Research - (2020) Advances in Dental Surgery
Study the Estradiol Add-on Benefit with Oral Ovarian Induction Medication in Subfertile Patients with PCOS or Unexplained Subfertility
Median Atia Alkhalaf Almamou1* and Asim Kurjak2
*Correspondence: Median Atia Alkhalaf Almamou, Sarajevo Medical School, University of Sarajevo School of Science and Technology (SSST), Sarajevo, Bosnia and Herzegovina, Email:
Abstract
Background: Estrogen might help when there is a decrease in estrogen quantity or increase in estrogen receptor antagonization. These effects occur when we use oral agents for ovulation induction. The use of estradiol add-on needs more research to assess its value in ovulation induction. Doppler studies might be beneficial in the management of subfertility. Methods: This study is a randomized prospective study. We recruited subfertile Patients with PCOS or unexplained subfertility from the infertility unit at Al-Moosa Specialist Hospital in eastern province in the Kingdom of Saudi Arabia. We randomized patients into four groups: clomiphene group, letrozole group, clomiphene with estrogen add-on group or letrozole with estrogen add-on group. We did a uterine and sub-endometrial doppler study on the hCG trigger day and on the mid-luteal phase day. We also studied endometrial thickness, pattern and vascularity zones. Results: When adding estradiol, there was a high cancellation rate for both clomiphene and letrozole groups (27.8%, 46.67% respectively) with a p-value of (0.007, 0.001 respectively). Conclusions: When using oral ovulation induction medications, it is not wise to add estradiol from day nine till ovulation day as it will be associated with a high cancellation rate.
Keywords
Estrogen, PCOS, Unexplained subfertility, Clomiphene, Letrozole
Introduction
It is a common practice to use clomiphene citrate and letrozole as ovulation inducers. We think that letrozole is better than clomiphene due to the weight of the studies that suggest that letrozole should be the first oral ovulationinducing treatment [1]. We think that doppler would change our view to subfertility when we have sufficient studies to justify its vital role in infertility management. Doppler of uterine arteries and sub-endometrial arteries can predict successful pregnancy. Clomiphene bind to estrogen receptors, causing mainly antagonizing effects [2]. So, it will cause pseudo estrogen deficiency by occupying the estrogen receptors [3]. Side effects of clomiphene are thinning of the endometrium [4] and decreased quality and quantity of cervical mucus [5] due to pseudo deficiency of estrogen. On the other hand, letrozole causes a real decrease in estrogen due to its suppression of androgen transformation to estrogen. Both pseudo and real estrogen deficiency will cause an increase in FSH and LH by positive hypothalamus feedback. Increased FSH and LH are responsible for the growth and maturation of follicles.
Add back of estrogen is a strategy to counteract the adverse clomiphene effect on the endometrium and cervical mucus. Also, in letrozole, it was tried to help to increase the success rate by increase the estrogen levels. Adding estradiol need more research to assess its benefits in ovulation induction.
Objective
We are aassessing the beneficiary of oestradiol add-back in oral subfertility ovulation induction agents.
Hypothesis
Oestradiol add-back increase pregnancy rate without affecting the quality of fertilization and implantation.
Materials and Methods
Selection of study subjects
Our study is a randomized comparative prospective study. All couples gave informed consent with detailed study information, and if they agree they will sign for that to guarantee their participation in our study. Also, we applied and took an ethical committee approval at Al Moosa Specialist Hospital. The IVF unit outpatient department was the source of the couples.
Inclusion criteria were patients in reproductive age, healthy pelvis organs and acceptance to participate. In contrast, exclusion criteria were: distorted pelvic organs, big leiomyomas (>4 cm), sub endometrial leiomyomas, primary or secondary ovarian failure (hypogonadism), male subfertility, Unmanaged Thyroid diseases or hyperprolactinemia, Non-responsive cases, cases with early ovulation (before triggering) and obesity.
We used the 2003 Modified Rotterdam consensus to diagnose PCOS using [6]. To avoid the inter-observer variability, we did all doppler sonography studies by the same doctor. We used a GE Voluson P80 ultrasound machine with a transvaginal probe of 5-9 MHz.
Randomization groups
Clomiphene with estrogen add-on group or letrozole with estrogen add-on group.
Group A: clomiphene induced group.
Group B: letrozole induced group.
Group C: clomiphene induced group with estrogen add-on.
Group D: letrozole induced group with estrogen add-on.
We gave 100 mg of clomiphene once daily from the third day of the cycle for five days. Letrozole was given with an amount of 5 mg once daily for five days starting from the third day of the cycle. In group C and D we gave 2 mg of oestradiol valerate twice daily form day 9 till trigger day.
Evaluation of study subjects
Trigger criteria were the follicular size of 18 mm or more. We measure follicular size using the mean of the right angles intersecting diameters [7]. Ten thousand units of Choriomon or Pregnyl used to trigger ovulation in the morning of the same day. We timed intercourse at 1 and 1.5 days from triggering.
If we found no follicle more than 12 mm at day 16 from the start of the period, we would cancel the cycle. We did a doppler study on the trigger day and the mid-luteal day.
Sub endometrial arteries studied at 1-2 mm distal from the endometrial outer edges. Uterine arteries studied on the lateral edges of the cervix. The minimum filter PRF was used (usually 1.3 for the uterine arteries and 0.3 for the sub endometrial arteries). We used the mean of both sides of the uterine arteries doppler indices.
We studied endometrial thickness, pattern and vascularity zones. We conducted an ultrasonographic assessment of the endometrial thickness, pattern, blood flow and endometrial scoring system, uterine artery, and sub endometrial arteries doppler indices done on the day of hCG and nine days after the day of hCG triggering. Measurement of the stripe of the endometrium was at the broadest part at the median plane, from side to side from the outer echogenic border.
We used Gonen and Casper in 1990 criteria for endometrial pattern [8]
Type A: high echogenic endometrium.
Type B: uterine muscle-like echogenicity.
Type C: multilayered high echogenic triple lines with hypo-echogenicity between the lines.
We recorded vascularity on the trigger day in 4 zones
Zone 1: vascularity is distal to external endometrial lines.
Zone 2: vascularity reaches external endometrial lines.
Zone 3: vascularity reaches the area between external and internal lines.
Zone 4: vascularity reaches the internal line.
On the mid-luteal phase day, we recorded two vascular zones
Zone 1: vascularity is distal to external endometrial lines.
Zone 2: vascularity reaches at least the external endometrial lines.
And finally, we calculate applebaum score [9]. This system includes
Endometrial thickness
≤ 7 millimeter=0.
7-9 millimeter=2.
10-13 millimeter=3.
≥ 14 millimeter=1.
Endometrial layering
Absent of layers=0.
Blurry 5 lines=1.
Clear 5 lines=3.
The endometrial waves
<3 wave movments in 60 seconds=0.
≥ 3 wave movments in 60 seconds=3.
Myometrial homogeneity
Inhomogenic=1.
homogenic=2.
Uterine artery PI
≥ 3.0=0.
2.5-2.99=0.
2.2-2.49=1.
≤ 2.19=2.
Endometrial zone 3 vasculairty
No vascilarity=0.
dispersed vascularity=2.
multi-spots vascularity=5.
Myometrial vascularity
Absent=0.
Present=2.
So, the maximum score 20.
We used 10 mg of Dydrogesterone (Duphaston) twice daily orally for 14 days, starting from day three after the trigger day. We did test for pregnancy 16 days after hCG triggering. We did a pelvic scan after four weeks to confirm embryo viability. We repeated the same steps (up to 2 successive cycles) in unsuccessful cycles if the couple agrees.
Data analysis
We used Excel and SPSS for data analysis and the G Power software for calculating the required sample size and power.
Results
Using G Power software, we calculated Fisher’s exact test sample size with control group proportion of .05, experimental group proportion of .4, two-sided α error of .05, the power value of 80% and an allocation ratio of 1. The calculated sample size was 48 cycles.
The calculated sample size for the point biserial correlation test was 52 cycles. We also used G Power software with an α error of .05, moderate effect size of .37 and an 80% power.
In our study, we used 54 cycles of ovulation induction; in each group, there were 27 cycles. There were differences in the mean BMI (p=0.153), and the mean age (p=0.109) but they were not significant in both groups (Table 1).
| Clomiphene citrate group | Letrozole group | |||||
|---|---|---|---|---|---|---|
| Mean | N | Std. Deviation | Mean | N | Std. Deviation | |
| AGE | 26.4 | 27 | 4.9 | 28.3 | 27 | 4.5 |
| BMI | 25.6 | 27 | 5.1 | 27.9 | 27 | 6.2 |
Table 1: Age and BMI with letrozole and clomiphene citrate groups.
There was a high cancellation rate when adding estradiol to clomiphene group. We performed Fisher’s Exact Test to examine if this cancellation rate is significant. We found sufficient evidence suggesting a moderate relationship with a p-value of 0.007 and a moderate phi test effect size of -0.433. The cancellation rate increased when adding estradiol to (27.8%) from 0% with the clomiphene group. On both trigger day and day of mid-luteal phase day, there was no sufficient evidence to suggest a difference after adding estradiol in terms of endometrial thickness, RI and PI of sub endometrial arteries, RI and PI of the uterine arteries, endometrial receptivity score system, endometrial pattern and endometrial vascularity with p values >0.05 (Tables 2 and 3).
+| Day of trigger | Protocol | Statistical Test | P | RS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Clomiphene group | Clomiphene+E2 group | ||||||||
| Mean | N | Std. Deviation | Mean | N | Std. Deviation | ||||
| Endometrial Thickness | 8.55 | 27 | 2.4 | 7.95 | 13 | 2.64 | Rank-biserial Spearman's correlation | 0.265 | NS |
| Endometrial receptivity score | 11.44 | 27 | 3.33 | 12.54 | 13 | 4.2 | Point-Biserial Pearson's Correlation | 0.377 | NS |
| Sub endometrial artery PI | 1.07 | 27 | 0.33 | 1.11 | 13 | 0.29 | Rank-biserial Spearman’s correlation | 0.495 | NS |
| Sub endometrial artery RI | 0.62 | 27 | 0.1 | 0.64 | 13 | 0.1 | Rank-biserial Spearman’s correlation | 0.425 | NS |
| Uterine Artery PI | 2.8 | 27 | 0.49 | 2.6 | 13 | 0.58 | Rank-biserial Spearman’s correlation | 0.298 | NS |
| Uterine Artery RI | 0.88 | 27 | 0.04 | 0.86 | 13 | 0.04 | Point-Biserial Pearson’s Correlation | 0.197 | NS |
Table 2: Clomiphene Group vs Clomiphene Group with Estradiol add-on (Correlations) on the trigger day.
| Mid-Luteal Phase day | Protocol | Std. Deviation | Statistical Test | P | RS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Clomiphene group | Clomiphene+E2 group | ||||||||
| Mean | N | Std. Deviation | Mean | N | |||||
| Endometrial Thickness | 12.16 | 27 | 2.89 | 11.02 | 13 | 3.84 | Rank-biserial Spearman's correlation | 0.355 | NS |
| Sub endometrial artery PI | 1.25 | 27 | 0.37 | 1.15 | 13 | 0.28 | Rank-biserial Spearman’s correlation | 0.325 | NS |
| Sub endometrial artery RI | 0.66 | 27 | 0.09 | 0.64 | 13 | 0.07 | Point-Biserial Pearson’s Correlation | 0.638 | NS |
| Uterine Artery PI | 2.63 | 27 | 0.41 | 2.75 | 13 | 0.57 | Rank-biserial Spearman’s correlation | 0.37 | NS |
| Uterine Artery RI | 0.86 | 27 | 0.03 | 0.87 | 13 | 0.05 | Point-Biserial Pearson’s Correlation | 0.325 | NS |
Table 3: Clomiphene Group vs Clomiphene Group with Estradiol add-on (Correlations) on the mid-luteal day.
When adding oestradiol to clomiphene there was: Increase in type C endometrium on the trigger day (92.3% vs 66.7%, p-value=0.124), increase in type A endometrium on the mid-luteal phase day (57.5% vs 48.1%, p-value=0.103) and decrease in zone 3 and zone 4 vascularity at the trigger day (61.5% vs 74.1%, p-value>0.05) and also decrease in zone 2 on the mid-luteal phase day (84.6% vs. 85.2%, p-value=1). however, there was no sufficient evidence that there was a difference regarding these parameters using Chi-Square test with p values more than 0.05 (Tables 4 and 5).
| Protocol | Statistical Test | P | Phi | ||||
|---|---|---|---|---|---|---|---|
| Clomiphene group | clomiphene+E2 GROUP | ||||||
| Endometrial Pattern | Pattern | % within Protocol | Pattern | % within Protocol | |||
| Type A | 3.70% | Type A | 0% | chi-square test “Fisher's Exact Test” | 1 | NS | |
| Type B | 29.60% | Type B | 7.70% | chi-square test “Fisher's Exact Test” | 0.226 | NS | |
| Type C | 66.70% | Type C | 92.30% | chi-square test “Fisher's Exact Test” | 0.124 | NS | |
| Endometrial vascularity | Zone | % within Protocol | Zone | % within Protocol | |||
| Zone 1 | 7.40% | Zone 1 | 0% | chi-square test “Fisher's Exact Test” | 1 | NS | |
| Zone 2 | 18.50% | Zone 2 | 38.50% | chi-square test “Fisher's Exact Test” | 0.246 | NS | |
| Zone 3 | 63% | Zone 3 | 38.50% | chi-square test “Fisher's Exact Test” | 0.185 | NS | |
| Zone 4 | 11.10% | Zone 4 | 23.10% | chi-square test “Fisher's Exact Test” | 0.37 | NS | |
Table 4: Clomiphene Group vs Clomiphene Group with Estradiol add-on (Fisher’s Exact Test) on trigger day.
| Protocol | Statistical Test | P | Phi | ||||
|---|---|---|---|---|---|---|---|
| Clomiphene group | Clomiphene + E2 group | ||||||
| Pattern | % within Protocol | Pattern | % within Protocol | ||||
| Endometrial Pattern | Type A | 48.10% | Type A | 76.90% | chi-square test “Fisher’s Exact Test” | 0.008 | 0.393 |
| Type B | 22.20% | Type B | 15.40% | chi-square test “Fisher's Exact Test” | 0.25 | NS | |
| Type C | 29.60% | Type C | 7.70% | chi-square test “Fisher's Exact Test” | 0.076 | NS | |
| Endometrial vascularity | Zone | % within Protocol | Zone | % within Protocol | |||
| Zone 1 | 14.80% | Zone 1 | 15.40% | chi-square test “Fisher's Exact Test” | 1 | NS | |
| Zone 2 | 85.20% | Zone 2 | 84.60% | chi-square test “Fisher's Exact Test” | |||
Table 5: Clomiphene group vs. Clomiphene group with estradiol add-on (Fisher’s Exact Test) on mid-luteal day.
When adding estradiol to letrozole, there was also a high cancellation rate, and when we performed a Fisher’s Exact Test, it confirms the high cancellation rate statistically when adding estradiol. The evidence was enough to confirm a robust negative relationship. The p-value for the two-sided Fisher’s Exact test was 0.001, with a strong negative Phi test effect size of -.528. The cancellation rate increased when adding estradiol to (46.67%) whereas the cancellation rate was only 3.57% in the letrozole group. There were eight cancelled cases: 4 cases for no response, 3 for early ovulation and 1 for risk of ovarian hyperstimulation syndrome.
The evidence was not sufficient to suggest that there was a difference in terms of pregnancy rate when adding estradiol with a p-value of 1. The pregnancy rate was 22.2% with letrozole group alone compared to 12.5% when adding estradiol to the letrozole group.
The evidence was not sufficient to suggest a difference when adding estradiol in terms of endometrial thickness, PI and RI of sub endometrial arteries on both the day of the trigger and the mid-luteal phase day, mean of PI of the uterine arteries on both the day of the trigger and the mid-luteal phase day, endometrial receptivity score system, endometrial pattern and endometrial vascularity with p values of more than 0.05.
The mean of uterine artery RI on the mid-luteal phase day when using letrozole alone was 0.85 (SD=0.04) vs. 0.88 (SD=0.03) when adding estradiol. There was evidence to suggest that uterine artery RI on the mid-luteal phase day when using letrozole alone was lower than when adding estradiol. There was a moderate correlation, rs=0.362, p=0.033 (Tables 6 and 7).
| Protocol | Statistical Test | P | RS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Letrozole group | Letrozole+E2 GROUP | ||||||||
| Mean | N | Std. Deviation | Mean | N | Std. Deviation | ||||
| Endometrial Thickness | 8.6 | 27 | 1.97 | 7.44 | 8 | 1.97 | Point-Biserial Pearson's Correlation | 0.155 | NS |
| Endometrial receptivity score | 12.37 | 27 | 3.01 | 12.88 | 8 | 4.02 | Rank-biserial Spearman's correlation | 0.289 | NS |
| Sub endometrial artery PI | 1.35 | 27 | 0.35 | 1.15 | 8 | 0.41 | Rank-biserial Spearman’s correlation | 0.154 | NS |
| Sub endometrial artery RI | 0.69 | 27 | 0.06 | 0.65 | 8 | 0.13 | Rank-biserial Spearman’s correlation | 0.268 | NS |
| Uterine Artery PI | 2.8 | 27 | 0.59 | 2.61 | 8 | 0.38 | Point-Biserial Pearson’s Correlation | 0.405 | NS |
| Uterine Artery RI | 0.87 | 27 | 0.05 | 0.87 | 8 | 0.04 | Rank-biserial Spearman’s correlation | 0.589 | NS |
Table 6: Letrozole Group vs Letrozole Group with Estradiol add-on (Correlations) on trigger day.
| Protocol | Statistical Test | P | RS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Letrozole group | Letrozole + E2 group | ||||||||
| Mean | N | Std. Deviation | Mean | N | Std. Deviation | ||||
| Endometrial Thickness | 11.47 | 27 | 3.23 | 11.26 | 8 | 3.32 | Point-Biserial Pearson's Correlation | 0.877 | NS |
| Sub endometrial artery PI | 1.28 | 27 | 0.33 | 1.05 | 8 | 0.22 | Point-Biserial Pearson’s Correlation | 0.076 | NS |
| Sub endometrial artery RI | 0.68 | 27 | 0.08 | 0.63 | 8 | 0.09 | Rank-biserial Spearman’s correlation | 0.191 | NS |
| Uterine Artery PI | 2.56 | 27 | 0.49 | 2.8 | 8 | 0.23 | Rank-biserial Spearman’s correlation | 0.154 | NS |
| Uterine Artery RI | 0.85 | 27 | 0.04 | 0.88 | 8 | 0.03 | Rank-biserial Spearman’s correlation | 0.033 | 0.362 |
Table 7: Letrozole Group vs Letrozole Group with Estradiol add-on (Correlations) on mid-luteal day.
Adding estradiol to letrozole causes more type c on the trigger day (87.5 vs77.8 with a p-value of 1), less type A endometrium on the midluteal phase day (75% vs 85.2% with a p-value of .602) and more vascularity at both the day of the trigger (Zone 3 or 4 was 75% vs74.1% with a p-value of 1) and the mid-luteal phase day (type 2 vascularity 87.5% vs 55.6 with a p-value of 0.21). However, there was no sufficient evidence that there was a difference regarding these parameters using Chi-Square test with p values more than 0.05 (Tables 8 and 9).
| Protocol | Statistical Test | P | Phi | ||||
|---|---|---|---|---|---|---|---|
| Letrozole group | Letrozole+E2 GROUP | ||||||
| Pattern | % within Protocol | Pattern | % within Protocol | ||||
| Endometrial Pattern | Type A | 7.40% | Type A | 0% | chi-square test “Fisher's Exact Test” | 1 | NS |
| Type B | 14.80% | Type B | 12.50% | chi-square test “Fisher's Exact Test” | 1 | NS | |
| Type C | 77.80% | Type C | 87.50% | chi-square test “Fisher's Exact Test” | 1 | NS | |
| Endometrial vascularity | Zone | % within Protocol | Zone | % within Protocol | |||
| Zone 1 | 7.40% | Zone 1 | 0% | chi-square test “Fisher's Exact Test” | 1 | NS | |
| Zone 2 | 18.50% | Zone 2 | 25% | chi-square test “Fisher's Exact Test” | 0.648 | NS | |
| Zone 3 | 63% | Zone 3 | 62.50% | chi-square test “Fisher's Exact Test” | 1 | NS | |
| Zone 4 | 11.10% | Zone 4 | 12.50% | chi-square test “Fisher's Exact Test” | 1 | NS | |
Table 8: Letrozole Group vs Letrozole Group with Estradiol add-on (Fisher’s Exact Test) on trigger day.
| Protocol | Statistical Test | P | Phi | ||||
|---|---|---|---|---|---|---|---|
| Letrozole group | letrozole + E2 group | ||||||
| Endometrial Pattern | Pattern | % within Protocol | Pattern | % within Protocol | |||
| Type A | 85.20% | Type A | 75% | chi-square test “Fisher’s Exact Test” | 0.6 | NS | |
| Type B | 7.40% | Type B | 12.50% | chi-square test “Fisher's Exact Test” | 0.553 | NS | |
| Type C | 7.40% | Type C | 12.50% | chi-square test “Fisher's Exact Test” | 0.553 | NS | |
| Endometrial vascularity | Zone | % within Protocol | Zone | % within Protocol | |||
| Zone 1 | 44.40% | Zone 1 | 12.50% | chi-square test “Fisher's Exact Test” | 0.21 | NS | |
| Zone 2 | 55.60% | Zone 2 | 87.50% | chi-square test “Fisher's Exact Test” | |||
Table 9: Letrozole Group vs Letrozole Group with Estradiol add-on (Fisher’s Exact Test) on the mid luteal day.
Finally, the total pregnancy rate was 18.52%. The pregnancy rate in the clomiphene group was 14.8% while it was 22.2% for letrozole group. However, the evidence was not sufficient to suggest a difference with a p-value of 0.728 (Figure 1).
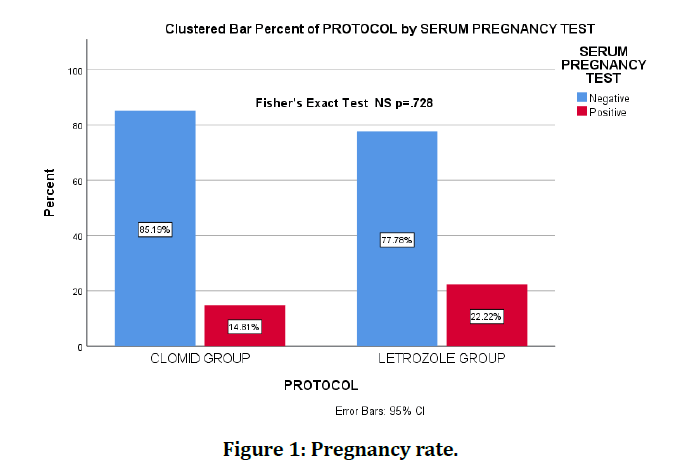
Figure 1: Pregnancy rate.
Discussion
Adding estradiol to clomiphene group was accompanied by a significant high cancellation rate of 27.8%.
The evidence was not sufficient to suggest a difference when adding estradiol in terms of endometrial thickness, sub endometrial arteries doppler indices, uterine arteries doppler indices, endometrial receptivity score system, endometrial pattern and endometrial vascularity neither on the trigger day nor on the mid-luteal phase day.
Interestingly, when adding estradiol to clomiphene group there was more type C endometrium on the trigger day, more type A endometrium on the mid-luteal phase day but less vascularity on both the day of the trigger and the mid-luteal phase day. However, there was no sufficient evidence that there was a difference regarding these parameters.
On the other hand, adding estradiol to letrozole group was associated with a significantly higher cancellation rate than adding it to the clomiphene group (46.67%).
When adding oestradiol to letrozole, there was no sufficient evidence to suggest a difference in terms of endometrial thickness, sub endometrial doppler indices, uterine arteries PI and endometrial receptivity score system, endometrial pattern and endometrial vascularity on both the trigger day and the mid-luteal phase day and also uterine artery RI on the trigger day.
The mean of uterine artery RI on the mid-luteal phase day was significantly higher when adding oestradiol.
Also, there was more type c on the trigger day and less type A endometrium on the mid-luteal phase day and more vascularity at both the day of the trigger day and the mid-luteal phase day. However, the evidence was not sufficient to suggest a difference.
These study does not encourage using estradiol to enhance oral ovulation induction.
Conclusion
When adding estradiol to clomiphene group, there was a high cancellation rate (27.8%) with a p-value of 0.007 and a moderate phi test effect size of -0.433. There is no sufficient evidence to suggest that there is an effect when adding estradiol other than increased cases with type A endometrium pattern on the mid-luteal phase day with a p-value of .008 and Phi effect of .393. When adding estradiol to letrozole group, there was a high cancellation rate (46.67%) with a p-value of 0.001 and a high phi test effect size of -0.528. There is no sufficient evidence to suggest that there is an effect when adding estradiol other than higher uterine artery RI at the midluteal phase day (p=0.033, rs=0.362).
The pregnancy success rate was: 18.52% for both groups, 22.2% for letrozole group, 14.81% for clomiphene group.
Acknowledgement
The authors would be keen on expressing gratitude to the members of the school of Science and Technology (SSST) university and the administration of Almoosa Specialist Hospital for their support towards making this research a success.
Ethical Considerations
We took consent of involvement in the research from every participating couple. Moreover, we also obtained the approval of the ethical and research Committee in Almoosa specialist hospital.
Funding
The authors bore the entire cost of the research and did not obtain any economic assistance from any organization.
Conflict of Interest
The authors state no conflicts of interest.
References
- Legro RS, Brzyski RG, Diamond MP, et al. Clomiphene versus letrozole for infertility in the polycystic ovary syndrome. New England J Med 2014; 371:119-129.
- Use of clomiphene citrate in women. Fertil Steril 2003; 80:1302–1308.
- Kettel LM, Roseff SJ, Berga SL, et al. Hypothalamic-pituitary-ovarian response to clomiphene citrate in women with polycystic ovary syndrome. Fertil Steril 1993; 59:532-8.
- Gadalla MA, Huang S, Wang R, et al. (2017). Effect of clomiphene citrate on ovulation, endometrial thickness, and live birth in anovulatory women: Systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017; 51:64–76.
- Thompson LA, Barratt CLR, Thornton SJ, et al. The effects of cyclofenil and clomiphene citrate on cervical mucus volume and receptivity over the periovulatory period. Fertil Steril 1993; 59:125–129.
- Revised 2003 consensus on polycystic ovary syndrome diagnostic criteria and long-term health risks related to it. Fertil Steril 2004; 81:19–25.
- Schild RL, Knobloch C, Dorn C, et al. Assessing receptivity of endometrium in an in vitro fertilization program by endometrial thickness, endometrial volume, spiral artery blood flow and uterine artery blood flow. Fertil Steril 2001; 75:361–366.
- Gonen Y, Casper RF. Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). In Vitro Fertilization Embryo Transfer J 1990; 7:146–152.
- Applebaum M. The uterine biophysical profile. Ultrasound Obstet Gynecol 1995; 5:67–68.
Author Info
Median Atia Alkhalaf Almamou1* and Asim Kurjak2
1Sarajevo Medical School, University of Sarajevo School of Science and Technology (SSST), Sarajevo, Bosnia and Herzegovina2IVF Unit, Obstetrics and gynaecology Department, Almoosa Specialist Hospital, Saudi Arabia
Citation: Median Atia Alkhalaf Almamou, Asim Kurjak, Study the Estradiol add-on Benefit with Oral Ovarian Induction Medication in Subfertile Patients with PCOS or Unexplained Subfertility, J Res Med Dent Sci, 2020, 8 (7): 57-63.
Received: 26-Sep-2020 Accepted: 13-Oct-2020 Published: 20-Oct-2020
