Research - (2022) Volume 10, Issue 2
Study of Cardiac Changes in Patients with Iron Deficiency Anaemia and its Correlation
Vimlesh Patidar, Ashish Sharma, Mustafa MalvI*, Ajay Adhikari and Ajay Prakash Tripathi
*Correspondence: Mustafa MalvI, Department of Medicine, RDGMC, Ujjain, India, Email:
Abstract
Introduction: Iron deficiency is the commonest nutritional deficiency worldwide; it is the most common type of microcytic anemia. Aim: To study the cardiac profile in patients with iron deficiency anemia and to evaluate in detail the electrocardiography and echocardiographic abnormalities in patients with iron deficiency anemia. Methods: An observational study in patients with iron deficiency anemia aged >18 years. All diagnosed cases underwent electrocardiography and 2D-echocardiography studies, and statistical analysis was performed. Results: A total of 140 patients with iron deficiency anaemia were enrolled in which females were 93 with a mean age of 37.34 ± 11.46 and males were 47 with a mean age of 37.9 ± 13.2. Out of all cases which turned out to be positive for electrocardiography showing ST-segment changes, majority 80.2% had ST-segment depression. In t wave morphology changes, 94.1% showed inversion. Among 45 cases of severe anemia, 44 had echocardiography findings suggestive of enlarged cardiac chambers whereas only 27 cases out of 95 in moderate anemia category showed similar findings. There was statistically significant association with severity of Iron deficiency anemia with abnormality in echocardiography parameters like left Ventricular mass, left ventricular internal dimension-systole, left ventricular internal dimension-diastole, right ventricular internal dimension-systole with significant 2 tailed Pearson Correlation coefficient p<0.01 Conclusion: Our study found that majority of the patients with Iron deficiency anaemia is having electrocardiographic and echocardiographic changes. Cardiovascular complications of anaemia can be easily diagnosed with these investigations which ultimately help in making necessary plan for appropriate treatment.
Keywords
Anaemia, Electrocardiography, Echocardiography, Ventricular hypertrophy
Introduction
Iron deficiency is the commonest nutritional deficiency worldwide, affecting more than one-third of the population [1]. Although not commonly acknowledged, iron deficiency adversely affects the biological functions of the body and limits the survival of humans at every complexity level. In the last decade, anemia was recognized as an important comorbid factor in heart failure, a factor limiting physical activity, responsible for a poor quality of life, and a predictor of unfavourable outcomes. Iron deficiency was hypothesized to be the cause of erythropoietin resistance in heart failure which could be responsible for the unsatisfactory effects of erythropoietin therapy in heart failure [2]. The erythropoietin receptor is widely distributed in the cardiovascular system, including endothelial cells, smooth muscle cells and cardiomyocytes and preclinical studies have established erythropoietin to be a pleiotropic cytokine with anti-apoptotic activity and tissue-protective actions in the cardiovascular system, early studies in heart failure patients with anemia suggest that erythropoietin therapy is safe and effective in reducing left ventricular hypertrophy, enhancing exercise performance and increasing ejection fraction. Most of the available literature have studied the effects of chronic anemia of any etiology on the cardiac function and have use M mode parameters for the same [3,4]. There are numerous studies on heart failure in iron deficiency anemia but there is lack of sufficient data regarding left ventricular mass cavity dilation/ejection fraction, wall thickness and volume in iron deficiency anemia. Hence, we would like to emphasize on these parameters based on clinical and echocardiographic findings in iron deficiency anemia for early detection of heart failure.
Methodology
This observational study was conducted at R.D.Gardi Medical College Ujjain, India from November 2017 to May 2019 . A total of 140 patients who was diagnosed with Iron deficiency anaemia were recruited. A written informed consent was obtained from all the participants. The study was conducted according to Good Clinical Practice and the Declaration of Helsinki. Patient identity was always kept confidential and was approved by the Institutional Ethics Committee.
Inclusion criteria (All must be satisfied)
Any patient above 18 years of age attending Medicine OPD or admitted in wards.
Moderate (Hb 7-9.9) or severe (Hb<7) iron deficiency anemia.
Exclusion criteria (Anyone)
Patients of chronic renal failure, chronic liver disease, anemia in pregnancy, dimorphic anemia and other cardiac diseases like ischemic heart disease, rheumatic heart disease.
Patients not willing to sign informed consent for the study.
Anemia due to any other pathophysiology other than Iron deficiency.
Patients in which a clear diagnosis of Iron deficiency was not established after investigations.
Study variables
Iron deficiency anemia profile including complete blood picture, peripheral smear study, RBC indices (MCV, MCH, MCHC), serum iron (cut off values in microgram/dl–37.0 for females, 49.0 for males), serum ferritin (cut off values in ng/ml–6.24 for females <50yrs, 11.1 for females >50yrs, 17.9 for males) serum transferrin (cut off value in microgram/dl - >400 ) levels were done. Further all study subjects underwent electrocardiography (STsegment, T-wave changes) and echocardiography (LVIDs, LVIDd, RVIDs, RVIDd, LV mass, LA(d) ) studies to correlate with severity of iron deficiency anemia.
Statistical analysis
Analysis was done using SPSS data sheet version 23. Frequency tables and measures of central tendency (mean) and measures of dispersion (Standard Deviation) were calculated. Correlation was assessed using the chisquare test for comparing mean of different group independent sample t-test and ANOVA were applied. Karl Pearson correlation coefficient was calculated for measuring linear relationship between Hb level and other echocardiograhy variables (LVIDs, LVIDd, RVIDs, RVIDd, LV mass, LA(d)). P<0.05 was considered as significant.
Results
Descriptive
In our study, a total of 140 patients with iron deficiency anaemia were enrolled in which females were 93 with a mean age of 37.34 ± 11.46 and males were 47 with a mean age of 37.9 ± 13.2 (Figure 1).
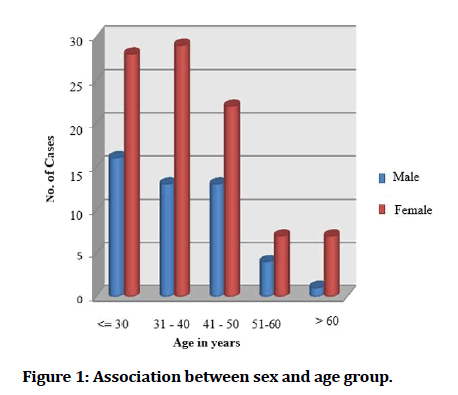
Figure 1: Association between sex and age group.
Majority of cases had moderate anemia, that is hemoglobin between 7.0-9.9 gm/dl. (32%) and rest had severe anemia that is hemoglobin below 7.0 gm/ dl (68%).
ECG changes
Majority of cases of Severe anemia are associated with ECG changes, there was significant association between severity of hemoglobin levels and ECG abnormalities (Figure 2).
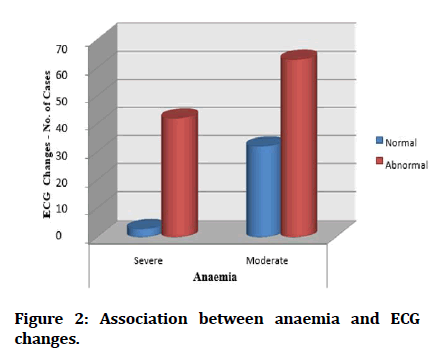
Figure 2: Association between anaemia and ECG changes.
Similarly, out of all cases which turned out to be positive for ECG showing ST-segment changes, majority that is 80.2% had ST-segment depression in their ECG (Figure 3).
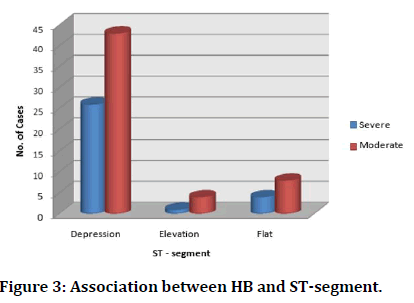
Figure 3: Association between HB and ST-segment.
In t wave morphology changes, inversion was the commonest in the study group, amounting Upto 94.1%.
Echocardiographic parameters
Among the 45 cases of severe anemia, 44 cases had ECHO findings suggestive of enlarged cardiac chambers whereas only 27 cases out of 95 in moderate anemia category showed similar findings.
There was statistically significant association with severity of Iron deficiency anemia with abnormality in ECHO parameters like LVIDs, LVIDd, RVIDs, RVIDd, LV mass, LA(d), with significant 2 tailed Pearson Correlation coefficient p<0.01 (Table 1).
| Variables | Statistical analysis | Values |
|---|---|---|
| AGE | Pearson Correlation | -0.243** |
| Sig. (2-tailed) | 0.004 | |
| N | 140 | |
| Hear Rate | Pearson Correlation | -0.411** |
| Sig. (2-tailed) | 0.001 | |
| N | 140 | |
| LVIDs | Pearson Correlation | -0.879** |
| Sig. (2-tailed) | 0.001 | |
| N | 140 | |
| LVIDd | Pearson Correlation | -0.792** |
| Sig. (2-tailed) | 0.001 | |
| N | 140 | |
| RVIDd | Pearson Correlation | -0.754** |
| Sig. (2-tailed) | 0.001 | |
| N | 140 | |
| LV Mass | Pearson Correlation | -0.738** |
| Sig. (2-tailed) | 0.001 | |
| N | 140 | |
| LA | Pearson Correlation | -0.799** |
| Sig. (2-tailed) | 0.001 | |
| N | 140 | |
| LV Mass- Left Ventricular mass, LVIDs-Left ventricular internal dimension-systole, LVIDd-Left ventricular internal dimension-diastole, RVIDs-Right ventricular internal dimension-systole, RVIDd-Right ventricular internal dimension-diastole, LA –Left atrium. | ||
Table 1: Correlation table for hemoglobin and different studied variables.
Discussion
Iron deficiency is the most common nutritional deficiency in developed and developing regions of the world. Approximately 10% of adult men and 50% of adult women in India have iron deficiency, and it is the most common cause of anaemia in our country [1]. Females are more predominantly affected with iron deficiency anaemia (IDA). There are several causes of IDA but factors contributing for IDA in females predominantly are blood loss due to menstruation, poor intake, malabsorption, and pregnancy related factors [5].
All patients with haemoglobin ≤ 9.9 gms were included in the study to determine the changes in cardiovascular system. All patients with low haemoglobin, with low serum iron and serum ferritin were included. However, the effects on cardiovascular system, is due to haemoglobin or low serum iron could not be determined. Since iron per se has several biological effects other than haemoglobin synthesis.
Nikitha Hegde et al in their study stated that the pathogenesis of the cardiomyopathy associated with anaemia has not been ascertained. It is unclear whether the anaemia itself contributes to the development of heart failure. Theoretically, severe anaemia leads to inadequate oxygen delivery to tissues, which in the heart could cause myocyte dysfunction. Additional diseasespecific factors may also contribute, including microinfarctions due to coronary vascular disease, and reduced myocyte iron stores in iron- deficiency anaemia [6].
The effects on cardiovascular system due to IDA was evaluated using electrocardiography (ECG) and 2D Echocardiography (ECHO). In our study, we correlated haemoglobin levels with ECG changes pertaining to ST-T segment, T wave and QT interval and volume overload changes by 2D Echocardiography. Changes seen in our study were ST segment depression and T wave flattening and inversion. Earlier studies have reported decreased QRS amplitude, T-wave flattening and minor degrees of atrioventricular conduction disturbances [7]. A study has also noted accentuation of T wave, appearances of Q wave in lead III, diminution, and enlargement of QRS complex [8]. But later many studies have reported changes in ECG like ST segment depression, flat or inverted T waves, but without corresponding changes in QRS complex [9-12]. The total prevalence of electrocardiographic changes in 140 patients was 75%, which was similar to high incidence of electrocardiographic changes of 62% in 183 patients reported by Mohit Khatri et al. [13] No changes in P wavelength of PR interval were found in our study and atrial extrasystole, atrial tachycardia, atrial fibrillation were not recorded.
Among the moderate anaemia group, we had 43(45.2%) who had ST segment depression and 4(4.21%) who had ST elevation and 8(8.42%) who had flat ST segment. There were 31(32.6%) who had T wave inversion and only 1 patient with tall T wave and 2 who had flat T wave. Among the severe anaemia group, we had 26 (57.7%) who have ST depression and 1 who had ST elevation and 4(8.8%) who had flat ST segment. There were 17 (37.7%) who had T wave inversion. The study by Neha H. Pandya colleagues [14] also had similar findings ECG abnormality as the current study. In ST segment changes were present in Lead I, II, III, avF, V4 -V6 and T wave changes were present in Lead V4 - V6. They also found 17 cases which had ST segment changes and the association of anaemia with ST segment changes was significant and further 8 cases having T wave abnormality which was found to be significant [14]. One of the ECG changes noted in our study was Left Ventricular Hypertrophy (LVH), indicating cardiac enlargement. Cardiac enlargement without other etiologies has been observed more frequently in patients with anaemia, particularly in patients with low Hb [15]. In anaemia there is combination of increased heart rate and stroke volume, to increase cardiac output, which in turn improves oxygen delivery. To accommodate this greater output, there is an increase in LV chamber size, both systolic and diastolic [16,17].
Echocardiographic parameters
Dimensions and volumes: The LVIDs in anemic patients were significantly higher compared to non-anemic population. Left ventricular end systolic volume and systolic radius/thickness ratio was also significantly increased in anemia [18-20]. Compared to non-anemic population in the present study, the anemic patients had significant increase in LVIDd. These findings reflect the changes in end diastolic volume in severe anemia and are suggestive of a chronic volume overload state, a known feature of chronic severe anemia.
The RVIDs and RVIDd was also increased in severe anemic patients. The septal thickness in systole and diastole did not show any significant changes. This represents the increase in preload, which is seen in chronic severe anemia. This finding suggests a role for the Frank Starling mechanism in the hyperdynamic state of chronic anemia [21,22].
The LV mass is significantly increased in patients with severe anemia (P<0.001). The mean LV mass was 253.76 gm ± 10.13 in cases of severe anemia and 181.20 gm ± 34.63 in cases of moderate anemia which is suggestive of hypertrophy. Trivedi et al [23] studied left ventricular mass in normal Indian population and found that the left ventricular mass in men was found to be 124 ± 32gm in males whereas in women it was 93 ± 37 gm. The increased LV mass reflects a hypertrophic response to chronic volume overload state.
Evaluation of systolic function: The percentage of fractional shortening and ejection fraction did not show significant differences. This finding in conjunction with increased end diastolic volume suggests that Starlings forces play an important role in the compensatory mechanisms seen in chronic severe anemia [9,24]. In patients with chronic severe anemia the increased preload and a decreased afterload (decreased blood pressure, hyperkinetic circulatory state) are the basic compensatory mechanisms. Due to these changes, the indices of left ventricular function are set at a higher level in the compensated state. Decompensation, therefore, probably occurs at a higher level of these indices as compared to normal individuals.
The resting heart can withstand acute severe isovolumic anemia with haemoglobin levels as low as 5 g/dl, without evidence of inadequate tissue oxygenation. The transition from a high- output (compensated) cardiac state to a state of LV dysfunction (decompensated) appears to begin at a haemoglobin level of approximately 7 g/dl in the iron deficient patient. As the haemoglobin level drops further, so does the LV function. In a study of irondeficient subjects (adolescents and adults) with a mean hemoglobin level of 5g/dL, 27% had CHF [25]. In our study, multiple linear regression analysis between haemoglobin levels and various ECHO parameters studied showed that with every 1gm% fall in haemoglobin the LV mass increased by 13.94 and chances of this occurring was 54.49% which is statistically significant. Similarly, LVIDs, LVIDd, RVIDs, RVIDd and LA(d) increased by 0.195, 0.255, 0.141, 0.148, 0.287 respectively.
Limitations of the study
The sample size of current study was small. Only cases of severe and moderate anemia were studied, whereas cases of mild anemia were not included.
Conclusion
Iron deficiency anaemia is the most common preventable nutritional deficiency in developing counties like India. Cardiovascular complications of IDA can be easily diagnosed with ECG and ECHO. In our study we found majority of the patients with IDA are having ECG and ECHO changes. Severe IDA leads to increase in Left Ventricular mass, left ventricular internal dimensionsystole, left ventricular internal dimension-diastole, right ventricular internal dimension-systole, right ventricular internal dimension-diastole all suggestive of volume overload state. In cases of mild-to-moderate anemia, hemodynamic adaptations permit adequate cardiovascular compensation. The combination of increased heart rate and stroke volume increases cardiac output, which, in turn, improves oxygen delivery whereas in severe IDA with HB < 6.9 gm/dl. diastolic and systolic LV chamber sizes increase to accommodate this greater output. In multiple logistic regression analysis, each 1g/dl decrease in haemoglobin was associated with 8% increase in risk of LV hypertrophy. Our study gives an idea on various ECG and ECHO changes in IDA patients. This study also helps in making necessary plan to diagnose cardiovascular complications of IDA with the help of ECG and ECHO and which helps in treatment planning. Further detailed studies are warranted to determine if decreased haemoglobin in IDA or decreased serum iron has diversified consequence on cardiovascular system.
References
- McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr 2009; 12:444-54.
- Opasich C, Cazzola M, Scelsi L, et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J 2005; 26:2232-2237.
- Al-Biltagi M, Tolba O, Elshanshory M, et al. Atrial function and glutathione in children with iron deficiency anemia-tanta-Egypt-2012. Int Blood Res Rev 2013; 1:72-86.
- Alvares JF, Oak JL, Pathare AV. Evaluation of cardiac function in iron deficiency anemia before and after total dose iron therapy. J Assoc Physicians India 2000; 48:204.
- Thankachan P, Muthayya S, Walczyk T, et al. An analysis of the etiology of anemia and iron deficiency in young women of low socioeconomic status in Bangalore, India. Food Nutr Bull 2007; 28s:328-336.
- Hegde N, Rich MW, Gayomali C. The cardiomyopathy of iron deficiency. Tex Heart Inst J 2006; 33:340-344.
- Porter WB. Heart changes and physiologic adjustments in hookworm anaemia. Am Heart J 1937; 13:550.
- Szekely P. Electrocardiographic findings in anaemia. Br Heart J 1940; 2:1-8.
- Hussein MAA. Relationship between anemia and diastolic dysfunction of the heart. Medical J Babylon 2012; 9:166-181.
- GV S, PK S, Herur A, et al. Correlation between haemoglobin level and electrocardiographic (ECG) findings in anaemia: A cross-sectional study. J Clin of Diagn Res 2014; 8:BC04-BC06.
- Wintrobe MM. The cardiovascular system in anaemia: With a note on the particular abnormalities in sickle cell anaemia. Blood 1946; 1:121-8.
- Gonzales-de-cassio A, Sanchez-Medal L, Smyth JF. Electrocardiographic modifications in anaemia. Am Heart J 1964; 67:166. Indexed at, Google Scholar, Cross Ref
- Khatri M, Deokar V, Patel J, et al. Study of electrocardiographic changes in mild, moderate and severe anaemia in a tertiary care hospital. Int J Contemp Med Res 2018; 5:9-13.
- Pandya NH, Desai KS, Naik S, et al. Effects of mild, moderate and severe anaemia on ECG. Indian J Applied Basic Med Sci 2011; 13:1-5.
- Wintrobe MM. The cardiovascular system in anaemia: With a note on the particular abnormalities in sickle cell anaemia. Blood 1946; 1:121-8.
- Georgieva Z, Georgieva M. Compensatory and adaptive changes in microcirculation and left ventricular function of patients with chronic iron deficiency anaemia. Clin Hemorheol Microcirc 1997; 17:21-30.
- Hayashi R, Ogawa S, Watanabe Z, et al. Cardiovascular function before and after iron therapy by echocardiography in patients with iron deficiency anaemia. Pediatr Int 1999; 41:13-7.
- Hunter A. The heart in anemia. Quart J Med 1945; 14:107.
- Varat MV, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J 1972; 83:415-426.
- Sanghvi LM, Sharma R, Misra SN. Cardiovascular disturbances in chronic severe anemia. Circulation 1957; 15:373-378.
- Bahl VK, Malhotra OP, Kumar D, et al. Noninvasive assessment of systolic and diastolic left ventricular function in patients with chronic severe anemia: A combined M-mode, two-dimensional, and doppler echocardiographic study. Am Heart J 1992; 124:1516-23. Indexed at, Google Scholar, Cross Ref
- Aessopos A, Deftereos S, Farmakis D, et al. Cardiovascular adaptation to chronic anemia in the elderly: An echocardio graphic study. Clin Invest Med 2004; 27:265-273.
- Trivedi SK, Gupta OP, Jain AP, et al. Left ventricular mass in normal Indian population. Indian Heart J 1991; 43:155-159.
- Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: Diagnosis, prognosis, and measurements of diastolic function. Circulation 2002; 105:1387-1393.
- Alvares JF, Oak JL, Pathare AV. Evaluation of cardiac function in iron deficiency anemia before and after total dose iron therapy. J Assoc Physicians India 2000; 48:204-206.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Vimlesh Patidar, Ashish Sharma, Mustafa MalvI*, Ajay Adhikari and Ajay Prakash Tripathi
Department of Medicine, RDGMC, Ujjain, IndiaCitation: Vimlesh Patidar, Ashish Sharma, Mustafa Malvi, Ajay Adhikari, Ajay Prakash Tripathi, Study of Cardiac Changes in Patients with Iron Deficiency Anaemia and its Correlation, J Res Med Dent Sci, 2022, 10(2): 788-793
Received: 29-Jan-2022, Manuscript No. JRMDS-22-55012; , Pre QC No. JRMDS-22-55012 (PQ); Editor assigned: 31-Jan-2022, Pre QC No. JRMDS-22-55012 (PQ); Reviewed: 14-Feb-2022, QC No. JRMDS-22-55012; Revised: 18-Feb-2022, Manuscript No. JRMDS-22-55012 (R); Published: 25-Feb-2022
