Research - (2022) Volume 10, Issue 7
Side Effects of COVID-19 Vaccines among Health Care Workers in Primary Health Care Centers in Jeddah 2021â2022
Lamies Mahmoud Omran* and Mohammed Sami Kutbi
*Correspondence: Lamies Mahmoud Omran, Department of Preventive Medicine, Ministry of National Guard, Health affairs, Western region, Jeddah, South Africa, Email:
Abstract
Background: A number of aspects of people's lives were affected by the COVID-19 pandemic, including their physical, social, emotional, and behavioral well-being. The WHO reported COVID‐19 as a pandemic in March 2020. Rapid herd immunity by vaccination is required to block mutation and prevent the emergence of variants. In December 2020, Saudi Arabia started its COVID-19 vaccination program to stop the spread of COVID-19. As healthcare workers are on the frontline of the COVID-19 pandemic, they require adequate protection to prevent onward transmission and to decrease the burden on the healthcare system. This study aimed to investigate the short-term side effects of Covid-19 vaccines and the association with sociodemographic factors among health care workers in Primary Health Care Centers in Jeddah 2021–2022. Subject and methods: A cross-sectional study was conducted among health care workers in Primary Health Care Centers of the Ministry of Health (MOH) in Jeddah. Using an online questionnaire. Multi-stage sampling techniques were applied. The first stage was to select the included PHCs, then a sampling of health care staff category in the PHC. Results: The type of vaccination received by healthcare professionals included in this study varied with Pfizer vaccine being most common (n=191, 43%). Study participants who received two doses reported several side effects (N=45). The most common side effect among them was injection site pain (n=14, 31.1%). Symptoms after second dose were more severe among participants who received Pfizer vaccination (P=0.001). Conclusion: Among study participants, the most prevalent adverse effects of the Pfizer–BioNTech COVID-19 vaccine were injection site discomfort, weariness, headache, muscle soreness, chills, and joint pain. In terms of their relationship with the (25-35) age group and the second dosage, they were very compatible with the manufacturer's data. The overall frequency of several local and systemic adverse effects was great, which might be due to the unique demographic involved in this research.
Keywords
COVID-19, WHO, Saudi Arabia, Sociodemographic factors
Introduction
COVID-19 pandemic has affected many aspects of people’s lives including physical, social, emotional, and behavioral wellbeing [1]. In March 2020, the WHO reported COVID-19 as a pandemic [2]. Clinically, COVID-19 presents with a range of symptoms extending from asymptomatic, to fever, dry cough, and fatigue to more severe or lethal diseases with a SOB and severe acute respiratory syndrome [3]. Concerning the WHO report, over 252 million confirmed cases and over 5 million deaths have been reported globally [4]. To date, COVID-2019 is becoming more aggressive with the emergence of variants. Rapid herd immunity by vaccination is required to block mutation and prevent the emergence of variants that may completely escape immune surveillance [5]. Pfizer/BioNTech was the first vaccine authorized by the United States FDA in December 2020 [6], followed by Oxford/AstraZeneca which was approved in January 2021 by the European Commission [7].
In Saudi Arabia, COVID-19 cases reached 748,915 confirmed cases by mid-March 2022 and 9018 total deaths [8]. The country started its COVID-19 vaccination program in December 2020 to stop the spread of COVID-19 [7]. Healthcare workers, the elderly, and those with chronic and autoimmune diseases are scheduled to receive the vaccine early [9]. Three vaccines are currently available in Saudi Arabia: Pfizer/BioNTech, Oxford/AstraZeneca, and Moderna COVID19 vaccines [10]. About 62 million COVID-19 vaccine doses have been administered so far, with nearly 70% of the country’s total population are fully vaccinated [8]. Healthcare workers stand at the frontline for fighting the COVID-19 pandemic. This puts them at higher risk of getting the infection than other individuals in the community [11]. So, they need adequate protection to avoid onward transmission and decrease the burden on the healthcare system [12]. HCWs play an important role in the success of the immunization program to achieve herd immunity in society [9].
Rationale
Although several researches have been published on the safety and effectiveness of covid-19 vaccines, there is a need for more information on adverse events to better understand the factors associated with different vaccine-specific adverse events and to help guide future investigations and proper management of the cases [7].
Aim
This study aims to investigate the short-term side effects of Covid-19 vaccines and the association with sociodemographic factors among health care workers in Primary Health Care Centers in Jeddah 2021-2022.
Objectives
✔ To calculate the prevalence of post-covid-19 vaccine side effects among health care workers in Primary Health Care Centers in Jeddah 2021–2022.
✔ To compare the prevalence of post-covid-19 vaccine side effects between male and female healthcare workers in Primary Health Care Centers in Jeddah 2021–2022.
✔ To assess the severity of post-covid-19 vaccine side effects among health care workers in Primary Health Care Centers in Jeddah 2021–2022.
Literature review
Various side effects such as fatigue, headache, fever, and pain at the injection site have occurred in the post- Covid 19 vaccination period [13]. Despite being very rare, a few clinical trials reported life-threatening and serious adverse events, such as thrombosis and VIIT, after taking the first dose of certain vaccines [14,15]. A study by Luxi et al. showed that myocarditis/pericarditis was recognized as a rare complication of COVID-19 vaccinations, particularly in young adult and adolescent males. According to the CDC, myocarditis/pericarditis reporting rates are approximately 12.6 cases per million doses for the second dose of an mRNA vaccine among 12 to 39-year olds [16]. A study by Kadali et al. mentioned that 64.5% of all participants received the Pfizer-BioNTech vaccine and reported at least one or more symptoms post-vaccination. Of these, 79.7% were able to continue daily activities, 12.83% had difficulty performing, 12.33% took a sick leave, 2.49% needed help from an outpatient provider, 0.62% visited an emergency department, and 0.25% required hospitalization [17]. A research by Riad et al. revealed that the most commonly reported side effects were a pain in the injection site (89.8%), fatigue (62.2%), headache (45.6%), muscle pain (37.1%), and chills (33.9%). The duration of the side effects following the vaccine was mainly one day (45.1%) or three days (35.8%). The people who received two doses were associated with a higher frequency of side effects [18].
In Saudi Arabia, a study done by Ahazmi et al. showed that the side effects associated with COVID-19 vaccines have been reported by 60% of the study subjects, and 90% of them reported fatigue, 85% reported pain at the site of the injections [19]. Also, a study by Darraj et al. mentioned that a total of 57.2% of the HCWs who participated in the study reported at least one sideeffect. The most commonly reported side-effects were redness and pain in the injection site (80.0%), fever (73.2%), body pain and fatigue (56.4%), and headache (48.8%). Also, 12.4% of the participants who reported side effects needed to be seen by a physician, and only one female participant needed a hospital admission [7]. Another study by El-Shitany et al. revealed that the most common post-vaccination symptoms were injection site pain, headache, flu-like symptoms, fever, and fatigue. Less common side effects included rapid heartbeat, whole body pain, difficulty breathing, joint pain, chills, and drowsiness. Rare side effects included Bell’s palsy and lymph nodes swelling and tenderness [20]. A study by Alghamdi et al. showed that the most commonly reported side effects were muscle pain (49%), followed by fever (42%) and headache (40%). HCWs had more muscle pain, headache, sore throat, and abdominal pain compared to non-HCWs [21].
Another study by Alghamdi et al. reported that the side effects among all participants during the first day included myalgia, fever, headache, sore throat, numbness, eye muscle pain, palpitations, SOB, and GI symptoms. No serious side effects were reported during the study period as anaphylaxis or thrombotic events. Female participants appeared to have more COVID-19 vaccine-associated symptoms [22].
Methodology
Study design
A retrospective analytical cross-sectional study.
Study population
Health Care Workers of Ministry of Health in Primary Health Care Centers in Jeddah during the proposed study period from April 2022 to June 2022 were the targeted population from which the sample was selected.
Eligibility criteria
Inclusion criteria
All health care workers of Ministry of Health in Primary Health Care Centers in Jeddah who received one of the approved Covid-19 vaccines in Saudi Arabia (Pfizer/ BioNTech, AstraZeneca, or Moderna).
Those included: Physicians, nurses, dental staff, pharmacists, radiology staff, laboratory specialist, and managerial.
Exclusion criteria
None.
Study area
The study was conducted in Jeddah City, which is the second largest city in Saudi Arabia after the capital city and represents the principal gateway to Mecca. The population in Jeddah is about four million people. MOH Services in Jeddah city is divided into five sectors (north, south, center, east, and west) and the total number of primary health care centers found in these sectors is around 47 centers.
Sample size (Figure 1)
✔ The sample size was calculated by using a website of sample size calculation (Raosoft).
✔ Prevalence of post-covid-19 vaccine side effects considered: 50%
✔ Confidence interval: 95%.
✔ The margin of error: 5%
✔ The researcher added 10% to compensate for item non-response.
✔ The total sample size was 415.
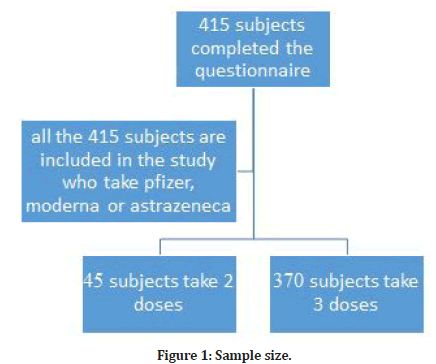
Figure 1: Sample size.
Sampling technique
A multistage stratified sampling technique was used. Stratification of PHC centers into five clusters (north, south, center, east, and west) of Jeddah city. Two centers were selected randomly from each clusters sector (cluster) using a simple random technique. The total sample size of 415 was divided equally into the 10 centers, from each center the calculated number of 42 participants were obtained proportionally by simple or systematic random sampling from each HCWs category.
Data collection tool
The researcher collected data through a selfadministered questionnaire. Questionnaires are simple to use, especially while assessing a large representative population. The questionnaire contained several sections. The first section contained an introduction about the purpose of the study, contact details for the researcher to facilitate the communication between the researcher and participants, and a consent section for agreeing to participate in the study. The second section was designed to collect general information about the study participants. The third section was formulated to focus mainly on the COVID-19 vaccine-related data. Participants were also allowed to report no symptoms by leaving the box unchecked. Also, it provided a section for reporting other unlisted side effects which might have been experienced by the study participants. Further, it also questioned the participants to report doctors’ visits after vaccination, any admissions to hospitals postvaccination, or the use of any medications taken postvaccination [19]. The questionnaire was taken from previously published research and the permission is taken from the first author (Dr. Alhazmi).
Data collection technique
The researcher distributed the questionnaire after getting approval from the ethical committee. Data was collected during May and June 2022. The questionnaire was self-administrated which was created on Google Forms and in the Arabic language and distributed to the health care workers in the primary health care centers in Jeddah to their mobile phone numbers through an online link by SMS and the response was collected automatically into an excel sheet. When required, communication between the investigator and the study participants established via electronic mail.
Study variables
✔ Sociodemographic data like the age, gender of the participants and the timing of the appearance of side effects post Covid-19 vaccine were explored as independent variables.
✔The side effects following vaccination were considered as the dependent variable.
Study outcomes
✔The prevalence of post covid-19 vaccine side effects among health care workers in Primary Health Care Centers in Jeddah 2021-2022.
✔ The difference in the prevalence of post covid-19 vaccine side effects between male and female healthcare workers in Primary Health Care Centers in Jeddah 2021-2022.
✔ The severity of post-covid-19 vaccine side effects among health care workers in Primary Health Care Centers in Jeddah 2021-2022.
Data entry and analysis
Demographic and related data were analyzed using descriptive statistics, including means, standard deviations, and frequencies.
Quantitative comparison between male and female health care workers was done by using a t-test or repeated-measures analysis of variance (ANOVA) for continuous data. The Chi-square test or Fisher exact test was used to test for the significance of statistical association between variables of categorical and nominal data measurements. The data was statistically analyzed using SPSS, version 21 for Windows. The level of significance was set at p ≤ 0.05.
Pilot study
The researcher conducted a pilot study on 10% of the sample size to test the questionnaire applicability and understanding before starting the actual research. And the participants were excluded from research analysis.
Ethical considerations
Consent was taken from all participants through the Google Form; the participants were informed that as they completed the questionnaire, they were accepted to be enrolled in the study. All data were processed confidentially. The research was conducted after approval from the Research Committee of the Saudi program of preventive medicine in Jeddah and the ethical research committee (IRB) of the health directorate in Jeddah.
Results
The study included 415 participants. The mean age among study participants was 45.57 + 13.43 years with median age of 36 years (Figure 2). Most of study participants were females (n=269, 65%) and the rest 146 were males. There were 107 participants with comorbid conditions (25.7%). The most frequent chronic condition was hypertension (n=32) followed by diabetes mellitus (n=29). Other chronic conditions included asthma, cardiovascular disease, peptic ulcer disease and others. Among study participants, 184 participants had previous COVID-19 infection.
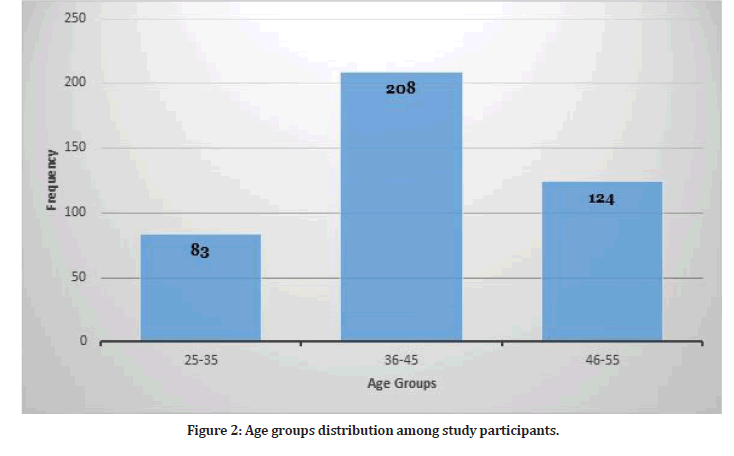
Figure 2: Age groups distribution among study participants.
The type of vaccination received by healthcare professionals included in this study varied with Pfizer vaccine being most common (n=191, 43%). Figure 3 shows the type of vaccine received by study participants. Most of study participants received three doses of the vaccine (n=370, 89.15%) while 45 participants received two doses.
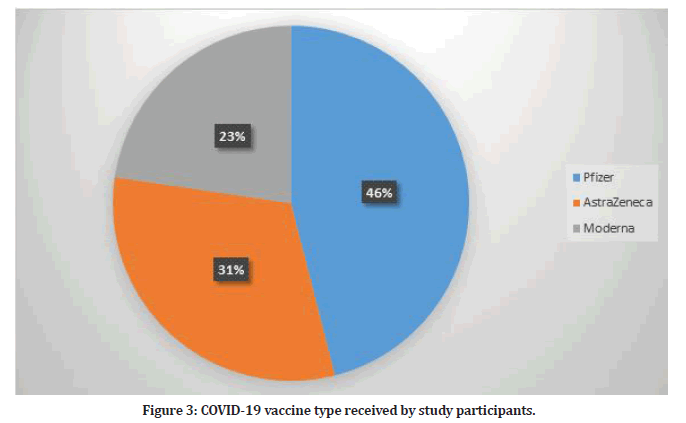
Figure 3: COVID-19 vaccine type received by study participants.
Study participants who received two doses reported several side effects (N=15). The most common side effect among them was injection site pain (n=14, 31.1%). Table 1 shows the frequency of side effects among participants who received two doses. Hence, some participants reported more than one side effect. The duration of symptoms lasted for maximum of two days. Figure 4 shows the distribution of side effects by gender.
| Side effect | Frequency | Percent |
|---|---|---|
| Injection site pain | 14 | 31.10% |
| Fever | 9 | 20% |
| Fatigue | 9 | 20% |
| Headache | 7 | 15.56% |
Table 1: Vaccine side effects among participants who received two doses (N=45).
Study participants who received three doses reported several side effects (N=370). Table 2 shows the frequency of side effects among participants who received three doses. Hence, some participants reported more than one side effect. The presence of side effects was statistically significant for the third dose as illustrated in Table 2. Figure 5 shows the distribution of side effects by gender. Figure 6 shows the frequency of participants who had side effects after the second and third dose and the participants who didn’t have any side effects.
| Side effect | Frequency | Percent | P-value |
|---|---|---|---|
| Fever | 137 | 37.02% | 0.048 |
| Fatigue | 189 | 51.08% | 0.001 |
| Headache | 231 | 62.43% | <0.001 |
| Shortness of breath | 71 | 19.18% | <0.001 |
Table 2: Vaccine side effects among participants who received three dose (N=370).
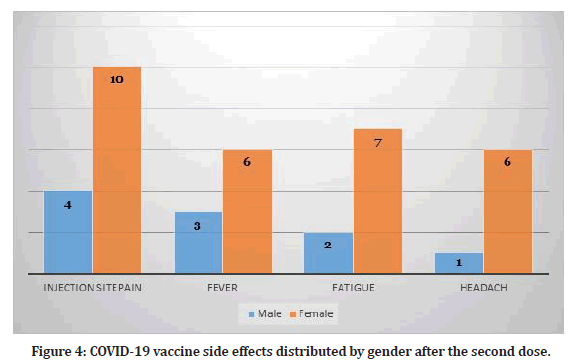
Figure 4: COVID-19 vaccine side effects distributed by gender after the second dose.
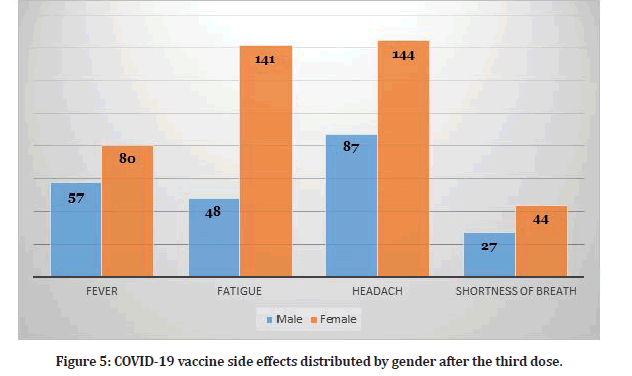
Figure 5: COVID-19 vaccine side effects distributed by gender after the third dose.
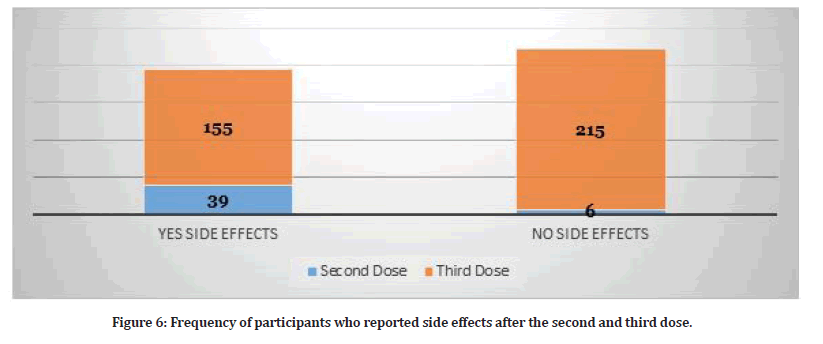
Figure 6:Frequency of participants who reported side effects after the second and third dose.
There were 42 participants who visited doctors for their side effects (9.5%). On the other hand, 4 participants required hospitalization (0.9%) due to severe chest pain, shortness of breath, high grade fever and severe headache. Furthermore, 308 participants used medications in order to relieve their symptoms (Figure 7).
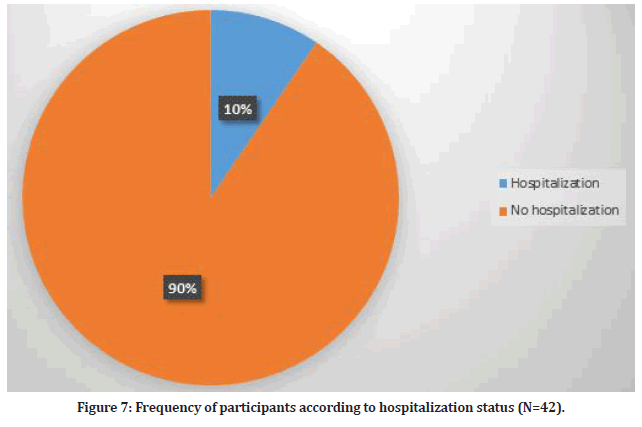
Figure 7:Frequency of participants according to hospitalization status (N=42).
Discussion
Immunization reluctance (VH) is characterized as the "delay in tolerating or denying antibodies notwithstanding the accessibility of antibody administrations"; it is a developing general medical problem powered by deception about antibody viability and security [23]. Repugnance for the likely unfavorable impacts of immunizations was distinguished as the most widely recognized reason of VH among segment bunches in the United Kingdom (U.K.) in a new cross country review [24]. This end was supported with regards to COVID-19 inoculations, since dread of unfriendly impacts was the most widely recognized justification for medical care staff and understudies in Poland to be reluctant to get the vaccination [25]. Subsequently, a methodical evaluation of VH-battling strategies observed that rising public information on inoculation viability and trustworthiness about secondary effects is basic for expanding immunization take-up [26]. The beginning of the COVID-19 immunization organization in December 2020 was a turning point in the battle against this pandemic; consequently, it was recommended that the pandemic history be separated into pre-immunization (B.V.; before immunization) and post-immunization (A.V.; after immunization) periods. Coronavirus related material ought to be marked as B.V. or then again A.V. [27].
The expanded articulation of angiotensin-changing over chemical 2 (ACE2) receptors in the tongue's epithelial cells, buccal and gingival mucosa has been connected to COVID-19-related oral side effects [28,30,31].
Up to this point, all known information on COVID-19 immunization unfriendly impacts has been distributed through producer supported preliminaries that observe drug authority guidelines and are checked by outsiders [32]. An absence of free examination on immunization security might destructively affect immunization takeup, which should be facilitated before very long to get away from the infection's and its varieties' horrendous circle [33].
A randomized controlled study (RCT) that enlisted 43,000 workers with a middle age of 52 years of age gave the primary information to assess the viability of the Pfizer- BioNTech COVID-19 immunization. Early discoveries from this RCT demonstrated that the immunization's viability was around 95%, for certain unfriendly reactions happening soon after the inoculation portion (CDC, 2021). The immunization's antagonistic impacts may be delegated either nearby or fundamental, with seriousness going from gentle to critical [34].
As per the FDA's evaluation, the general recurrence of fundamental side effects, for example, fever, fatigue, migraine, chills, regurgitating, loose bowels, muscle torment, and joint agony was a lot more prominent in more youthful people than in more seasoned ones (82.8% versus 70.6% ). Neighborhood reactions, for example, infusion site distress, expanding, and redness, showed the comparative pattern, with 88.7% of more youthful people influenced contrasted with 79.7% of more established grown-ups. In the study example [30], the mean number of side effects after first, second and third doses were (4 side effects, 2 side effects and 3 side effects) respectively and all out recurrence of tormented people were significantly more prominent in the more youthful than in the more established [30].
In general, the Czech medical care experts in [30] had impressively more noteworthy paces of infusion site uneasiness (89.8% versus 75.35%), infusion site edema (25.6% versus 6.4%), and infusion site expanding (23% versus 5.5%) than the Pfizer-BioNTech preliminary members [35]. The absolute recurrence of cerebral pain, then again, was somewhat reliable between the Czech example [30] and the FDA's report (45.6% versus 40.06%, separately).
The FDA's examination observed that the occurrence of nearby incidental effects was to some degree more prominent after the subsequent measurement contrasted with the primary portion while looking at the first and second dosages of the immunization. On account of fundamental unfriendly impacts, the comparable example was more articulated [35]. The Czech information confirmed this propensity in completely detailed antagonistic impacts aside from infusion site redness, which was more normal in people who got just a single measurements.
Since they were fundamentally impacted by infusion site redness (2 =6.27; p =0.012), migraine (2 =7.5; p =0.006), sickness (2 =4.97; p =0.026), fever (2 =6.62; p =0.01), chills (2 =6.1; p =0.014), and lymphadenopathy (2 =5.54; p =0.019), the unfavorably susceptible populace who utilized allergy med drugs were the most weak gathering for encountering aftereffects [30]. Inside its in-between time guidelines for the COVID-19 immunization sending, the Centers for Disease Control and Prevention (CDC) educated that anybody having a set of experiences regarding any fast unfriendly reaction to earlier inoculations or injectable meds ought to be vaccinated with intense watchfulness. Individuals having a past filled with extreme hypersensitive reactions, like hypersensitivity, to an earlier portion of the immunization or a part of the antibody, like polyethylene glycol (PEG), are not allowed to get the inoculation at this stage [36]. In spite of the fact that individuals with sensitivity to oral meds, food, pets, bugs, toxin, plastic, and other ecological affronts, as well as family backgrounds, are encouraged to get the immunization to no one's surprise, it is actually important that allergy medicine utilization increments altogether in spring in Europe; subsequently, exceptional consideration ought to be paid to the remedy of these medications during this season with regards to inoculation [30].
The middle postpone between the first and second dosages was 21 days, which is predictable with the World Health Organization (WHO) suggested period. The middle patency span between the recuperation date and the primary antibody measurements was 65 days, which meets the WHO's suggestion of seven days between the positive test and vaccination.
According to the findings of this study [20], injection site discomfort was reported more frequently among those aged 60 and above (80.8 percent) than among younger people (68.6%). A relatively low number of people reported injection-site edema and redness. Following the second dosage, the percentage of those experiencing local symptoms climbed dramatically. Our findings contradict the findings of [31-35], who found that injection site discomfort was more common in those under the age of 55 compared to those over the age of 55.
They also discovered that the proportions of persons who reported local symptoms following the first and second doses of the vaccination were substantially identical. Our investigation and their study, however, found the identical outcomes in terms of injection-site edema and redness. Our findings are consistent with those of [36- 38], since both studies found that vaccination-associated systemic adverse effects were more prevalent in younger persons and more common after the second vaccine dosage. According to the two studies, headaches were the most prevalent complaint following a vaccination injection. In addition, fever was more likely among young person's following the second dosage.
Conclusion
Among study participants, the most prevalent adverse effects of the Pfizer–BioNTech COVID-19 vaccine were injection site discomfort, weariness, headache, muscle soreness, chills, and joint pain. In terms of their relationship with age groups. Headache, nausea, muscular discomfort, fever, and lymphadenopathy were all substantially linked with side effects. More independent research on vaccination safety is urgently needed to boost public trust in the vaccine and give a better knowledge of the potential risk factors for vaccine adverse effects.
Strengths and Limitations
The current study is one of the most comprehensive investigations on the negative effects of the COVID-19 vaccines. Few or no studies have recorded the vaccine's negative effects, particularly the vaccine's post-marketing follow-up. The present study's disadvantage is that it only looked at the vaccines’ short-term negative effects. In contrast, the vaccines’ medium and long-term effects are yet uncertain. Furthermore, the study was limited to Saudi participants, excluding people of other races. The modest number of research participants who had previously been infected with the coronavirus suggests that additional investigations with an appropriate and compatible sample size for both previously infected and non-infected people are needed.
Acknowledgement
The authors are thankful to all study participants.
References
- Kumari A, Ranjan P, Chopra S, et al. Development and validation of a questionnaire to assess knowledge, attitude, practices, and concerns regarding COVID-19 vaccination among the general population. Diabetes Metabolic Syndrome Clin Res Rev 2021; 15:919-925.
- Abbas AM, Ahmed OA, Shaltout AS. COVID-19 and maternal pre-eclampsia: A synopsis. Scand J Immunol 2020.
- Ahn RW, Mootz AR, Brewington CC, et al. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imag 2021; 3:e210008.
- https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---16-november-2021
- Liu Q, Qin C, Liu M, et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 2021; 10:1-5.
- https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-COVID-19/comirnaty-and-pfizer-biontech-COVID-19-vaccine
- Darraj MA, Al-Mekhlafi HM. Prospective evaluation of side-effects following the first dose of oxford/astrazeneca COVID-19 vaccine among healthcare workers in Saudi Arabia. Vaccines 2022; 10:223.
- https://covid19.moh.gov.sa/
- Qattan AM, Alshareef N, Alsharqi O, et al. Acceptability of a COVID-19 vaccine among healthcare workers in the Kingdom of Saudi Arabia. Front Med 2021; 8:644300.
- https://www.moh.gov.sa/en/Ministry/HotTopics/Pages/COVID-19-Vaccine.aspx
- Alserehi HA, Alqunaibet AM, Al-Tawfiq JA, et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: Comparing case and control hospitals. Diagn Microbiol Infect Dis 2021; 99:115273.
- Noushad M, Nassani MZ, Alsalhani AB, et al. COVID-19 vaccine intention among healthcare workers in Saudi Arabia: A cross-sectional survey. Vaccines 2021; 9:835.
- Hernández AF, Calina D, Poulas K, et al. Safety of COVID-19 vaccines administered in the EU: Should we be concerned?. Toxicol Rep 2021; 8:871-879.
- See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26. COV2. S vaccination, March 2 to April 21, 2021. JAMA 2021; 325:2448-2456.
- Tobaiqy M, MacLure K, Elkout H, et al. Thrombotic adverse events reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 vaccines: Comparison of occurrence and clinical outcomes in the EudraVigilance database. Vaccines 2021; 9:1326.
- Luxi, N, Giovanazzi A, Capuano A, et al. COVID-19 vaccination in pregnancy, paediatrics, immunocompromised patients, and persons with history of allergy or prior SARS-CoV-2 infection: overview of current recommendations and pre-and post-marketing evidence for vaccine efficacy and safety. Drug Safety 2021; 44:1247-1269.
- Kadali RA, Janagama R, Peruru S, et al. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis 2021; 106:376-381.
- Riad A, Hocková B, Kantorová L, et al. Side effects of mRNA-based COVID-19 vaccine: nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals 2021; 14:873.
- Alhazmi A, Alamer E, Daws D, et al. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines 2021; 9:674.
- El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: A retrospective cross-sectional study. Int J Gen Med 2021; 14:1389.
- Alghamdi AA, Alkazemi A, Alissa A, et al. Adverse events following AstraZeneca COVID-19 vaccine in Saudi Arabia: A cross-sectional study among healthcare and nonhealthcare workers. Intervirol 2022; 65:104-109.
- Alghamdi A, Ibrahim A, Almutairi R, et al. A cross-sectional survey of side effects after COVID-19 vaccination in Saudi Arabia: Male versus female outcomes. J Adv Pharm Edu Res 2021; 11.
- Babamahmoodi F, Saeedi M, Alizadeh-Navaei R, et al. Side effects and Immunogenicity following administration of the Sputnik V COVID-19 vaccine in health care workers in Iran. Sci Rep 2021; 11:1-8.
- Bae S, Lee YW, Lim SY, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci 2021; 36.
- Indexed at, Google Scholar, Cross Ref
- Butler R, MacDonald NE. Diagnosing the determinants of vaccine hesitancy in specific subgroups: The Guide to Tailoring Immunization Programmes (TIP). Vaccine 2015; 33:4176.
- COVID C, Team R, Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Morbidity Mortality Weekly Rep 2021; 70:46.
- Doroftei B, Ciobica A, Ilie OD, et al. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics 2021; 11:579.
- Dziedzic A, Riad A, Attia S, et al. Self-reported adverse events of COVID-19 vaccines in polish healthcare workers and medical students. Cross-sectional study and pooled analysis of CoVaST project results in Central Europe. J Clin Med 2021; 10:5338.
- Gonçalves AK, Cobucci RN, Rodrigues HM, et al. Safety, tolerability and side effects of human papillomavirus vaccines: A systematic quantitative review. Br J Infect Dis 2014; 18:651-659.
- Riad A, Klugar M, Krsek M. COVID-19 related oral manifestations, early disease features?. Oral Dis 2020.
- Harrison EA, Wu JW. Vaccine confidence in the time of COVID-19. Eur J Epidemiol 2020; 35:325-330.
- Hocková B, Riad A, Valky J, et al. Oral complications of ICU patients with COVID-19: Case-series and review of two hundred ten cases. J Clin Med 2021; 10:581.
- Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy–A systematic review. Vaccine 2015; 33:4180-4190.
- Kadali RA, Janagama R, Peruru S, et al. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis 2021; 106:376-381.
- Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. Am J Transplant 2021; 21:1332.
- Kaur RJ, Dutta S, Charan J, et al. Cardiovascular adverse events reported from COVID-19 vaccines: A study based on WHO database. Int J Gen Med 2021; 9:3909–3927.
- Klugar M, Klugarová J, Pokorná A, et al. Use of epidemiological analyses in clinical practice guideline development focused on the diabetic patients treated with insulin. JBI Evid Implement 2019; 17:48-52.
- Smith L, Amlot R, Weinman J, et al. A systematic review of factors affecting vaccine uptake in young children. Vaccine 2017; 35:6059–6069.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Lamies Mahmoud Omran* and Mohammed Sami Kutbi
Department of Preventive Medicine, Ministry of National Guard, Health affairs, Western region, Jeddah, South AfricaReceived: 07-Jun-2022, Manuscript No. JRMDS-22-68399; , Pre QC No. JRMDS-22-68399 (PQ); Editor assigned: 09-Jun-2022, Pre QC No. JRMDS-22-68399 (PQ); Reviewed: 24-Jun-2022, QC No. JRMDS-22-68399; Revised: 29-Jun-2022, Manuscript No. JRMDS-22-68399 (R); Published: 06-Jul-2022
