Research - (2022) Future Prospects of clinical and Medical Research
Stem Cells for Treatment of Diabetes Mellitus
Tripti Arora1*, Namrata Arya2, Jayanand3 and Manju4
*Correspondence: Tripti Arora, Department of Pharmaceutical Chemistry, SGT University, Gurugram (HR), India, Email:
Abstract
Diabetes mellitus is a collection of metabolic illnesses which is described by a persistently high level of sugar in the blood. Diabetes is generally categorized into 3 types viz. diabetes type-1, type-2 & gestational. Discrete β-cells are extremely dedicated microscopic insulin-generating units found in the pancreatic islets of Langerhans, proϔcent of managing properly measured insulin discharged in answer to a meal-triggered upsurge in plasma glucose with extraordinary accuracy during a normal life span. Undifferentiated cells are clonogenic cells that could self-renew & discriminate into many ancestries. The current study focuses on the treatment of diabetes using stem cells by reviewing some of the scentϔc ϔndns and discussing future strategies based on the close dialogue between stem cell biology and the ϔed of developmental biology, with the goal of transferring the ϔndns related to development biology of β-cell into the ϔed of practical stem cell biology & cure.
Keywords
Β-Cell, Diabetes mellitus, Stem cell, Diabetes type-1, Diabetes type-2
Introduction
Diabetes Mellitus (DM)
DM, universally denoted as diabetes, exists as cluster of metabolic diseases described by an elevated plasma glucose concentration that persists. Symptoms include recurrent urination, extreme thirst, & increased hunger. Diabetes may cause a host of health problems if left untreated. Acute consequences include diabetic ketoacidosis, hyperosmolar hyperglycemia, and death. Chronic effects include cardiovascular illness, nerve damage, stroke, foot ulcers, eye damage, nerve damage& cognitive impairment.
Diabetes is characterized by either a shortage of insulin synthesis by the pancreas or an incapability of the cells to reply to insulin. Diabetes mellitus is divided into three categories:
Diabetes Type-1: It is defined through the death of pancreatic islet's insulin-generating β-cells, resulting in insulin inadequacy. Immune-arbitrated or idiopathic are 2 subtypes of this kind. The mainstream of diabetes type-1 is triggered by immune-arbitrated autoimmune bout on T cells, which fallouts in the demise of β-cells & consequently insulin. Numerous genes, particularly some HLA genotypes, are recognized to affect the danger of diabetes in individuals with type-1 diabetes. Diabetes could be carried on due to 1 or more ecological parameters, including a viral contagion or a modification in diet, in genetically vulnerable persons. Numerous viruses have been involved; however, there is no decisive indication in people to support this notion. Gliadin appears to play a part in the growth of diabetes type-1, although the contrivance is unknown. Diabetes type-1 can strike at any stage, but it is most commonly diagnosed in grown-ups. When diabetes type-1 progresses in grownups, it is denoted as LADA. It develops more slowly than it does in children.
Diabetes Type-2: Insulin resilience is a feature of type 2 diabetes, which can be accompanied with a reduction in insulin production. The insulin receptor is believed to be involved in the diminished insulin responsiveness of bodily tissues. The particular flaws, however, are unknown. Cases of diabetes mellitus caused by a recognized deficiency are categorized differently. Diabetes type-2 is the utmost frequent form of the illness. Before reaching the criteria for type-2 diabetes, many persons with diabetes type-2 may have indications of pre-diabetes. Lifestyle adjustments or drugs that increase insulin compassion or decrease the liver's glucose synthesis can halt or stop the advancement of prediabetes to overt type-2 diabetes.
Gestational diabetes: In many respects, gestational diabetes resembles type 2 diabetes in that it is characterized by poor insulin production & response. It affects around 2 percent to 10 percent of all pregnancies and may improve or vanish once the baby is born. All pregnant women must be tested at 25–27 weeks of pregnancy. It is most frequently observed in the 2nd or 3rd trimester due to an upsurge in insulin-competitor hormone echelons at this moment. Nevertheless, percent to 10 percent of females having gestational diabetes are later diagnosed with a new form of diabetes, the most common of which is type-2 diabetes. Although gestational diabetes is totally curable, it does necessitate close medical monitoring during the pregnancy. Dietary adjustments, blood glucose checking, & in rare circumstances, insulin may be necessary for treatment.
Untreated gestational diabetes may harm the fetus or mother's wellbeing, even if it is only temporary. Macrosomia, congenital heart & skeletal muscle deformities, & central nervous system aberrations are all risks for the newborn. Amplified insulin levels in a fetus's blood can cause foetal surfactant making to be inhibited, resulting in infant respiratory distress syndrome. The breakdown of red blood cells might result in a high blood bilirubin level. Perinatal death can occur in severe situations, most typically as an outcome of insufficient placental perfusion caused by vascular dysfunction. Reduced placental function may need labor induction.
Regulation of β-cells and the cause of diabetes
Discrete β-cells are extremely dedicated microscopic insulin-generating units found in pancreatic islets of Langerhans, proficient of synchronizing a properly dosed insulin discharge in reply to a diet-persuaded upsurge in plasma glucose with extraordinary accuracy during a normal life span. While uncontrolled excessive insulin secretion can be dangerous, insulin is necessary for capacity of the body to move glucose from the bloodstream to the cells, the place where it is digested. Hyperglycemia and diabetes are caused by a lack of insulin. The two most common types of diabetes are distinguished by outright or comparative paucity of β- cells. In type-1 diabetes, the immune system's autoaggressive lymphocytes target and destroy the β-cells, resulting in a lifelong reliance on life-saving regular insulin injections. The status of autoimmune diabetes doesn’t return to usual, implying that enduring autoimmunity may negate whichever efforts at endogenous β- cell revival, or that β-cells don’t redevelop at all. It's been proposed recently that type-1 individuals with remaining β-cells are really attempting to replenish β-cell bulk in struggle with active annihilation. The repeated β-cellspecific obliteration after fractional pancreas replacement amid indistinguishable twins dissenting for diabetes type-1 highlights the lifelong durability of diabetes autoimmunity (β-cell specificity).
Current animal studies have revealed a remarkable dynamic potential for adjusting the functional β-cell mass in reply to a diversity of stimuli, including peripheral insulin resistance, pregnancy & delivery, & transplanted insulinoma. Obesity and increasing insulin resistance have been linked to larger Langerhans islets in humans, as has been shown in animal research. To keep plasma glucose in check, more insulin is required. Type 2 diabetes develops when this compensatory process flops, that is, once the upkeep of increasing β-cell mass can’t keep up with the upsurge in peripheral insulin resilience. Likewise, people with diabetes type-2 may develop a leisurely-acting type of auto-immunity, which causes β- cell loss and, eventually, insulin dependency that is indistinguishable from type-1 diabetes.
Hypertrophy, apoptosis, replication, & neoformation are all ways in which the dynamic regulation of -cell mass may be regulated in healthy individuals. Recent convincing research in mice has revealed that novel β- cells originate solely from prevailing β-cells throughout adulthood & that islet neogenesis doesn't really arise in mice. Likewise, Bock et al. found that significant hyperplasia of β-cell seen in obese mice is completely described by a rise in the size of current islets rather by islet neogenesis.
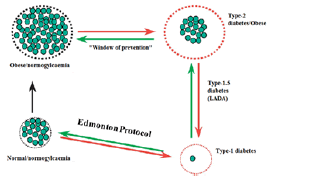
Figure 1 illustrates the correlation between β-cell mass regulation & diabetes. The loss of functioning β-cell mass, & thus insulin, is the mutual factor in all kinds of diabetes. In type-1 diabetes, there is an absolute loss of β-cells, but in type-2 diabetes, there is a relative shortage of β-cells. At the bottom of the image, to the left, is a healthy Langerhans islet. Type-1 diabetes is prompted by the immune system's aggressive killing of β-cells. Higher β-cell mass compensates for emerging insulin resistance (overweight, amplified calorie intake, not at all exercise). Type-2 diabetes develops when this compensatory response is unable to keep up with rising insulin demands. A small percentage of type-2 diabetics may mislay even more β-cell mass; ultimately causing total insulin need (as in type-1 diabetes). The reinstatement of functioning β-cell mass will reestablish normal plasma glucose management, according to this simplified diagram [1-3].
Figure 1: Representing the β-cell Mass & Diabetes
Stem cells
Undifferentiated cells are clonogenic cells that can autorenew & discriminate into many ancestries. Self-renewal does not, however, describe stem cells, which can be nondividing or gradually separating cells. Adult stem cells are typically characterized as cells that could be stimulated to proliferate in response to tissue injury in order to restore function. There is regularly a transitional population of devoted precursors with inadequate proliferative potential & constrained disparity probable between the stem cell & its terminally discriminated progeny; these cells show the most proliferative action under physiological & pathophysiological conditions. Distinct species and/or tissues have different differentiation methods & intermediate stages, but they all have two things in common: the ability to make alike daughter cells ('self-regeneration') & the ability to yield daughter cells that will differentiate. While the former refers to their ability to expand, the latter refers to their capability to discriminate into the appropriate cell type, either in vivo or in vitro.
They are categorized as, pluripotent, or unipotent. Unipotent stem cells are cell ancestry precursors that have chosen to grow into a certain cell type. These features have incredible therapeutic promise since they may be utilized as an endless supply of cells for transplantation & tissue restoration treatments, as well as for rigorous pharmacological & toxin evaluation instead of animal studies. The totipotent cell (from the zygote towards the blastocyst's inner cell mass) is the earliest undifferentiated cell in ontogenesis; totipotent cells quickly give birth to somatic stem, forerunner cells, & primeval germ-line undifferentiated cells.
In the neurula stage, the processes between somatic undifferentiated cells & development of tissue- or organprecise stem cells are poorly known. At this point, hematopoietic stem cells emerge. Most of the ideas have been based on these undifferentiated cells as they are the most well-known, but it is uncertain if direct speculations to other undifferentiated cell types are feasible. Besides, stem cells may be utilized to study how in vitro cell differentiation is controlled.
Apart from their ability to auto-renew & differentiate, undifferentiated cells have also been characterized as having the ability to divide asymmetrically, exist in a mitotically inactive state, & clonally revive. The cytokine leukemia inhibitory factor (LIF), that stimulates STAT3 & the receptor-associated Janus kinase (JAK) via binding to the LIFR & the gp130 signaling complex, is needed for the self-replenishment of primordial mouse ESC. Oct-3/4, a POU transcription factor that controls lineage commitment; operates as a major regulator of pluripotency by activating the LIF signalling pathway. Human ESC, on the other hand, which express Oct-4 but do not require an exogenic source of LIF to maintain their propagation as indistinguishable stem cells do not know the specific nature of these signals.
In this regard, during ordinary passaging, human ESC underwent modest levels of Stem cells and diabetes spontaneous differentiation, which was enhanced by cultivating cultures at great density for 5 to 8 weeks. Lastly, when human ESC are grafted onto immunocompromised severe joint immunodeficient mice, they spontaneously differentiate into cell types coming from all 3 nascent germ layers in vivo[1, 4–6].
Sources of stem cells
Pluripotent and totipotent stem cell lines: ESC generated from the inner cell mass of human blastocyst phase embryos, EGC generated from the gonadal province of five to nine week-old terminated human foetuses, & ECC generated from teratocarcinoma tissue have all been shown to give upswing to pluripotent cell lines. ECC lines are converted and often aneuploid, while ESC and EGC lines are diploid & genetically healthy. Their expression of cell surface markers, cell culture environment, pluripotency, & clinical use are also distinct. ESC & EGC lines have been employed in cell distinction readings of early human progress as a foundation of anticipated cells, or enucleated receivers of nuclei from cells of patient rather than enucleated oocytes. This process will result in ‘customized' pluripotent stem cells, which would daze the current challenges of immunological compatibility and transplant rejection, as well as the need for immunosuppressive medicines.
Adult stem cell lines: Because stem cells may renew for the remainder of their lives, they are found in specific tissues. The epidermis, intestinal epithelia, & blood cells of a healthy adult exhibit a high pace of cell revitalization, which involves both cell growth & death, even in the lack of damage. Other tissues, like the liver or muscle, may rejuvenate if they are damaged. Ultimately, terminally differentiated cells are thought to have halted their differentiation potential and retained a stable phenotype with low division capacity. This has been the established model for many years.
Certain long-held beliefs, however, have lately been challenged. According to many investigations, adult stem cells may be generated from tissues that are believed to be incapable of regeneration. The number of tissues that contain stem cells has been expanded to include brain, retinal, & bone marrow tissues. Moreover, some of these grownup stem cells do not seem to be permanently committed to a particular destiny and have much higher differentiation potential than previously believed. Multipotent adult stem cells have been found in adult mouse brain tissue, according to two studies. Mentioned two teams have shown that cells arising from various sources may develop into a variety of brain cells [7,8].
Clarke et al. have discovered that grownup neural undifferentiated cells may assist in the production of chimeric chicks & mice, showing that grownup neural undifferentiated cells have a extensive range of progressive potential. The search for adequate data to support the premise that "differentiation requires continuous management" is not hampered by this rejection of the dominant paradigm. Moreover, adult tissue molecular indicators of the stem cell morphology are poorly known. The presence of high amounts of β1 integrin in epidermal stem cells indicates that the cells have stem cell features, & β1-arbitrated cell adhesion slows terminal maturation [9].
Literature Review
Tingxia Guo & Matthias Hebrok's research emphasized the many techniques utilized to control the maturation of hESCs into pancreatic & b-cells. Around the globe, the number of individuals with diabetes mellitus is rising at upsetting pace. Insulin-generating β-cells in the islet of Langerhans are killed in diabetic patients to different degrees, either as a consequence of an autoimmune response in diabetic type 1 patients or owing to intrinsic β-cell activity anomalies in type 2 diabetic patients. Islet transplantation as a cell replenishment method may provide diabetes patients more options. The inconsistency between the restricted number of donor islets available and the huge number of patients who might profit from such a therapy, on the other hand, emphasizes the urgent need for more high-quality β-cell sources. Self-renewing hESCs could be developed into gears of all 3 germ layers, together with all pancreatic ancestries. The capacity to develop hESCs into β-cells points to a hopeful technique for addressing the β-cell deficit [4].
G. Bernaô??´ et al. in their paper concluded that diabetes mellitus is a metabolic ailment that affects 3 percent to 6 percent of the populace. Transplanting isolated Langerhans islets from a donor pancreas may be used to cure diabetes; however, the shortage of donor material & the long-term immunosuppressive side effects make this an impractical approach. Such glitches may be addressed by consuming a renewable cell supply, such as islet cells resulting from stem cells. Undifferentiated cells are selfrenewing clonogenic cells that can develop into a variety of lineages. This implies that these cells may be differentiated & amplified to produce the appropriate cell type in vivo or in vitro. When a marker gene is generated by means of in vitro differentiation & screening methods, embryonic undifferentiated cells may be directed into certain cell ancestries & chosen via genetic assortment. Differentiation & cell choice methods have recently been used to generate insulin-generating cells that regulate plasma glucose when embryonic stem cell-derived insulin-generating cells are transplanted into diabetic animals. Mature stem cells' functional flexibility may be higher than previously believed, according to numerous recent researches. Adult stem cells will enable autotransplantation without the ethical concerns that come with embryonic stem cells. These findings have given rise to the possibility that cell therapy may one day be used to treat diabetes [2].
In their study, KO Lee et al. said that stem cell treatment has a lot of promise in the treatment of diabetes patients. Research has discovered the developing phases & transcription contributing aspects in the differentiation of human embryonic undifferentiated cells into islet cells. However, ethical concerns as well as the danger of teratoma development restrict the usage of human embryonic stem cells in therapeutic settings. As a consequence, alternative stem cell treatments including induced umbilical cord stem cells, pluripotent stem cells, & bone marrow-derived mesenchymal undifferentiated cells have become a popular study subject. Recent progresses in stem cell treatment may make this a feasible diabetes cure option in the near future [10].
Discussion
Following techniques could be used for treatment of diabetes mellitus:
Maturation of pancreatic stem cells into β-cells
When it comes to diabetes treatment, the possibility of pancreatic stem cells has gotten a lot of attention because an expanded somatic stem cell may be an understandable preliminary place for the creation of novel β-cells. EScells, on the other hand, are the least specialized stem cells and thus the farthest removed from developed cell type seen in organs & tissues. Pluripotent ES-cells, on the other hand, have the ability to develop in any direction, including β-cells.
Long-standing growth of mouse pancreatic tissue directed in the preferential endurance of "isletgenerating undifferentiated cells," which were subsequently capable to generate islet-like assemblies in culture & had a beneficial impact on diabetic mice after transplantation, according to a study published in 2000. More stringent controls, on the other hand, would have been preferable to back up these findings. Later that year, researchers reported similar results using more specified culture settings in which secluded human pancreatic duct samples as inoculum permissible for the creation & dissemination of human islet-like edifices. Although additional labeling analysis of human ducts clearly indicated that presumed endocrine precursors live in ducts (CK-19+) & could indeed proliferate to begin forming islet-like cells, it is unclear whether such findings are elucidated by the presence of a suitable pancreatic undifferentiated cells. The relatively low generation of islet-alike material in such studies is a significant problem, which may be related to poor cultivation conditions or the duct cell preparations' restricted proliferative ability.
As previously mentioned, the absence of efficient somatic pancreatic endocrine SC in mice has lately been shown using very strong evidence (lineage tracing). The pancreatic ductal epithelium, according to Bonner-Weir, may serve as a group of precursor cells, & further ancestry tracing readings are being performed to validate this specific hypothesis [12]. Similarly, the Bouwens group recently reported that EGF and LIF (leukemia inhibitory factor) were used to produce β-cells from exocrine rat pancreatic preparations. In such a arrangement, genetic lineage tracing is achievable, and it would deliver definitive indication of β-cell progenitor capacity in the mature non-endocrine pancreas.
Trans-differentiation is interesting because it has the ability to dedifferentiate cells into a progenitor-like phase with proliferative capacity. The research groups of Gerschengorn & Habener demonstrated epithelial-tomesenchymal transitions in islet-consequent tissue cultures with high propagation, which can consequently be re-differentiated into endocrine cells. Clonogenic tests may have directly confirmed which cells had both propagative capability & the potential to produce insulin in all of these studies.
Use of multipotent stem cells
The desired option for somatic stem cells over ES-cells exemplifies a political/public desire, which may have resulted in some journals' strict scientific standards being relaxed, allowing studies with non-replicable results to be published. As a result, much energy has gone into testing the constraints of somatic stem cells' mellowing repertoire, and a huge count of journals have surfaced since the late 1990s displaying that the effectiveness of somatic undifferentiated cells would seem to confront the otherwise unprecedented pluripotency of ES-cells: mesenchymal cells may start generating both hepatic (endoderm) & intestinal (mesoderm) cells. These are ground-breaking research that show bone marrow stem cells may be utilized to cure a wide range of degenerative illnesses induced by tissue breakdown in any germ layer [1].
MAPC culture is challenging and has not been readily reproduced in many labs, thus comprehensive comparisons with ES-cell equivalents, for example, are not possible. Other labs have reported MAPC-like cultures with minor differences, thus relative global gene expression readings are needed to establish these cells' accurate nature in contrast to known pluripotent ES-cell cultures. It's uncertain if the MAPC phenotype is the consequence of in vitro somatic stem cell dedifferentiation or a naturally arising very uncommon stem cell type. If this is the case, these stem cells may be pluripotent pre-gastrulation phases that persist as rare latent cells in adult tissues. To sum up, the popular implementation of MAPC cultures as easily obtainable, versatile, pluripotent undifferentiated cell reports has yet to be revealed, & well ES-cells seem to to be the most easily accessible & appealing foundation of pluripotent undifferentiated cells for ex vivo-cell creation for diabetes cure at the moment.
Maturation of embryonic stem cell into β-cell
In a variety of animals, including humans, EM cell lines may be cultivated from the blastocyst phase of development. ES-cells may develop indeterminately (telomerase+) & persist pluripotent under specific growth circumstances. Mouse ES-cells may be put back into the blastocyst & re-embedded in mice to determine if they subsidize to the development of all tissues in the emerging embryo chimaera [1].
A study published in 2001 stated that a five-step procedure may be utilized to create islet of Langerhanslike colonies from mouse ES-cells. The approach was based on the research group's experience developing neurons in vitro from nestin-carrying stem cells, & the idea that endocrine & neuronal maturation are comparable in certain ways. Though it is still unclear if these cells are neuron-like phenotypes of ectodermal ancestries since they only express bit quantities of insulin-2 mRNA rather than insulin-1, the findings were consistent across labs and were adjusted to enhance the generation of insulin-containing cells. Insulin-2 is mostly articulated in non-β-cells throughout expansion.
Despite the fact that the cells in query embedded & released insulin, the connection to pancreatic insulingenerating β-cells is indeed called into question after the discovery of a cell type proficient of discharging insulin which was vigorously consumed from the medium & thereby not generated; insulin is prevalent in huge amounts in the β-cells' medium. They are unlikely to resemble endodermally produced pancreatic β-cells since such cells don’t co-discharge C-peptide with insulin.
Finally, it is essential to pursue a fixed disparity of EScells that follows the route of usual β-cell growth. As a consequence, a deep understanding of all phases of β-cell development is critical for future guided differentiation success, & extremely similar techniques have been effective in trying to direct the divergence of motor neurons from mouse ES cells & dopaminergic neurons from human ES cells through reiterating discrete series of stages of usual ontogeny [1].
Conclusion
Over last few decades, diabetes is a disease which has been found is a large number of individuals. The absence of insulin-generating β-cells has emerged as a mutual factor in all types of diabetes. Insulin cure for diabetes is life-increasing but it is not a treatment, & insulin injection is crucial as overdose (hypoglycemia and coma) can be fatal, and insulin insufficiency and hyperglycemia are intimately associated to the development of serious late diabetic complications over time.
Stem cells have received a lot of consideration recently, and they offer a lot of promise for treating and maybe curing a variety of human disorders, including diabetes. Somatic (sometimes known as "adult") vs. embryonic stem cells is the topic of communal discussion, both ethically & scientifically. Thus, stem cells from various sources could be used to produce β-cell mass responsible for the production of insulin thereby, providing a cure for all the types of diabetes.
Directed differentiation of therapeutically relevant functional b cells from stem cells persist a viable possibility for the future. In light of the recent debate over whether is the ideal undifferentiated cell foundation: somatic or embryonic, it seems clear that gladly available ES-cells are the ideal inoculum at this time. Such cells may be of alike interest if MAPC cultures become more widely used, as long because they preserve pluripotency. The guided distinction of a selected stem cell into ripe β-cells, which reflects the ontogenic course, poses a significant scientific problem.
References
- Madsen, O. D. (2005). Stem cells and diabetes treatment. APMIS, 113(11â??12), 858â??875.
[Crossref], [GoogleScholar], [Indexed]
- Berná, G., León-Quinto, T., Enseñat-Waser, R., Montanya, E., MartÃn, F., & Soria, B. (2001). Stem cells and diabetes. Biomedicine and Pharmacotherapy, 55(4), 206â??212.
[Crossref], [GoogleScholar], [Indexed]
- Zeeshan, N., Naveed, M., Asif, D. F., Ahsan, A., Abrar, M., & Ghafoor, S. (2017). Stem cell technology for the treatment of diabetes. Journal of Cell Science and Therapy, 08(2).
[Crossref], [GoogleScholar], [Indexed]
- Guo, T., & Hebrok, M. (2009). Stem cells to pancreatic β-cells: New sources for diabetes cell therapy. Endocrine Reviews, 30(3), 214â??227.
[Crossref], [GoogleScholar], [Indexed]
- Solis, M. A., Moreno Velásquez, I., Correa, R., & Huang, L. L. H. (2019). Stem cells as a potential therapy for diabetes mellitus: A call-to-action in Latin America. Diabetology and Metabolic Syndrome, 11, 20.
[Crossref], [GoogleScholar], [Indexed]
- Noguchi, H. (2007). Stem cells for the treatment of diabetes. Endocrine Journal, 54(1), 7â??16.
[Crossref], [GoogleScholar], [Indexed]
- Scadden, D. T. (2019). Stem cells. In Molecular Hematology.
- Zakrzewski, W., DobrzyÅ?ski, M., Szymonowicz, M., & Rybak, Z. (2019). Stem cells: Past, present, and future. Stem Cell Research and Therapy, 10(1), 68.
[Crossref], [GoogleScholar], [Indexed]
- Clarke, D. L., Johansson, C. B., Wilbertz, J., Veress, B., Nilsson, E., Karlström, H., Lendahl, U., & Frisén, J. (2000). Generalized potential of adult neural stem cells. Science, 288(5471), 1660â??1663.
[Crossref], [GoogleScholar], [Indexed]
- Stem celltherapy for diabetes. (2010). [Crossref], [GoogleScholar], [Indexed]
- Dang, L. T.-T., Phan, N. K., & Truong, K. D. (2017). Mesenchymal stem cells for diabetes mellitus treatment: New advances. BiomedicalResearch and Therapy, 4(1).
[Crossref], [GoogleScholar], [Indexed]
- Bonner-Weir, S., Toschi, E., Inada, A., Reitz, P., Fonseca, S. Y., Aye, T., & Sharma, A. (2004). The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatric Diabetes, 5 Suppl. 2, 16â??22.
[Crossref], [GoogleScholar], [Indexed]
Author Info
Tripti Arora1*, Namrata Arya2, Jayanand3 and Manju4
1Department of Pharmaceutical Chemistry, SGT University, Gurugram (HR), India2Department of Biotechnology , Sanskriti University, Mathura, Uttar Pradesh, India
3School of Biomedical Engineering, Shobhit Institute of Engineering and Technology (Deemed to be Univ, India
4Department of Biotechnology, RIMT University, Mandi Gobindgarh, Punjab, India
Received: 02-May-2022, Manuscript No. JRMDS-22-58460; , Pre QC No. JRMDS-22-58460(PQ); Editor assigned: 04-May-2022, Pre QC No. JRMDS-22-58460(PQ); Reviewed: 14-May-2022, QC No. JRMDS-22-58460; Revised: 18-May-2022, Manuscript No. JRMDS-22-58460(R); Published: 25-Jun-2022