, Irvine, CA, USA) was used to perform the laser irradiation. It is of 2780 nm, a pulse width of 140 µs, 20 Hz repetition rate, and 0.25 to 0.5 W of power. The energy is delivered via a fiberoptic system with a sapphire terminal with a diameter of 600 µm and a length of 6 mm (MZ6 tip), used with 10% air and 1% water spray. The laser hand piece was stabilized with the clamp during lasing while the tooth was attached to a CNC machine during laser irradiation. The tip of the fiber was positioned at a standard distance of 2 mm from the enamel surface, and laser irradiation was performed in selected areas of the enamel surface.
Surface treatment
Fluoride application: For group 2, acidulated phosphate fluoride 1.23%, which composed of (0.79% from sodium fluoride and 0.44% from hydrogen fluoride) in 0.1 molar phosphoric acid (Lincolnood, USA) was applied with a cotton bud for 4 minutes, and then the gel was removed using cotton rolls according to the manufacturer's recommendations.
Laser irradiation: The samples in group 3 were irradiated with a pulsed Er, Cr:YSGG laser the following parameters: 0.25 W of power, 20 Hz repetition frequency, pulse duration of 60 µs and 10 s exposure time (10 percent and 1% of air pressure and water level, respectively). Samples mounted on the CNC machine and the hand piece was also fixed 2 mm away from the enamel surface. By using the CNC, laser across the full selected surface area, the samples were irradiated. Group 4 is the same as group 3 but with a different power (0.5 w, 20 Hz, 10% air, 1% water).
Laser treatment and fluoride: For group 5, of laser irradiation at 0.25 watts, 20 Hz, 10% air, 1% water followed by a fluoride gel applied for 4 minutes according to manufacture instruction. For group 6, 10 seconds of laser irradiation at 0.5 W, 20 Hz, 10% air, 1% water followed by a fluoride gel was applied to each specimen for 4 minutes [18].
pH cycling
Each group's samples were exposed to a validated 7 days in vitro pH cycling technique. Every day, the samples were immersed in a demineralization solution (2.2 mM NaH2PO4, 2 mM CaCl2, 1 M KOH, 0.05 M CH3COOH pH: 4.5) for 6 hours and then in a remineralization solution (0.9 mM NaH2PO4, 1.5 mM CaCl2 and 0.15 M KCL, pH: 7.0) for the remaining 18 hours. The pH cycle was completed after 5 days, and then the samples were placed in remineralization solution for 2 days. The solutions were renewed every three days.
Micro hardness test
Vickers micro hardness tester was used to determine hardness (digital micro vikers hardness tester, TH 715 2008, China). It composed of a diamond indenter with a square base and a high-resolution optical microscope with a magnification of 400. A load of 1000 g (9.8 N) was applied for 15 seconds at three distinct locations on each sample during the measurement measuring at a baseline before and after laser shutting, also after demineralization and remineralization. The micro hardness value of each sample was determined by taking the average of three measurements.
Scanning Electronic Microscope (SEM): Sample of groups were tested under Field Emission Scanning Electron Microscope (FE-SEM) at a magnification of 3000 to analyse the morphology of the enamel surface of the control sample and after the teeth treated with laser only, fluoride only and combination [19].
Results
The mean micro hardness values for the six groups are shown in Table 1. The difference between baseline and post-ph-cycling challenge data was statistically significant for all groups (all P-values 0.05). In comparison to the other groups, Group 6 (Laser 0.5 fluoride) had the greatest surface micro hardness value (213.139 ± 20.877) after the ph-cycling challenge. After the ph-cycle, the micro hardness values in Groups Control, fluoride, laser 0.25, laser 0.5, and laser0.25+Fluoridedecreasedsignificantly(81.209+14.285,121.847+15.845,106.184+17.031, 110.156+13.242, and 149.350+17.795 respectively.
| Groups | Before | Surface treatment | After PH | Statistics | P value* | ES | |
|---|---|---|---|---|---|---|---|
| G1 control # | Min. | 303.4 | 58.53 | 54.969 | 0 | 17.383 | |
| Max. | 376.73 | 109.8 | |||||
| Mean | 341.367 | 81.209 | |||||
| ± SD | 21.92 | 14.285 | |||||
| G2^ | Min. | 203.9 | 303.7 | 107.1 | 137.45 | 0 | 0.862 |
| Max. | 383.8 | 411.1 | 150.7 | ||||
| Mean | 296.38 | 355.96 | 121.847 | ||||
| ± SD | 53.907 | 39.274 | 15.845 | ||||
| G3^ | Min. | 224.9 | 308.1 | 88.85 | 189.084 | 0 | 0.896 |
| Max. | 387.6 | 456.9 | 138 | ||||
| Mean | 322.04 | 381.79 | 106.184 | ||||
| ±SD | 47.456 | 48.296 | 17.031 | ||||
| G4^ | Min. | 234.2 | 291.9 | 91.63 | 152.701 | 0 | 0.874 |
| Max. | 356.4 | 398.3 | 135.6 | ||||
| Mean | 281.677 | 354.94 | 110.156 | ||||
| ±SD | 38.86 | 40.019 | 13.242 | ||||
| G5^ | Min. | 205.9 | 303 | 119.4 | 126.318 | 0 | 0.852 |
| Max. | 348.2 | 456.1 | 186 | ||||
| Mean | 295.336 | 369.79 | 149.35 | ||||
| ±SD | 42.129 | 49.002 | 17.795 | ||||
| G6^ | Min. | 271.5 | 357.1 | 173.2 | 141.517 | 0 | 0.865 |
| Max. | 390.9 | 496.7 | 241.1 | ||||
| Mean | 329.29 | 434.02 | 213.139 | ||||
| ±SD | 39.65 | 43.046 | 20.877 | ||||
| ANOVA (F) | 2.37 | 5.433 | 76.706 | ||||
| P value | 0.051 NS | 0.001 Sig. | 0.000 Sig. | ||||
| #=Paired T test, ^=Repeated measure ANOVA, NS=not significant at p>0.05*, Sig=Significant at p<0.05. | |||||||
Table 1: Descriptive and statistical test of micro hardness among groups and treatment.
However, when compared to baseline, both group Fluoride and group laser 0.25 w with Fluoride showed a significant increase in micro hardness values, demonstrating that demineralization was prevented to some extent. After therapy (post-treatment), there were statistically significant differences between groups (P<0.05). To compare differences in mean micro hardness values between groups, a Tukey's HSD analysis was used (Table 2). Control group had a significantly lower mean micro hardness than G2, G3, G4, G5, and G6 as displayed in Table 3.
| Treatment | (I) Groups | (J) Groups | Mean difference (I-J) | p value |
|---|---|---|---|---|
| After laser levene test=0.214, p value=0.930 NS | G3 | G6 | -52.23 | 0.079^ |
| G4 | 26.85 | 0.655^ | ||
| G5 | 12 | 0.973^ | ||
| G2 | 25.83 | 0.687^ | ||
| G6 | G4 | 79.08 | 0.002* | |
| G5 | 64.23 | 0.017s | ||
| G2 | 78.06 | 0.002* | ||
| G4 | G5 | -14.85 | 0.943^ | |
| G2 | -1.020- | 1.000^ | ||
| G5 | G2 | 13.83 | 0.955^ | |
| ^=not significant at p>0.05, *=significant at p<0.05. | ||||
Table 2: Pairwise comparisons of micro hardness between groups using Turkey HSD after laser.
| (I) Groups | (J) Groups | Mean difference (I-J) | P value |
|---|---|---|---|
| G1 control | G3 | -24.9748 | 0.018* |
| G6 | -131.9298 | 0.000* | |
| G4 | -28.9468 | 0.004* | |
| G5 | -68.1408 | 0.000* | |
| G2 | -40.6378 | 0.000* | |
| G3 | G6 | -106.955 | 0.000* |
| G4 | -3.972 | 0.995^ | |
| G5 | -43.166 | 0.000* | |
| G2 | -15.663 | 0.304^ | |
| G6 | G4 | 102.983 | 0.000* |
| G5 | 63.789 | 0.000* | |
| G2 | 91.292 | 0.000* | |
| G4 | G5 | -39.194 | 0.000* |
| G2 | -11.691 | 0.624^ | |
| G5 | G2 | 27.503 | 0.007* |
| ^=not significant at p>0.05, *=significant at p<0.05. Levene test=0.506, p value=0.770 NS | |||
Table 3: Multiple comparisons of micro hardness after PH between groups using Tukey HSD.
Group G5 had considerably greater mean microhardness than Groups fluoride (P 0.007). Finally, Group G6 had considerably higher mean microhardness than Groups laser 0.5, laser 0.25 with fluoride, and fluoride (P 0.000, P 0.000, and P 0.000, respectively).
Scanning electronic microscope
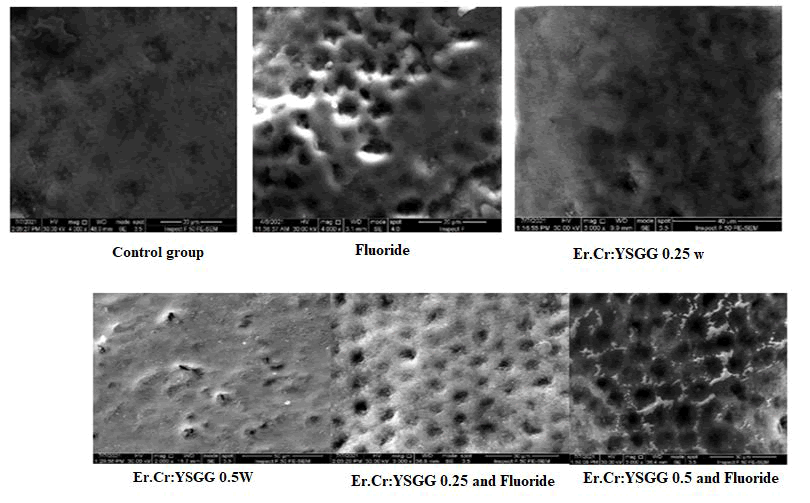
Immediately after surface treatment, typical micrographs of the control, Fluoride, Laser 0.25 w, Laser 0.5, Laser 0.25 with fluoride, and Laser 0.5 with fluoride groups are shown in Figure 1.
Figure 1: Scanning electron microscope images prepared from the sample surface of each study group at x 3000 magnifications.
Because of the melting caused by the laser irradiation, the enamel in the groups that were laser irradiated had an uneven and rough surface after treatment. The enamel crystals appear to have re-solidified after melting, resulting in larger crystallites and no cracks or carbonization on the enamel surface. Surfaces in the control and fluoride groups were more regular and smoother.
Discussion
Fluoride plays an important role in tooth structural resistance to demineralization. In developed countries, fluoride has proven to be an effective approach for reducing the prevalence and severity of dental caries [20]. One of the most cost-effective measures of caries reduction is fluoride, which is accessible in public water supplies at 0.7 mg/L. Fluoridation of drinking water was first introduced in the United States in 1940. It can also be found in a variety of oral hygiene products or applied by a professional. While little levels of fluoride are beneficial for caries prevention, large amounts cause fluorosis, which causes enamel discoloration and mottled enamel abnormalities [21]. The fluoride ion enters tooth structure and changes hydroxyl. The effect of several lasers on caries prevention has been explored. Each laser uses a different method, as well as different laser settings and irradiation parameters, to strengthen the resistance of tooth structure to caries.
The 2780 nm wavelength of the erbium, chromium: Yttrium-scandiuiu-gallina-garnet laser is considered as a helpful laser for ablating enamel and dentin without damaging the tooth pulp. This laser's ablation potential is determined by its high absorption by water and hydroxyapatite hydroxyl groups [22,23]. “This type of laser should be utilized with a lower energy density than that used for ablation to achieve caries prevention effects. Only chemical changes would occur as a result of the increased temperature of the enamel surface discovered that while an energy density of 8.5 J/cm can improve enamel acid resistance, smaller fluencies can also be used as fluoride dentifrice alternatives." In this study, water level in the experimental groups was 0% to enhance laser effect, this agree with the suggestion of Meister et al. external water of the enamel and dentine do not play a significant role in ablation. As a result, water cooling should not be employed during irradiation in order to get caries prevention effaces from this type of laser. Using a low-energy laser with air cooling, enamel caries resistance can be achieved without increasing pulpal temperature” [24].
With laser irradiation, the advantages of fluoride on reducing tooth demineralization have been shown to improve. However, no consensus has been reached in the dental literature on the efficacy of combining laser irradiation and fluoride administration in increasing enamel hardness. The use of an Er,Cr: YSGG laser, either combined with fluoride or not, was evaluated against enamel demineralization advancement in a prior study conducted by our research group (unpublished data). The combination of fluoride with this laser with parameter (Er,Cr: YSGG laser 0.5 W, 5.7 J/cm2) produced promising results. In this study, it was noticed that considerable reduction in enamel surface loss, which prompted us to investigate the anti-erosive properties of this laser in enamel.
According to the readings, the baseline measurement of the micro hardness of the groups in the baseline values were similar, and that any differences in the following time period could only be related to laser irradiation. The optimal parameter for laser irradiation should increase enamel hardness while without altering surface roughness in the best case scenario. According to the findings, the surface micro hardness of the enamel increased after a single application of fluoride or a combination of laser irradiation and fluoride application, with mean micro hardness differences (P<0.000 and P<0.000, respectively) compared to the control group. These two surface treatment procedures were the only ones that could stop enamel demineralization from progressing. The finding that fluoride increased the micro hardness of demineralized enamel is in line with previous research findings. However, research has shown that combining Er,Cr: YSGG laser irradiation with fluoride application reduces enamel demineralization more effectively than either laser or fluoride alone.
This could be explained by the chemical and morphological changes induced by laser irradiation on the surface. Chemical reaction takes place when carbonated apatite is extracted, whereas morphological changes take place as the surface temperature rises. These changes occur after the application of fluoride gel, enhancing fluoride uptake at the tooth's surface and improving enamel protection. Ana et al. discovered that prior to fluoride application, exposing the tooth to Er,Cr: YSGG laser treatment increased the formation of calcium fluoride-like material on the enamel surface, which could explain why this combination treatment was able to control the progression of enamel demineralization in the current study. Found that groups treated with fluoride alone or a combination of fluoride and irradiation with the same type of laser used in this study had higher calcium concentration levels, and these two groups had lower dental enamel solubility than laser irradiation alone, so i agree. Fluoride application preceding laser irradiation, on the other side, did not prevent demineralization in this study; in fact, it lowered the micro hardness of the enamel surface. According to a prior study, when fluoride was given to enamel before laser irradiation, there was no significant increase in the uptake of calcium fluoride-like substance. This could be due to fluoride's mechanical barrier, which lowers the warmth and energy sent to the enamel surface, preventing any alterations [25].
Although laser groups without fluoride showed no highly meaningful control over the advancement of enamel demineralization when examined alone but still better than control, the combination of Er,Cr: YSGG laser irradiation with fluoride had a protective effect, greatly lowering surface loss when compared to the control group. The combination of laser irradiation with fluoride treatment demonstrated that this combination can result in even less enamel demineralization and increased fluoride retention on the tooth surface. This finding is in agreement with the results of other studies, Single irradiation with the kind of laser used here was found to be not highly effective in avoiding enamel demineralization. On the other hand, presented contradictory results, claiming that irradiation with this type of laser alone might maintain enamel micro hardness after pH challenge. Differences in laser settings, irradiation techniques, and irradiating a degraded enamel surface rather than an intact surface could explain the disparate outcomes.
The APF gel-treated group did not exhibit any ability to inhibit the advancement of enamel demineralization in this investigation. This could be due to the fact that the gel was only administered once before cycling, resulting in a lesser frequency of application. Despite the fact that highly concentrated and acidic fluoridated formulations, such as APF gel, can predispose to the formation of more CaF2-like deposits on tooth substrates, a study suggests that its protection may be short-lived in highly acidic environments, necessitating frequent application of the agent. We employed a commonly used demineralization-remineralization cycle approach to reproduce the conditions typically seen in clinical instances, where those who drink extremely acidic beverages have a higher risk of enamel breakdown. A comprehensive simulation of clinical enamel demineralization is impossible owing to variation in biological parameters such as saliva flow rate, content, and buffering capacity. These variables are essential in the remineralization of tooth structure and should be taken into account in future studies. The enamel specimens were smoothed and polished to give a flat base and allow uniform micro hardness measurements, and the specimen preparation may have influenced the in vitro results and conclusions. As a result, these in vitro studies have limited clinical implications.
Conclusion
Lasers can enhance micro hardness of the enamel and boosting acid resistance and preventing cavities from developing and progressing. Furthermore, the most promising treatment for caries prevention appears to be a combination of laser and fluoride. Further research is needed to see if these changes are maintained over time with regular monitoring of a patient's dental health.
References
- Ana PA, Bachmann L, Zezell DM. Lasers effects on enamel for caries prevention. Laser Phys 2006; 16:865-875.
- Apel C, Birker L, Meister J, et al. The caries-preventive potential of subablative Er: YAG and Er: YSGG laser radiation in an intraoral model: A pilot study. Photomed Laser Ther 2004; 22:312-317.
- Apel C, Meister J, Schmitt N, et al. Calcium solubility of dental enamel following subâ?ablative Er: YAG and Er: YSGG laser irradiation in vitro. Laser Surg Med 2002; 30:337-341.
- Clark MB, Slayton RL, Segura A, et al. Fluoride use in caries prevention in the primary care setting. Pediatrics 2014; 134:626-633.
- da Silva VR, Viana ÃE, Lopes RM, et al. Effect of Er, Cr: YSGG laser associated with fluoride on the control of enamel erosion progression. Arch Oral Biol 2019; 99:156-160.
- de Freitas PM, Rapozo-Hilo M, Eduardo CD, et al. In vitro evaluation of erbium, chromium: Yttrium-scandium-gallium-garnet laser-treated enamel demineralization. Lasers Med Sci 2010; 25:165-170.
- de Oliveira RM, de Souza VM, Esteves CM, et al. Er, Cr: YSGG laser energy delivery: Pulse and power effects on enamel surface and erosive resistance. Photomed Laser Surg 2017; 35:639-646.
- Dionysopoulos D, Tolidis K, Strakas D, et al. Evaluation of a clinical preventive treatment using Er, Cr: YSGG (2780 nm) laser on the susceptibility of enamel to erosive challenge. Lasers Med Sci 2019; 34:1089-1097.
- Eversole LR, Rizoiu I. Pulpal response to cavity preparation by an erbium, chromium: YSGG laser-powered hydrokinetic system. J Am Dent Assoc 1997; 128:1099-1106.
- Fekrazad R, Ebrahimpour L. Evaluation of acquired acid resistance of enamel surrounding orthodontic brackets irradiated by laser and fluoride application. Lasers Med Sci 2014; 29:1793-1798.
- Fried D, Featherstone JD, Visuri SR, et al. Caries inhibition potential of Er: YAG and Er: YSGG laser radiation. Lasers Dent II 1996; 2672:73-78.
- Ganss C, Lussi A, Schlueter N. The histological features and physical properties of eroded dental hard tissues. Erosive Tooth wear 2014; 25:99-107.
- Geraldo-Martins VR, Lepri CP, Faraoni-Romano JJ, et al. The combined use of Er, Cr: YSGG laser and fluoride to prevent root dentin demineralization. J Appl Oral Sci 2014; 22:459-464.
- Ghelejkhani A, Nadalizadeh S, Rajabi M. Effect of casein-phosphopeptide amorphous calcium phosphate and fluoride with/without erbium, chromium-doped yttrium, scandium, gallium, and garnet laser irradiation on enamel micro hardness of permanent teeth. Dent Res J 2021; 18.
- Hossain M, Kimura Y, Nakamura Y, et al. A study on acquired acid resistance of enamel and dentin irradiated by Er, Cr: YSGG laser. J Clin Laser Med Surg 2001; 19:159-163.
- Hossain M, Nakamura Y, Kimura Y, et al. Caries-preventive effect of Er: YAG laser irradiation with or without water mist. J Clin Laser Med Surg 2000; 18:61-65.
- Huysmans MC, Young A, Ganss C. The role of fluoride in erosion therapy. Erosive Tooth Wear 2014; 25:230-243.
- Karlinsey RL, Mackey AC, Blanken DD, et al. Remineralization of eroded enamel lesions by simulated saliva in vitro. Open Dent J 2012; 6:170.
- Meister J, Franzen R, Forner K, et al. Influence of the water content in dental enamel and dentin on ablation with erbium YAG and erbium YSGG lasers. J Biomed Optics 2006; 11:34030.
- Pereira LG, Joao-Souza SH, Bezerra SJ, et al. Nd: YAG laser irradiation associated with fluoridated gels containing photo absorbers in the prevention of enamel erosion. Lasers Med Sci 2017; 32:1453-1459.
- Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J Dent 2007; 35:695-698.
- Saxegaard E, Rolla G. Fluoride acquisition on and in human enamel during topical application in vitro. Euro J Oral Sci 1988; 96:523-535.
- Seka WD, Featherstone JD, Fried D, et al. Laser ablation of dental hard tissue: From explosive ablation to plasma-mediated ablation. Lasers Dent II 1996; 2672:144-158.
- Ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res 1982; 16:201-210.
- Tuloglu N, Bayrak S, Tunc ES, et al. Effect of fluoride varnish with added casein phosphopeptide-amorphous calcium phosphate on the acid resistance of the primary enamel. BMC Oral Health 2016; 16:1-7.
Author Info
Ahmed Adnan Hadi* and Basima Mohammed Ali
Department of Physics, Institute of Laser for Postgraduate Studies, University of Baghdad, IraqCitation: Ahmed Adnan Hadi, Basima Mohammed Ali, Role of Er.Cr: YSGG Laser and Fluoride in Caries Resistance, J Res Med Dent Sci, 2022, 10 (7): 000-000.
Received: 23-May-2022, Manuscript No. JRMDS-22-47129; , Pre QC No. JRMDS-22-47129; Editor assigned: 26-May-2022, Pre QC No. JRMDS-22-47129; Reviewed: 07-Jun-2022, QC No. JRMDS-22-47129; Revised: 25-Jul-2022, Manuscript No. JRMDS-22-47129; Published: 02-Aug-2022