Research - (2022) Volume 10, Issue 6
Study of Maternal and Neonatal Outcome
*Correspondence: G Jayalakshmi, Department of Obstetrics and Gynaecology, Sri Lakshmi Narayana Institute of Medical Sciences Affiliated to Bharath Institute of Higher Education and Research, India, Email:
Abstract
In the present work, we intend to examine the characteristics of early against prolonged cord clamping on maternal- and neonatal-effect. Primary consequences were assessment of maternal blood-loss intra-partum, neonatal packed cell volume and serum bilirubin at 72 hours after delivery. The average maternal pre-labor packed cell volume (PCV)–33.13, and post-partum PCV- 32.65 were similar across groups and was not statistically significant. PCV difference across groups was not statistically significant. Moreover, our results showed that DCC has no effect on the length of the third trimester of pregnancy or the requirement for manual placenta-removal.
Keywords
Pregnancy, Umbilical Cord, Clamping
Introduction
The human umbilical contraction and cutting is one of the distinctive properties of pregnancy and delivery, with many treatments offering benefits and drawbacks. The umbilical cord must be clamped and cut in order to avoid maternal loss of blood and to enable the newborn to be taken from the woman for rescue. In the first few months after birth, the placenta may provide considerable transfusions to the baby. A term child held 10 cm below the region of the uterine increases its intravascular capacity by 32 percent on average during first three minutes of life [1-3]. In young infants, postponing human umbilical cutting elevates blood levels at pregnancy and increases iron stores over the first several months following birth, perhaps enhancing children's health. Longer umbilical cord cutting in premature infants is associated with enhanced transitional circulatory, improved red blood cell volume growth, fewer blood transfusions, and a decreased risk of necrotizing-enterocolitis and intraventricularhaemorrhage [4,5]. There is a modest increase in the occurrence of jaundice in grown infants who have had their umbilical cord clamped for an extended period of time, necessitating photo-therapy. As a consequence, obstetrician–gynecologists and healthcare practitioners should double-check that postponed umbilical cord cutting precautions for newborns are in effect. As per the 2015 ILCOR scientific report and other review articles, anaemia, hypotension, respiratory failure, enteritis, retinopathy, and right ventricular hemorrhage of any degree are all linked to decreased cord cutting [6-9].
Materials and Methods
Study period
June 2019-February 2021.
Sample size
The representative sample was 200, which was obtained using a procedure for comparing two proportions, and the projected sample group for each category was 100.
Inclusion criteria
Females who have delivered a baby to a term newborn through vaginal delivery.
Exclusion criteria
9 Women who have delivered to a preterm neonates.
9 HIV affected mothers.
9 Multiple pregnancies.
9 RH incompatibility mothers.
9 IUGR babies.
9 Fetus with Congenital anomalies.
Statistical analysis
IBM SPSS statistics.v20 programme has been used for statistical analysis. The Chi-square test was employed to examine categorical data. The Student unpaired T-test and the Man Whitney U test have been employed to examine quantitative variables.
Results
The average motherhood age in groups A and B was 27.0 2.6 and 27.1 2.8, accordingly. In groups A and B, 55% of the respondents were primigravida, but in both groups A and B, 45 percent of respondents were multigravida. The B positive blood type was shown to contribute to 74 percent and 76 percent of the group sessions, respectfully (Figure 1).
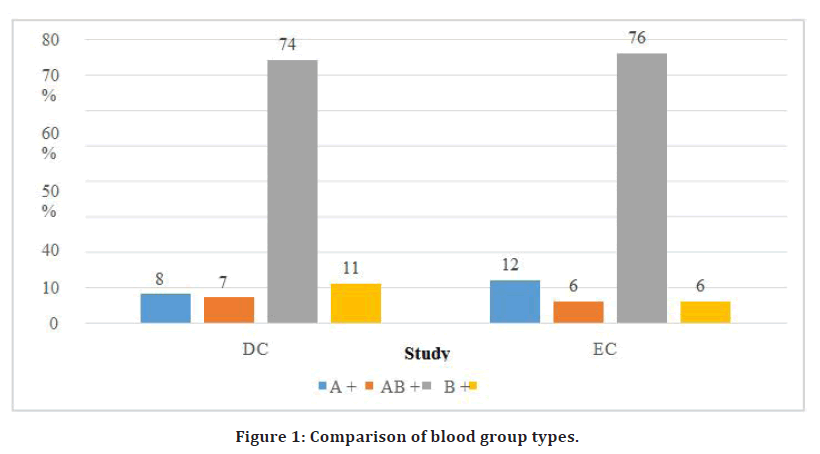
Figure 1. Comparison of blood group types.
DCC newborns had identical immediate neonatal results as newborns in the other group in terms of birth weight. ECC newborns weighed 2847.9 ± 28 grams on average, while DCC infants weighed 2852.28 ± 25 grams on average. In both ECC and DCC groups, the majority of the newborns were all in the 2500-3000 gram grouping (Figure 2).
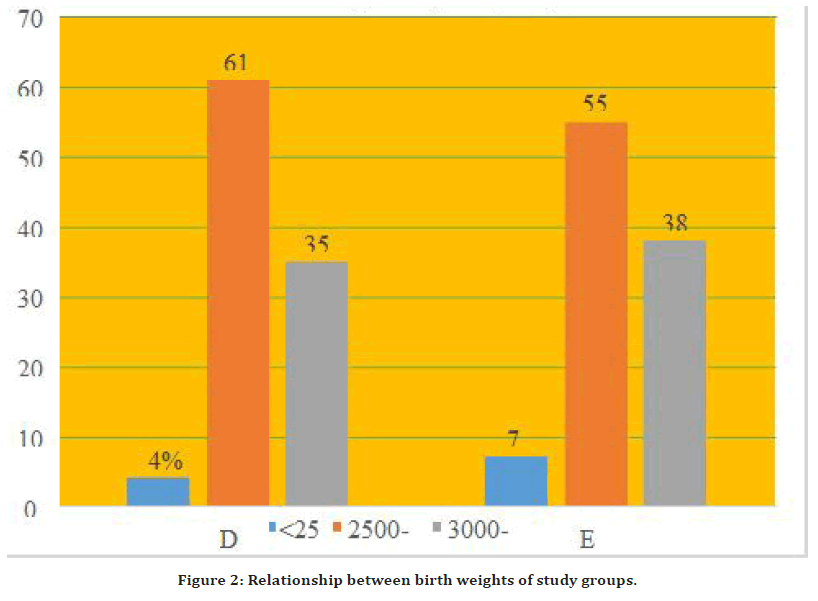
Figure 2. Relationship between birth weights of study groups.
The ECC team had a gestational age of 38.47 0.87 weeks, while the DCC group had a gestational age of 38.61 0.7 weeks. In the ECC arm, 75% of babies all between the ages of 38 and 39 weeks, while in the DCC arm, 88 percent of infants were between the ages of 38 and 39 weeks. Both research arms had similar APGAR scores at one minute and five minutes (Figure 3).
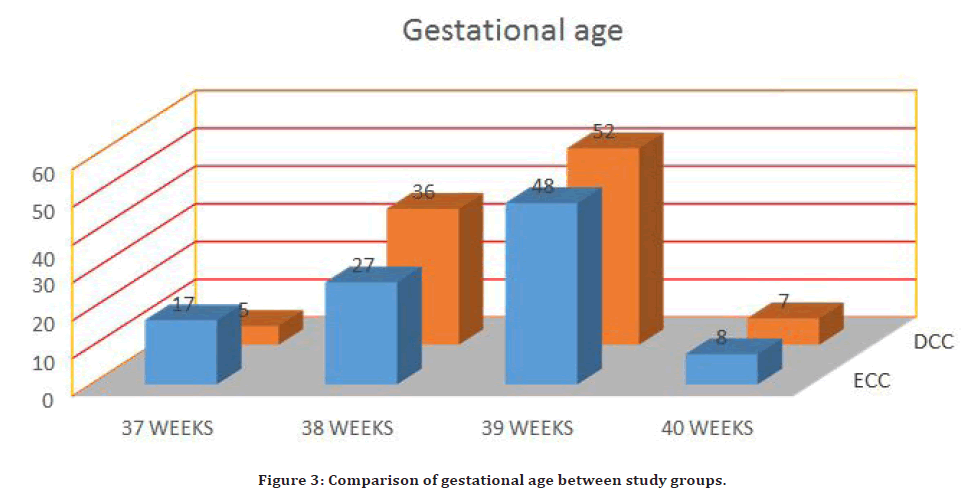
Figure 3. Comparison of gestational age between study groups.
The pre-labour PCV of the woman and the post-partum PCV of the woman were not clinically important (p=0.17 and 0.5, respectfully). Extended cord cutting is not linked with an increased incidence of pregnant blood loss or postpartum haemorrhage, according to this investigation (PCV difference: p=0.2). Despite the inclusion of the PCV variation to improve the accuracy of the anticipated loss of blood, no significant variations between the testing arms were found. This is in line with previous research [1,2,5-16]. In a Cochrane review of 15 randomized clinical trials involving a total of 3911 new - borns, McDonald et al. (2013) showed no significant association in postpartum haemorrhage while ECC and DCC were examined (Figure 4).
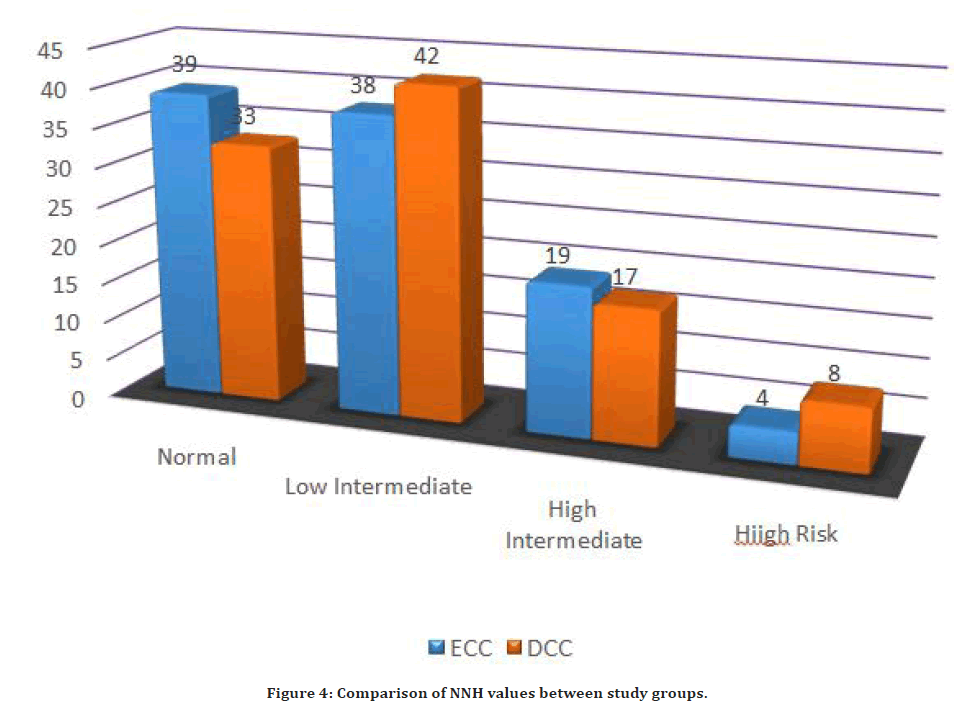
Figure 4. Comparison of NNH values between study groups.
The DCC sample had four infants with polycythemia, but none of the newborns in either category required an exchanged transfusion. Similarly, there was no significant difference in the rate of polycythemia between the 2 categories.
Discussion
In our investigation, the average motherhood was 27 years in both the ECC and DCC groups. Jombo et al (7) performed a randomized open study in which the average maternal age was 27 years in the ECC group and 28 years in the DCC group. In both the DCC and ECC groups, the percentage of the respondents was primigravida (55 percent). In several researches, the prevalence of primigravida has been found.
In our investigation, 12 percent of the moms in group A (ECC) had A blood type and 76 percent had "B blood group," with the remaining 12 percent shared by "AB" and "O" blood groups. Likewise, the majority of the moms in the DCC group had the blood group "B," whereas 8% had the blood group "A," and 11% had the blood group "O." In our research, we discovered that 58 (58%) of the DCC respondents had mild anaemia. Individuals in the ECC group experienced mild anaemia 46 percent of the time and moderate anaemia 1 percent of the time. Furthermore, all groups' mean maternal haemoglobin levels before childbirth were 11.04 0.91 and 10.88 0.73, correspondingly [13].
The DCC group had a mean body mass of 2852.28 0.7 gramme, whereas the ECC group had a mean birth weight of 2847.9 0.87 gramme, and the mean distinction was statistically insignificant (P value 0.210). 4 (4%) of DCC newborns weighed less than 2500 gm, 61 (61%) of DCC babies weighed between 2500 and 2999 gm, and 35 (35%) of DCC babies weighed between 3000 and 3999 gm. 7 (7%) of ECC newborns weighed less than 2500 gm, 55 (55%) of ECC babies weighed between 2500 and 2999 gm, and 38 (38%) of ECC babies weighed between 3000 and 3999 gm. The difference in neonate low birth weight proportions between group sessions was not statistically significant [14]. This discrepancy can be attributable to VLBW newborns, who had a large weight difference after cord clamping was delayed. Farrar et al. compared the birth weights of DCC and ECC newborns that were weighed with the placental undamaged and found that DCC babies had a higher birth weight [15-17].
In the DCC subgroup, 5% of females were 37 weeks pregnant, 36% of females were 38 weeks pregnant, 52% of females were 39 weeks pregnant, and 7% of females were 40 weeks pregnant. In the ECC group, 17% of women were in their 37th week, 27% were in their 38th week, 48% were in their 39th week, and 8% were in their 40th week. The variation in gestational age proportions between both the study groups was statistically significant (P value 0.045). Although the mean birth weight for group B (DCC) is larger than for group A (ECC), this difference is not statically important because the average birth weight in our environment is 3.1 kg, which is the same for both groups [18,19]. The mean PCV difference of DCC group was 1.09 ± 1.22 % and ECC group was 1.32 ± 1.3 %, and the mean difference between two groups was statistically not significant (P value 0.206) [21,22]. In our study, 2 neonates had hematocrit greater than 65% in the ECC group (20). DCC did not enhance the risk of maternal PPH, the duration of the phase of labor, or the requirement for manual placenta removal, according to the result of this research. Additionally, neonatal haemoglobin, hematocrit, and RBCs are all much higher [23-27].
Conclusion
Our analysis revealed that DCC raises the PCV of infants at 72 hours after birth, which stayed larger in the DCC group when compared to the ECC group, with no significant risks of post-partum haemorrhage or neonatal-hyperbilirubinemia. Moreover, our findings stated that DCC had no effect on the length of the phase of labor or the requirement for manual-placenta exclusion. It also demonstrated that it raised neonatal haemoglobin, hematocrit, and RBCs considerably. As a result, it is recommended that delaying cord clamping is benefited rather than harmful to both the mother and the neonate.
References
- ACOG. Timing of the umbilical cord clamping at birth. Committee Opinion NO. 543. 2012; 120:1522-1526.
- Hutton EK, Hassan ES. Late versus early clamping of the umbilical in full term neonates: Systematic review and meta–analysis of controlled trials. JAMA 2007; 297:1241.
- Elgzar WT, Ibrahim HA, Elkhateeb HH. Effects of deferred versus early umbilical cord clamping on maternal and neonatal outcomes. Am J Nurs 2017; 5:115-128.
- https://www.rcog.org.uk/en/guidelines-research-services/guidelines/sip14/
- https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/prevention-and-management-of-postpartum-haemorrhage-green-top-guideline-no-52/
- Mc Donald SJ. Effect of timing of umbilical cord of term infants on maternal and fetal outcomes. Cochrane Data Base Systemic Rev 2008; 2.
- Jombo SE, Eifediyi RA. Effects of delayed umbilical cord clamping on maternal and neonatal outcomes in IRRUA: A randomized controlled (Open Label) trial. Int J Obstetr Gynaecol Res 2017; 4:491-517
- Raju TN. Timing of umbilical cord clamping after birth for optimizing placental transfusion. Curr Opinion Pediatr 2013; 25:180-187.
- Bolstridge J, Bell T, Dean B, et al. A quality improvement initiative for delayed umbilical cord clamping in very low-birthweight infants. BMC Pediatr 2016; 16:1-5.
- Wangwe PJ, Balandya B. Accuracy in diagnosis of PPH using visual estimation of blood loss versus change in haematocrit in a tertiary hospital in Tanzania. Tanzania J Health Res 2012; 14.
- Gharoro EP, Enabudoso EJ. Relationship between visually estimated blood loss at delivery and postpartum change in haematocrit. J Obstetr Gynaecol 2009; 29:517-520.
- Ogundeyi MM, Olarewaju DM, Njokanma OF. Haematological profile of apparently healthy term babies age one day, three days and 6 weeks delivered in Sagamu, Nigeria. Nig J Paediatr 2011; 38:125-130.
- Weeks A. Umbilical cord clamping after birth. Br Med J 2007; 335:312.
- Hutchon DRJ. Immediate or early cord clamping vs. delayed cord clamping. J Obstetr Gynaecol 2012; 32:724-729.
- Boere I, Roest AA, Wallace E, et al. Umbilical blood flow patterns directly after birth before delayed cord clamping. Arch Dis Childhood Fetal Neonatal 2015; 100:121-125.
- Dipak NK, Nanavati RN, Kabra NK, et al. Effect of delayed cord clamping on hematocrit, and thermal and hemodynamic stability in preterm neonates: A randomized controlled trial. Indian Pediatr 2017; 54:112-115.
- Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatr 2004; 114:297-316.
- Backes CH, Huang H, Cua CL, et al. Early versus delayed umbilical cord clamping in infants with congenital heart disease: A pilot, randomized, controlled trial. J Perinatol 2015; 35:826-831.
- Chidre YV, Chirumamilla V. Impact of early versus delayed umbilical cord clamping on post-partum blood loss: A randomized controlled trial. Int J Reprod Contraception Obstetr Gynecol 2015; 4:1103-1109.
- Korkut S, Oğuz Y, Bozkaya D, et al. Evaluation of the effects of delayed cord clamping in infants of diabetic mothers. Am J Perinatol 2021; 38:242-247.
- Kugelman A, Borenstein-Levin L, Riskin A, et al. Immediate versus delayed umbilical cord clamping in premature neonates born< 35 weeks: A prospective, randomized, controlled study. Am J Perinatol 2007; 24:307-315.
- Katheria AC, Lakshminrusimha S, Rabe H, et al. Placental transfusion: A review. J Perinatol 2017; 37:105-111.
- Garabedian C, Rakza T, Drumez E, et al. Benefits of delayed cord clamping in red blood cell alloimmunization. Pediatr 2016; 137.
- Raju TN. Timing of umbilical cord clamping after birth for optimizing placental transfusion. Curr Opinion Pediatr 2013 1;25:180-187.
- Aladangady N, McHugh S, Aitchison TC, et al. Infants' blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatr 2006; 117:93-98.
- Prendiville WJ, Harding JE, Elbourne DR, et al. The Bristol third stage trial: Active versus physiological management of third stage of labour. Br Med J 1988; 297:1295-1300.
- Eichenbaum‐Pikser G, Zasloff JS. Delayed clamping of the umbilical cord: A review with implications for practice. J Midwifery Women's Health 2009; 54:321-326.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Department of Obstetrics and Gynaecology, Sri Lakshmi Narayana Institute of Medical Sciences Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaReceived: 16-May-2022, Manuscript No. JRMDS-22-66693; , Pre QC No. JRMDS-22-66693 (PQ); Editor assigned: 18-May-2022, Pre QC No. JRMDS-22-66693 (PQ); Reviewed: 02-Jun-2022, QC No. JRMDS-22-66693; Revised: 07-Jun-2022, Manuscript No. JRMDS-22-66693 (R); Published: 14-Jun-2022
