Research - (2021) Volume 9, Issue 6
Role of Uric Acid as a Risk Factor in Metabolic Syndrome
K Gunanithi and AJ Manjula Devi*
*Correspondence: AJ Manjula Devi, Department of Biochemistry, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, India, Email:
Abstract
To evaluate the role of serum uric acid as a risk factor in diabetic metabolic syndrome. determine the level of serum uric acid in patients with diabetic metabolic syndrome. To compare the level of serum unc acid between patients with diabetic metabolic syndrome and normal healthy subjects and to find their significance of association and correlate the association of serum unc acid with various laboratory components of metabolic syndrome like fasting plasma glucose, post prandial plasma glucose, fasting serum triglycerides, fasting serum total cholesterol, fasting serum LDL cholesterol, fasting serum HDL cholesterol and fasting serum VLDL cholesterol.
Keywords
Diabetes mellitus, Uric acid, Glucose amd triglycerides
Introduction
Uric acid is a final end product in the degradation of purine metabolism Hyperuricemia results from increased production of uric acid, decreased excretion or a combination of both [1-3]. Hyperuricemia has been identified as having major clinical significance in the development of various co morbidities including gout, metabolic syndrome, coronary artery disease and type 2 diabetes mellitus [4-6], despite its major antioxidant property. Serum uric acid level is associated with the individual components of metabolic syndrome such as obesity, dyslipidemia and hypertension [7]. Hyperuricemia can be an accompaniment disorder with syndrome X (characterized by abdominal obesity, impaired glucose tolerance, increased LDL Cholesterol & decreased HDL Cholesterol). Its presence is an indication for screening and aggressively treating any accompanying obesity, hypercholesterolemia, diabetes or hypertension [8]. Diabetes mellitus type 2 is a common condition among middle age and older individuals. The Prevalence of diabetes mellitus type 2 is increasing globally. India have the max imum increase during the last few years. The prevalence of type 2 diabetes mellitus is 2.4% in rural population and 11.6% in urban population [9]. The metabolic syndrome is a cluster of most dangerous coronary artery disease risk factors: diabetes mellitus type 2, pre diabetes, abdominal obesity, high cholesterol and high blood pressure [10].
People with metabolic syndrome are twice as likely to die from, and three times as likely to have a heart attack or stroke compared with people without the syndrome. Cardiovascular disease is the leading cause of death throughout the world. It accounts for about 30% of death worldwide, including 40% in high income countries, and about 28% in low and middle income countries [11]. Both hyperuricemia and metabolic syndrome are associated with hyperinsulinemia. Hyper uncemia In diabetes mellitus type 2 though prevalent among those with metabolic syndrome, it is not within the diagnostic criteria for diabetic metabolic syndrome. Little is known about the prevalence of hyper uricemia among our population, which is prone for metabolic syndrome, particularly among middle aged and older populations in our society, where compliance and the glycemic control are poor. Serum uric acid a cost effective laboratory assessment could not only serve as an adjunctive, but could also become a criterion to be included 1n diagnosing metabolic syndrome.
This study was taken up in our population , of middle aged and older individuals, to investigate the association between serum uric acid levels and diabetic metabolic syndrome and to find the correlation between serum uric acid levels and various components of metabolic syndrome and thereby to study the role of serum uric acid as a risk factor in metabolic syndrome.
Materials and Methods
This study was conducted in the Department of Biochemistry, Sree Balaji Medical College and Hos pital , Chromepet, Chennai during the period of January 2012 - June 2012 among outpatients and healthy volunteers visiting the outpatient services of the Department of Medicine, Sree Balaji Medical College and Hospital , Chromepet , Chennai . The ground work for the study was started after getting clearance from the research committee and the Institutional human ethical committee (reference number for approval: 03/ IEC/ 20 11- 16 ) of Sree Balaji Medical College and Hospital, Chromepet, Chennai.
Inclusion criteria
Type 2 diabetes mellitus patients of both sexes (40-60 years of age) with metabolic syndrome and normal healthy controls of both sexes (40 - 60 years of age).
Exclusion criteria
Patients with chronic kidney disease and history of cancer and patients on treatment with systemic steroids. The sample size of this study includes 100 subjects involving 50 diabetic patients with metabolic syndrome and 50 normal healthy controls. The study parameters includes measurement of Height, Weight, Blood pressure and Waist circumference. The laboratory parameters includes Fasting plasma glucose, Post prandial plasma glucose, Serum fasting lipid profile, Serum urea, Serum creatinine, Serum uric acid levels, Electrocardiogram - 12 lead.
Sample collection
The study was explained to the participants and informed consent obtained from them before taking the blood sample. The blood samples were collected from subjects by venepuncture. Both fasting (8 hours overnight fasting) and post prandial samples were collected under aseptic precautions.
Data analysis
The statistical analysis was done with the help of epidemiologist / statistician and it includes the Pearsons correlation coefficient as the test of correlation and the Independent sample t test as the test of significance. The statistical software used for statistical interpretation was from SPSS data editor version 17.
Results
Serum uric acid levels among patients with metabolic syndrome (8.02+1.82 (mg%) were significantly higher than I normal controls ( 4.4 7+ 1.50 (mg%) with level of significance (t value= 10.612) (p < 0.05 ). Fasting plasma glucose levels among patients with metabolic syndrome (153.14+32.66(mg%) were significantly higher than normal controls ( 93.04+13.96(mg%)) with level of significance (t value = 11.963 ) (p < 0.05 ). Post prandial plasma glucose levels among patients with metabolic syndrome (240.96+54.49(mg%) were significantly higher than normal controls ( 123.90+23.45(mg%) with level of significance (t value = 13.951 ) (p < 0.05) . Fasting Serum total cholesterol levels among patients with metabolic syndrome (222.92+27.45(mg%) were significantly higher than normal controls ( 165.02+18.57(mg%)) with level of significance ( t value= 12.351) (p < 0.05).
Fasting Serum triglyceride levels among patients with metabolic syndrome (189.08+69.33(mg%) were significan tly higher than normal controls ( 130.78+28.58(mg%) with level of significance (t value = 5.497) ( p < 0.05 ).
Fasting Serum LDL cholesterol levels among patients with metabolic syndrome (138.70+23.56 (mg%) were significantly higher than normal controls (94.92+ 16.60 (mg%) with level of significance (t value=l0.739) (p <0.05) Fasting Serum VLDL cholesterol levels among patients with metabolic syndrome (37.92+13.82(mg%) were significantly higher than normal controls (26.08+5.70(mg%) with level of significance (t value = 5.597) (p<0.05).
But Fasting serum HDL cholesterol levels among patients with metabolic syndrome (46.32+5. 77(mg%) were significantly lower than normal controls ( 44.22+5.97 (mg%) with insignificant association (t value= 1.787) (p >0.05).
Serum unc acid levels were significantly higher in patients with diabetic metabolic syndrome when compared with normal healthy controls with level of significance (t value = 10.612) p = 0.000, (p < 0.05).
Serum uric acid levels showed positive correlation with various components of the metabolic syndrome including fasting plasma glucose, post prandial plasma glucose, serum total cholesterol, serum LDL cholesterol, serum triglycerides (pearsons correlation significant at p<0.05) except for serum HDL cholesterol (Tables 1 to Table 10) and Figures 1 and Figure 2.
| N | Minimum (mg%) | Maximum (mg%) | Mean (mg%) | SD | |
|---|---|---|---|---|---|
| FPG | 50 | 116 | 255 | 153 .14 | 32.664 |
Table 1: Comparison-Fasting plasma glucose. FPG-Fasting Plasma Glucose for cases (patients with diabetic metabolic syndrome): Group I.
| N | Minimum (mg%) | Maximum (mg%) | Mean (mg%) | SD | |
|---|---|---|---|---|---|
| FPG | 50 | 68 | 125 | 93.04 | 13.96 |
Table 2: Comparison-Fasting plasma glucose. PPG-fasting Plasma Glucose for controls (healthy volunteers): Group II.
| N | Minimum (mg %) | Maximum (mg%) | Mean (mg %) | SD | |
|---|---|---|---|---|---|
| CHOL | 50 | 167 | 280 | 222.92 | 27.456 |
Table 3: Comparison: Fasting serum total cholesterol. CHOL-Fasting serum total cholesterol for cases (patients with diabetic metabolic syndrome): Group I.
| N | Minimum (mg %) | Maximum (mg %) | Mean (mg%) | SD | |
|---|---|---|---|---|---|
| CHOL | 50 | 126 | 217 | 165.02 | 18.575 |
Table 4: Comparison: Fasting serum total cholesterol. CHOL-Fasting serum total cholesterol for controls: Group II.
| N | Minimum (mg¾) | Maximum (mg¾) | Mean (mg¾) | SD | |
|---|---|---|---|---|---|
| HDL | 50 | 37 | 58 | 46.32 | 5.773 |
Table 5: Comparison: Fasting serum HDL cholesterol. HDL-fasting serum HDL cholesterol for cases (patients with diabetic metabolic syndrome): Group I.
| N | Minimum (mg¾) | Maximum (mg¾) | Mean (mg¾) | SD | |
|---|---|---|---|---|---|
| HDL | 50 | 26 | 52 | 44.22 | 5.97 |
Table 6: Comparison: Fasting serum HDL cholesterol. HDL-Fasting serum HDL cholesterol for controls (healthy volunteers): Group II.
| N | Minimum (mg%) | Maximum (mg%) | Mean (mg%) | SD | |
|---|---|---|---|---|---|
| VLDL | 50 | 22 | 69 | 37.92 | 13.82 |
Table 7: Comparison-Fasting serum VLDL cholesterol. VLDL-Serum fasting VLDL cholesterol for cases (patients with diabetic metabolic syndrome): Group I.
| N | Minimum (mg%) | Maximum (mg%) | Mean (mg%) | SD | |
|---|---|---|---|---|---|
| VLDL | 50 | 16 | 43 | 26.08 | 5.7 |
Table 8: Comparison-Fasting serum VLDL cholesterol. VLDL-Fasting serum VLDL cholesterol for controls (healthy volunteers): Group II.
| N | Minimum (mg ¾) | Maximum (mg ¾) | Mean (mg¾) | SD | |
|---|---|---|---|---|---|
| UA | 50 | 4.8 | 14.31 | 8.0212 | 1.82578 |
Table 9: Comparison-Serum uric acid. UA-Serum uric acid for cases (patients with diabetic metabolic syndrome): Group I.
| N | Minimum (mg ¾) | Maximum (mg¾) | Mean (mg¾) | SD | |
|---|---|---|---|---|---|
| UA | 50 | 1.72 | 8.08 | 4.4708 | 1.50439 |
Table 10: Comparison-Serum uric acid. DA-serum uric acid for controls (healthy vo lu ntee rs): Group II.
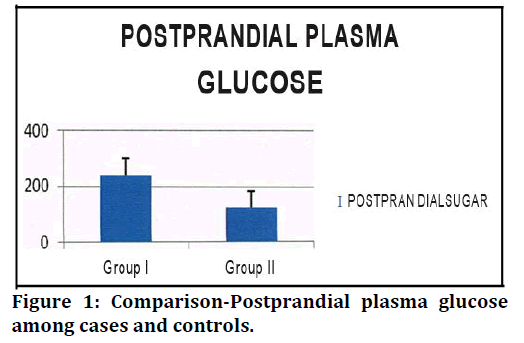
Figure 1. Comparison-Postprandial plasma glucose among cases and controls.
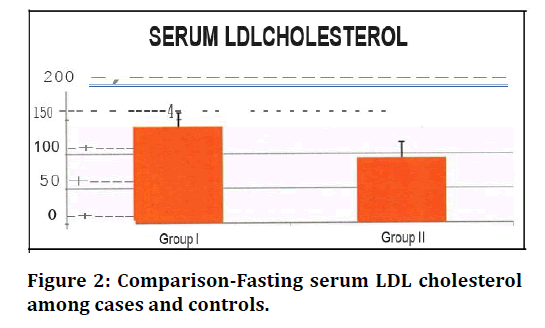
Figure 2. Comparison-Fasting serum LDL cholesterol among cases and controls.
Discussion
Meta-analysis has recently shown that uric acid elevation is related to an increase in myocardial infarction and mortality events and epidemiological studies showed that uric acid 1s an independent risk factor for cardiovascular diseases particularly in hypertensive and diabetic individuals. It is also speculated that uric acid is one of the determinants of the metabolic syndrome . Individuals with high uric acid levels have an odds ratio of 1.6-fold higher for developing metabolic syndrome. Uric acid is associated with metabolic [12]. Observed negative correlation between HDL-c and uric acid. The likely mechanism is the relationship between decreased HDL-C and insulin resistance [13-15]. In the adipose tissue, there 1s adipokine production, including that of leptin. One possible explanation for the association between higher waist circumference and hyperuricemia were [16,17]. Which studies found that uric acid serum concentrations are independently related to leptin concentration, thus suggesting that would be a pathogenic factor responsible for uric acid increase in obese patients.
A recent study showed an inverse relation between muscle mass and uric acid in healthy individuals older than 40 years. Chronic elevation of uric acid concentrations would be a causal factor for sarcopenia, especially through increased inflammation and oxidative stress [13]. The activation of the xanthine oxidase metabolic pathway, which increases uric acid production and the superoxide radical 132 could elevate the reactive oxygen species (ROS) and it could be the main mechanism for the reduction of muscle mass. Furthermore, uric acid exerts a pro-inflammatory effect, thus stimulating the production of interleukin- I, interleukin-6 the tumor necrosis factor which also can influence the muscle mass [18].
Several factors are associated as cause and consequences of high uric acid concentration. Higher waist circumference and body mass index are associated with higher insulin resistance and leptin production, and both reduce renal unc acid excretion, thus increasing its concentration. HDL-cholesterol concentration is negatively associated to insulin resistance, what can influence its negative correlation to uric acid. Obese individuals usually have Metabolic Syndrome diagnostic, which can also increase uric acid serum concentrations due to synthesis increase (triglycerides-TO concentration) and lower excretion [19-21].
Additionally, obesity and muscle mass reduction are associated with low-intensity chronic inflammation, and uric acid levels can increase in order to protect the organism against the moderate oxidative stress resulting from this situation. Low muscle mass (sarcopenia) is negatively associated with uric acid, However, it has not yet been clarified what the cause or effect is. Probably, oxidative stress produced by excessive uric acid can influence muscle mass reduction. The role played by diet on hyperuricemia has not yet been fully clarified, but high intake of fructose-rich industrialized food and high alcohol intake (particularly beer) seem to influence uricemia. The main mechanism of excretion of urate concentrations occurs by means of renal excretion; hence, glomerular function markers (urea and creatinine) are positively associated with uric acid [22].
Diversion of glycolytic intermediates toward R -5-P , PRPP, and uric acid will follow if there is diminished activity of GA3PDH (glyceraldehyde-3-phosphate dehydrogenase), which is regulated by insulin. Serum triglyceride concentrations may also increase, as might be expected from accumulation of glycerol-3-phosphate. Thus , intrinsic defects 1n GA3PDH and a loss of its responsiveness to insulin, by causing accumulation of glycolytic intermediates, may explain the association between insulin resistance, hyperuricemia, and hyper triglyceridemia [23,24].
The importance of hyperuricemia and the clustering phenomenon of the metabolic syndrome were first described [25]. when he described the clustering of three clinical syndromes: hypertension, hyperglycemia, and hyperuricemia. In 1988, Reaven GM described the important central role of insulin resistance in the seminal Banting lecture where he described Syndrome X, which has now become known as the metabolic syndrome and/or the insulin resistance syndrome. Seven decades after the clustering phenomenon was reported [26]. suggested that hyperuricemia be added to the cluster of metabolic and hemodynamic abnormalities associated with insulin resistance and/ or hyperinsulinemia of Syndrome. The four major players in the metabolic syndrome are hyperinsulinemia, hypertension, hyper lipidemia, and hyperglycemia. Each member of this deadly quartet has been demonstrated to be an independent risk factor for coronary heart disease and capable of working together in a synergistic manner to accelerate both non diabetic atherosclerosis and the atheroscleropathy [27].
In a like manner, hyperuricemia, hyperhomocysteinemia, reactive oxygen species, and highly sensitive C- reactive protein (hsCRP) each play an important role in expanding the original Syndrome X described by Reaven in the atherosclerotic process. The above quartet does not stand alone but interacts in a synergistic manner resulting in the progression of accelerated atherosclerosis and arterial vessel wall remodeling. The metabolic syndrome of clinical clustering has been renamed multiple times over the past 16 years indicating its central importance to cardiovascular disease and was included in the recent National Cholesterol Educational Program - Adult Treatment Panel III (NCEP ATP III) clinical guidelines in order to assist the clinician in using this important tool to evaluate additional cardiovascular risk [28].
The effect of hyperuricemia might be partially responsible for the low-grade inflammation and insulin resistance in the adipose tissue and for increased risk of cardiovascular disease induced by obesity. Glucotoxicity places an additional burden of redox stress on the arterial vessel wall and capillary endothelium. Hyperglycemia induces both an oxidative stress (glucose autoxidation and advanced glycosylation endproducts (AGE)-reactive oxygen species- oxidation products) and a reductive stress through pseudohypoxia with the accumulation of NADH and NADH in the vascular intima [29]. This redox stress consumes the natural occurring local antioxidants such as: superoxide dismutase, glutathione peroxidase, and catalase. Once these local intimal antioxidants are depleted uric acid can undergo the paradoxical antioxidant-prooxidant switch [30]. Uric acid, early on in the atherosclerotic process 1n physiologic ranges as antioxidant. But acts as pro oxidant 1n an elevated range. With the loss of supporting antioxidants above and in a milieu of oxidative-redox stress within the atherosclerotic intima. In metabolic syndrome, type 2 diabetes mellitus and advanced vulnerable atherosclerotic plaques superoxide dismutase, glutathione peroxidase, and catalase are depleted. Antioxidants may become prooxidants 1n certain situations. Serum unc acid in the early stages of the atherosclerotic process is known to act as an antioxidant and may be one of the strongest determinates of plasma antioxidative capacity. However, later 1n the atherosclerotic process when serum unc acid levels are known to be elevated (in the upper 1/3 of the normal range >4mg/dl and outside of the normal range >6mg/dl in females and 6.5-7mg/dl 1n males) this previously antioxidant (serum unc acid) paradoxically becomes prooxidant. The antioxidant - prooxidant urate redox shuttle is an important concept to understand regarding accelerated atherosclerosis. This shuttle is important in understanding the role of how the antioxidant uric acid becomes prooxidant in this environmental milieu, which results in its damaging role to the endothelium and arterial vessel wall remodelling with an elevated tension of oxidative - redox stress (ROS), accelerated atherosclerosis and arterial vessel wall remodelling [31-33].
Conclusion
This study has shown that hyperuricemia 1s significantly present in type 2 diabetic patients with metabolic syndrome and is significantly correlated with vanous components of metabolic syndrome including obesity, dys lip id emia , hypertension and insulin res istance. All these components of metabolic syndrome have a bidirectional causal effect with hyperuricemia. The relationship between hyperuricemia and metabolic syndrome might be linked to insulin resistance and thereby with increasing body mass index. The prooxidant and pro-inflammatory effect of hyperuricemia could also explain the clustering of factors found in metabolic syndrome. Hence, Serum unc acid, a cost effective laboratory assessment could not only serve as an adjunctive, but could also become a criterion to be included in diagnosing metabolic syndrome.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The encouragement and support from Bharath University, Chennai, is gratefully acknowledged. For provided the laboratory facilities to carry out the research work.
References
- Puig JG, Martínez MA, Mora M, et al. Serum urate, metabolic syndrome, and cardiovascular risk factors. A population-based study. Nucleosides Nucleotides Nucleic Acids 2008; 27:620-623.
- Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Current Opinion Rheumatol 2013; 25:210-216.
- Rho YH, Choi SJ, Lee YH, et al. The prevalence of metabolic syndrome In patients with gout: A multicenter study. J Korean Med Sci 2005; 20:1029-1033.
- Mellen PB, Bleyer AJ, Erling er TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension 2006; 48:1037-1042.
- Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med 2012; 125:679- 687.
- Kim TH, Lee SS, Yoo JH, et al. The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetol Metabol Syndrome 2012; 4:1-7.
- Robert I. Disorders of purine and primidine metabolism. In: Horrison,s Principles of internal medicine. Eds, Kasper and Braunwald. 15st. Mc Graw hill, New York, 2001; 2268-71
- Nebulas I, Yuko I, Toda EI, et al. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese Individuals. Arterioscler Thromb Vase Biol 2005; 25:1038-1044.
- Ramachandran A. Epidemiology of type II diabetes mellitus in India. Diabetes research centre & mv hospital for diabetes, Chennai. J Indian Med Assoc 2002; 100:425-427.
- Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. The metabolic syndrome new worldwide definition. Lancet 2005; 366:1059-62
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus: present and future perspectives. Nature Reviews Endocrinol 2011.
- www2.niddk.nih.gov
- https://diabetesatlas.org/en/
- https://www.cdc.gov/mmwr/pdf/wk/mm5345.pdf
- Barlow SE. Expert committee recommendations regarding the prevention, assessment and treatment of childhood and adolescent overweight and obesity: Summary report. Paediatrics 2007; 120:S164-S192.
- Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities 1n adults. JAMA 2008; 300:1303-1310.
- Rother KI. Diabetes treatment-bridging the divide. N Engl J Med 2007; 356:1499-1501.
- Fujioka K. Pathophysiology of type 2 diabetes and the role of incretin hormones and beta-cell dysfunction. JAAPA 2007; 3-8.
- Garcia-Roves PM. Mitochondrial pathophysiology and type 2 diabetes mellitus. Arch Physiol Biochem 2011; l17:177-187.
- Kim SY, Guevara JP, Kim KM, et al. Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Rheum 2009; 61:885-892.
- Nakagawa T, Tuttle KR, Short RA, et al. Hypothesis: Fructose-induced 9yperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 2005; :80-86.
- Takahashi MM, de Oliveira EP, de Carvalho AL, et al. Metabolic Syndrome and dietary components are associated with coronary artery disease risk score in free-living adults: A cross-sectional study. Diabetol Metab Syndr 2011; 3:7
- Roch-Ramel F, Guisan B. Renal transport of urate in humans. News Physiol Sci 1999; 14:80-84.
- Maxwell SR, Thomason H, Sandler D, et al. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1997; 27:484-490.
- Farquharson CA, Butler R, Hill A, et al. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 2002; 106:221-226.
- Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension 2003; 42:24 7-252.
- Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radie Biol Med 2006; 41:1727-1746.
- Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004, 350:1093- 1103.
- Ghadirian P, Shatenstein B, Verdy M, et al. The influence of dairy products on plasma uric acid in women. Eur J Epidemiol 1995; 11:275-281.
- Arion WJ, Canfield WK, Ramos FC, et al. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys 1997; 339:315-322.
- Wu T, Giovannucci E, Pischon T, et al. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women. Am J Clin Nutr 2004; 80:1043-1049.
- Pierine DT, Nicola M, Oliveira ÉP. Sarcopenia: Alterações metabólicas e conseqüências no envelhecimento. Revista brasileira de Ciência e Movimento 2009; 17:96-103.
- Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: Role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 2005; 288:R337-R344.
Author Info
K Gunanithi and AJ Manjula Devi*
Department of Biochemistry, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaCitation: K Gunanithi, AJ Manjula Devi, Role of Uric Acid as a Risk Factor in Metabolic Syndrome, J Res Med Dent Sci, 2021, 9(6): 174-179
Received: 08-May-2021 Accepted: 17-Jun-2021
