Review Article - (2023) Volume 11, Issue 1
Synthesis, Characterization and Evaluation of New Pyrazoline Derivatives Containing Sulfonamide Moiety as Anti-Microbial and Anti-inflammatory Agents
Zeyad D Najmuldeen* and Tagreed NA Omar
*Correspondence: Zeyad D Najmuldeen, Department of Pharmaceutical Chemistry, College of Pharmacy, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Nitrogen containing heterocyclic compounds has received considerable attention due to their wide range of pharmacological activity such as anti-bacterial, antifungal, antioxidant, antiviral, anticancer, anti-inflammatory, analgesic and anticonvulsant. New sulfonamide pyrazoline derivatives (Z1-6) have been synthesized, by four steps: The first step included synthesis of chalcone using cross aldol condensation (claisen schmidt), the second step included synthesis of 2-chloro-N-(4-sulfamoylphenyl) acetamide (S), the third step included the synthesis of 2-hydrazineyl-N-(4-sulfamoylphenyl) acetamide (SH), finally; the fourth step involve the synthesis of 2-(3,5-diphenyl-4,5-dihydro-1H-pyrazol-1-yl)-N-(4-sulfamoylphenyl) acetamide derivatives (Z1-6) the final compounds, the progress of reaction was monitored by the use of thin layer chromatography and the structures of the synthesized compounds were characterized by FT-IR and 1H-NMR spectroscopy and evaluated preliminarily for their anti-inflammatory and anti-microbial activities. All synthesized compounds were tested in vitro against gram positive, gram negative bacteria by using a well diffusion method in two different concentrations and the result, all compounds possess high to moderate anti-bacterial activity in high concentration, compound Z2 exert a high activity in both concentrations as well as some compounds show anti-fungal activity when tested against Candida albicans and compound Z4 has greater inhibition of the fungal growth when compared with fluconazole as reference. In vivo anti-inflammatory activity of the final compounds (Z1-6) was studied in rats using egg white induced edema method. All synthesized compound showed anti-inflammatory activity compared to the control group while compounds Z5, Z2 showed more anti-inflammatory activity compared with diclofenac as reference.
Keywords
Chalcones, Heterocyclic, Sulfonamide, Pyrazoline, Anti-bacterial, Anti-inflammatory
Introduction
Pyrazoline or dihydropyrazole is a five membered heterocyclic compound with chemical formula C3H6N2, which contain two nitrogen atoms adjacent to each other and only one endocyclic double bond; there are three different types of pyrazoline depending on the position the endocyclic double bond [1]. There are three isomers of pyrazoline and tautomerize one into another by heating and by acid catalysis (Figure 1) [2].
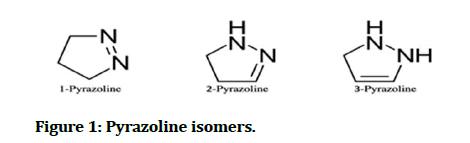
Figure 1: Pyrazoline isomers.
Due to high stability and variety of biological activity of 2- pyrazoline derivatives, it has a specific importance in pharmaceutical chemistry researches and drug discovery; it considered as potent medicinal scaffold to synthesis of new derivatives have anti-microbial, anti-oxidant, antidiabetic, anti-convulsant, anti-depressant, antiinflammatory, anti-cancer and mono amine oxidase inhibitor [3-10].
N-substituted 3,5-diphenyl pyrazoline derivatives show antibacterial activity against different bacteria. Phenyl substituted derivatives displayed a good activity against H. pylori strains. Previously studied derivatives, which containing the N-acetyl group and methoxy group at position 4 on the phenyl ring exhibited the greatest potential against metronidazole resistant H. pylori strains [11].
1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2- pyrazoline derivatives are showed a good antimicrobial activity when they are evaluated against E. coli, S. typhimurium, S. aureus, S. faecalis, B. cereus, A. hydrophila, C. albicans, C. glabrata(Figure 2)[12].
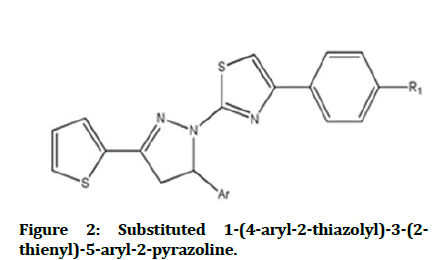
Figure 2: Substituted 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline.
Anti-inflammatory drugs can by classify to steroidal and Non-Steroidal Anti-inflammatory Drugs (NSAIDs) furthermore it can be subdivided in to COX1 and COX2 inhibitors depending on the selectivity of inhibitory effect on Cyclooxygenase enzyme (COX) subtypes [13].
Derivative of 2-pyrazolines were synthesized by reacting chalcones, with phenyl hydrazine possess different substituents in the presence of pyridine and ethanol activity of the synthesized compounds. They showed significant activity when compared to the different NSAIDs. For example, 3,4,5–tri-methoxyphenyl and 4- methoxyphenyl ring at the 5-position of the 2-pyrazoline ring showed the Figure 3 [14].
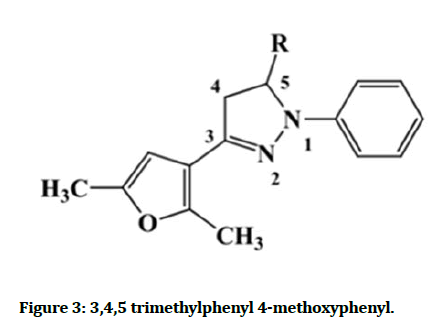
Figure 3: 3,4,5 trimethylphenyl 4-methoxyphenyl.
As a COX inhibitors, sulfonamide diaryl hetero cycle derivatives as well show selectivity toward COX-2 isozyme which is like COX-1 mediated the conversion of arachidonic acid to prostaglandins which are the key player in the inflammatory cascade, e.g. celecoxib and valdecoxib (Figure 4) [15].
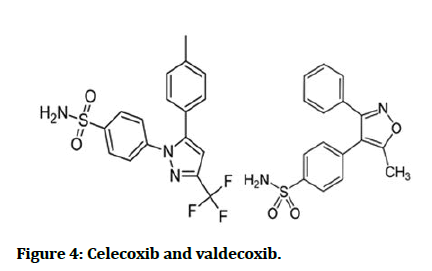
Figure 4: Celecoxib and valdecoxib.
Materials and Methods
The starting chemical substances and solvents used in this work where purchased from (Sigma-Aldrich, Fluka, TCI, Himedia), all solvents were used without further purification except further drying by using anhydrous magnesium sulphate. Thin Layer Chromatography (TLC) using type of silica gel used was 60 F254 manufactured by MERCK (Germany), TLC was used to ensure the purity of the synthesized intermediates, final compounds and to follow up the reactions progress. Visualization of all the synthesized compounds was done with aid of UV light apparatus. Chromatograms were eluted by different solvent systems.
Electro thermal melting point apparatus (stuart) and open capillary tubes were used to determine the melting points and the infrared spectra were performed using FTIR (IR affinity-1) spectrophotometer, shimadzu, Japan at University of Baghdad college of pharmacy. The 1H-NMR spectra were performed by using BRUKER and varian spectrometers, 500 MHz with (TMS) Tetra Methyl Saline as internal solvent and DMSO as solvent for samples, (δ=ppm) represent the chemical shift.
Chemical synthesis
General method of chalcones derivatives synthesis
Chalcone derivatives were synthesized by dissolving benzaldehyde derivatives (0.01 mol) in (15 ml) ETOH 99% in 250 ml round bottom flask and then acetophenone (1.17 ml, 0.01 mol) was added. The reaction mixture was kept in an ice bath then (3-5 ml) of 30% NaOH solution was gradually added over a period of 5 minutes and the reaction mixture was kept stirring overnight at 25° C [16]. The reaction mixture diluted with ice cold water filtered using buchner funnel, the solid product was further purified by recrystallization from ETOH 99% [17].
1,3-diphenylprop-2-en-1-one (compound CH1) (C15H12O)
Description: Pale yellow crystals. Yield: 94 %. MP: 55-57oC. RF=0.54, FT-IR: 3028, 3059 (Stretching vibration of CH aromatic), 1658 (C=O), 1600, 1573 (C=C aromatic).
3-(4-nitrophenyl)-1-phenylprop-2-en-1-one (compound CH2) (C15H11NO3)
Description: Mustard like yellow powder. Yield: 76%. MP: 155-156°C. RF=0.61. FT-IR: 3105, -3708 (stretching vibration of CH aromatic), 1658 (C=O), 1600 1573 (C=C aromatic), 1512 (NO2 asymmetric stretching), 1334 (NO2 symmetric stretching).
3-(4-Hydroxyphenyl)-1-phenylprop-2-en-1-one (compound CH3) (C15H12O2)
Description: Bright yellow crystals. Yield: 68%. MP: 184-185°C. RF=0.93. FT-IR: 3205 (OH stretching vibration), 3167,3070 (stretching vibration of CH aromatic), 1647 (C=O), 1597,-1554,-1508 (C=C aromatic), 1346 (OH bending), 1215 (C-O).
3-(4-methoxyphenyl)-1-phenylprop-2-en-1-one (compound CH4) (C16H14O2)
Description: Light yellow crystals. Yield: 95%. MP: 74-75°C. RF=0.55. FT-IR: 3059, 3012 (stretching vibration of CH aromatic), 2943 (asymmetric CH stretching of CH3), 2843 (symmetric CH stretching of CH3), 1654 (C=O), 1593,1573,-1508 (C=C aromatic), 1261(C-OCH3).
3-(4-Chlorophenyl)-1-phenylprop-2-en-1-one (compound CH5) (C15H11OCl)
Description: Off white powder. Yield: 99%. MP: 113-115°C. RF=0.5. FT-IR: 3059,-3032 (stretching vibration of CH aromatic), 1654 (C=O), 1600, 1589, 2562 (C=C aromatic), 821 (C-Cl).
3-(4-(Dimethyl amino) phenyl)-1-phenylprop-2-en-1- one (compound CH6) (C17H17NO)
Description: Bright orange crystal. Yield: 87%. MP: 110-111°C. RF=0.7. FT-IR: 308693,-3051 (stretching vibration of CH aromatic), 2908 (asymmetric CH stretching of CH3), 2858 (symmetric CH stretching of CH3), 1647 (C=O), 1597, 1558 (C=C aromatic), 1157 (C-N).
Synthesis of 2-chloro-N-(4-sulfamoylphenyl) acetamide (intermediate)
Chloro acetyl chloride (2.16 ml, 0.278 mol) was added drop wise with continuous stirring to a solution of sulfanilamide (4 g, 0.232 mol) in DMF (25 ml) in ice bath. After the completion of dropping, the mixture was stirred for 4 h and left over night, then poured into the iced water (125 ml). Precipitated product was filtered, washed with water, dried and recrystallized from ETOH 99% [18].
2-chloro-N-(4-sulfamoylphenyl) acetamide (compound S) (C8H9ClN2O3S)
Description: Pure white powder. Yield: 97%. MP: 117°C. RF=0.68. FT-IR: 3321(asymmetric stretching vibration of NH),-3299 (symmetric stretching vibration of NH), 3205 (Stretching vibration of NH amide), 3136, -3089 (stretching vibration of CH aromatic), 1685 (C=O amide), 1597, -1543, -1500 (C=C aromatic), 1338, -1315, -1149 (O=S=O stretching vibration), 833 (C-Cl). 1HNMR (500 MHz, DMSO-d6, ppm): 4.31 (2H, s, protons of methylene group ∝ to carbonyl), 7.29 (2H, s, protons of amino of sulfonamide), 7.74-7.76 (2H, d, aromatic protons ortho to amide), 7.78-7.8 (2H, d, aromatic protons ortho to sulfonamide), 10.63 (1H, s, NH proton of amide group).
Synthesis of 2-hydrazineyl-N-(4-sulfamoylphenyl) acetamide (intermediate)
Excess of hydrazine hydrate (5 ml) was added in portions into a solution of 2-chloro-N-(4-sulfamoylphenyl) acetamide (S) in ETOH 99%. The reaction mixture was stirred for 15 hours at 25°C. The precipitated product was filtered and washed with cold ETOH 99%. The product was dried and recrystallized from EtOH [19].
2-hydrazineyl-N-(4-sulfamoyl phenyl) acetamide (compound SH) (C8H12N4O3S)
Description: bright white powder. Yield: 54%. MP: 208-210oC. RF=0.21. FT-IR: 3429 (asymmetric stretching vibration of NH2 hydrazine), -3336 (asymmetric stretching vibration of NH2 sulfonamide), 3305 (stretching vibration of N-H hydrazine), 3248 (stretching vibration of NH amide), -168951 (C=O amide), 1573 (C=C aromatic), 1311, -1153 (O=S=O stretching vibration), 825 (C-Cl). 1HNMR (500 MHz, DMSO-d6, ppm): 3.4 (2H, s, protons of methylene group ∝ to carbonyl), 3.83 -3.86 (1H, dd, proton proton of secondary amine), 4.41–4.42 (2H, dd, protons of primary amine), 7.25 (2H, s, protons of amino of sulfonamide), 7.70–7.78 (2H, m, aromatic protons ortho to amide), 7.8–7.89 (2H, m, aromatic protons ortho to sulfonamide), 10.21 (1H, s, NH proton of amide group).
Synthesis of 2-(3,5-diphenyl-4,5-dihydro-1Hpyrazol- 1-yl)-N-(4-sulfamoylphenyl) acetamide derivative (final compounds)
Hydrazine derivative (SH) (2.44 g), (0.01 mol) was dissolved in ETOH 99% (20 ml) then add the appropriate aromatic chalcone derivative (0.01 mol) CH1-6 with catalytic amount of glacial acetic acid, the solution was refluxed for 6-8 hrs. After cool down the reaction mixture, the formed precipitate was filtered, washed, dried and crystallized from ETOH 99% to give the pyrazoline derivatives final compounds [20].
2-(3,5-diphenyl-4,5-dihydro-1H-pyrazol-1-yl)-N-(4- sulfamoylphenyl) acetamide (compound Z1) (C23H22N4O3S)
Description: Off white powder. Yield: 42%. MP: 200-202°C. RF=0.62. FT-IR: 3356, 3336 (stretching vibration of NH2), -3267 (stretching vibration of NH amide), 3136 (stretching vibration of CH aromatic), 1693 (C=O amide), 1637 (C=N 2-pyrazoline), 1597 (C=C aromatic), 1307, 1153 (O=S=O stretching vibration). 1HNMR (500 MHz, DMSO-d6, ppm): 3.16–3.24 and 3.62– 3.83 (2H, dd, protons of methylene pyrazoline), 3.83-4.19 (2H, dd, protons of methylene ∝ to carbonyl), 4.53-4.68 (1H, dd, proton of methine of pyrazoline), 7.29 (2H, s, protons of amino of sulfonamide), 7.08–7.79 (10H, m, aromatic protons ring A,B), 7.82–7.85 (2H, m, aromatic protons ortho to amide), 7.78-7.8 (2H, m, aromatic protons ortho to sulfonamide), 10.46 (1H, s, NH proton of amide group).
2-(5-(4-nitrophenyl)-3-phenyl-4,5-dihydro-1H-pyrazol- 1-yl)-N-(4-sulfamoylphenyl) acetamide (compound Z2)(C23H21N5O5S)
Description: Light yellow powder. Yield: 48%. MP: 238-239°C. RF=0.5. FT-IR: 3325, 3290 (stretching vibration of NH2), -3209 (stretching vibration of NsH amide), 3074 (stretching vibration of CH aromatic), 1685 (C=O amide), 1634 (C=N 2- pyrazoline), 1597_(C=C aromatic), 1519, 1315 (stretching vibration NO2), 1307, 1153 (O=S=O stretching vibration). 1HNMR (500 MHz, DMSO-d6, ppm): 3.18–3.21 and 3.45–3.51 (2H, dd, protons of methylene of pyrazoline), 3.80–4.19 (2H, dd, protons of methylene ∝ to carbonyl), 5.14–5.22 (1H, dd, proton of methine of pyrazoline), 7.17– 7.20 (2H, m aromatic protons meta to NO2), 7.23 (2H, s, Protons of amino of sulfonamide), 7.28–7.75 (5H, m, aromatic protons ring A), 7.77–7.80 (2H, m, aromatic protons ortho to amide), 7.88–7.89 (2H, m, aromatic protons ortho to sulfonamide), 8.13–8.32 (2H, m aromatic protons ortho to NO2, 10.46 (1H, s, NH proton of amide group).
2-(5-(4-hydroxyphenyl)-3-phenyl-4,5-dihydro-1H pyrazol-1-yl)-N-(4-sulfamoylphenyl) (compound Z3)(C23H22N4O4S)
Description: Light yellow powder. Yield: 68%. MP: 245-246°C. RF=0.6. FT-IR: 3321, 3294 (stretching vibration of NH2), -3205 (stretching vibration of NH amide), 3136 (stretching vibration of CH aromatic), 1685 (C=O amide), 1626 (C=N 2-pyrazoline), 1597_(C=C aromatic), 1519, 1315 (stretching vibration NO2), 1327, 1153 (O=S=O stretching vibration). 1HNMR (500 MHz, DMSO-d6, ppm): 2.95–3.01 and 3.43–3.47 (2H, dd, protons of methylene of pyrazoline), 3.72–3.86 (2H, dd, protons of methylene ∝ to carbonyl), 4.66–4.71 (1H, dd, proton of methine of pyrazoline), 6.71– 7.73 (2H, m aromatic protons ortho to OH), 7.17–7.19 (2H, m aromatic protons meta to OH), 7.20 (2H, s, protons of amino of sulfonamide), 7.37–7.48 (3H, m, aromatic protons meta and para to pyrazoline of ring A), 7.65–7.68 (aromatic protons ortho to pyrazoline ring A) and aromatic protons ortho to amide ring C), 7.70–7.73 (4H, m, aromatic protons ortho to sulfonamide), 9.74 (1H, s, phenolic protons), 10.23 (1H, s, NH proton of amide group).
2-(5-(4-methoxyphenyl)-3-phenyl-4,5-dihydro-1Hpyrazol- 1-yl)-N-(4-sulfamoylphenyl) acetamide (compound Z4) (C24H24N4O4S)
Description: bright white powder. Yield: 75%. MP: 225-227°C. RF=0.6. FT-IR: 3348, 3313 (stretching vibration of NH2),-3248 (stretching vibration of NH amide), 3128, 3101 (stretching vibration of CH aromatic), 1678 (C=O amide), 1622 (C=N 2-pyrazoline), 1589 (C=C aromatic), 1323, 1149 (O=S=O stretching vibration). 1HNMR (500 MHz, DMSO-d6, ppm): 2.95-3.01 and 3.56– 3.62 (2H, dd, protons of methylene of pyrazoline ), 3.72– 3.86 (2H, dd, protons of methylene ∝ to carbonyl), 3.73 (3H, s, protons of OCH3), 4.66 –4.71 (1H, dd, proton of methine of pyrazoline), 6.91-6.94 (2H, m aromatic protons ortho to OCH3), 7.34–7.43 (2H, m aromatic protons meta to OCH3), 7.36 (2H, s, protons of amino of sulfonamide), 7.46–7.65 (5H, m, aromatic protons of ring A), 7.65–7.68 (aromatic protons ortho to pyrazoline ring A), 7.66–7.67 (2H, m, aromatic protons ortho to amide), 7.71–7.72 (4H, m, aromatic protons ortho to sulfonamide), 10.29 (1H, s, NH proton of amide group) (Figure 5).
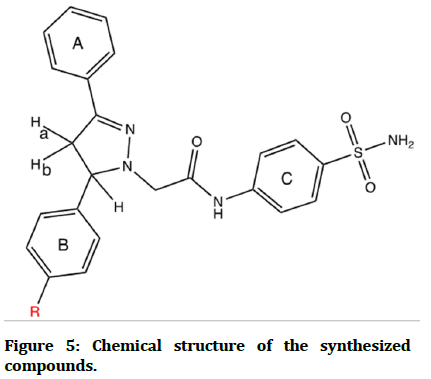
Figure 5: Chemical structure of the synthesized compounds.
2-(5-(4-chlorophenyl)-3-phenyl-4,5-dihydro-1Hpyrazol- 1-yl)-N-(4-sulfamoylphenyl) acetamide (compound Z5) (C23H21ClN4O3S)
Description: Creamy white powder. Yield: 81%. MP: 230-232°C. RF=0.55. FT-IR: 3356, 3336 (stretching vibration of NH2), 3267 (stretching vibration of NH amide), 3116 (stretching vibration of CH aromatic), 1687 (C=O amide), 1631 (C=N 2-pyrazoline), 1593 (C=C aromatic), 1307-1153 (O=S=O stretching vibration), 829 (C-Cl) 1HNMR (500 MHz, DMSO-d6, ppm): 3.12–3.19 and 3.43–3.47 (2H, dd, protons of methylene pyrazoline), 3.60–3.89 (2H, dd, protons of methylene ∝ to carbonyl), 4.67–4.72 (1H, dd, proton of methine of pyrazoline), 7.17– 7.19 (2H, m aromatic protons meta to Cl), 7.24 (2H, s, protons of amino of sulfonamide), 7.34–7.38 (2H, m aromatic protons ortho to Cl), 7.36–7.61 (5H, m, aromatic protons ring A), 7.65–7.67 (2H, m, aromatic protons ortho to amide), 7.70–7.72 (2H, m, aromatic protons ortho to sulfonamide), 10.46 (1H, s, NH proton of amide group).
2-(5-(4-dimethyaminophenyl)-3-phenyl-4,5-dihydro -1H-pyrazol-1-yl)-N-(4-sulfamoylphenyl) acetamide (compound Z6) (C25H27N5O3S)
Description: Light red powder. Yield: 94%. MP: 214-215°C. RF=0.54. FT-IR: 3340 (stretching vibration of NH2), 3267 (stretching vibration of NH amide), 3120 (CH stretching vibration diethylamino), 3082 (stretching vibration of CH aromatic), 1683 (C=O amide), 1625 (C=N 2- pyrazoline), 1593 (C=C aromatic), 1300-1149 (O=S=O stretching vibration). 1HNMR (500 MHz, DMSO-d6, ppm): 2.91 (6H, s, protons of dimethyl amino), 2.96– 3.00 and 3.02-3.05 (2H, dd, protons of methylene of pyrazoline ), 3.92–4.19 (2H, dd, protons of methylene ∝ to carbonyl), 4.38 (1H, dd, proton of methine of pyrazoline), 6.67–6.69 (2H, m aromatic protons ortho to dimethyl amino), 6.76–6.78 (2H, m aromatic protons meta to OCH3), 7.26 (2H, s, protons of amino of sulfonamide), 7.28–7.34 (3H, m, aromatic protons meta and para to pyrazoline of ring A), 7.64–7.77 (4H, m, aromatic protons ortho to pyrazoline ring A and aromatic protons ortho to amide ring C), 7.79–7.82 (2H, m, aromatic protons ortho to sulfonamide), 10.27 (1H, s, NH proton of amide group).
Pharmacological studies
Antimicrobial activity: The antimicrobial activity of the target compounds was done in college of biological science, university of Baghdad. A preliminary antibacterial activity has been performed according to well diffusion method. In vitro study used one fungus (Candida albicans) and four bacteria (Staphylococcus aureus, Streptococcus pyogenes), as a gram positive bacteria and (Pseudomonas aeruginosa, Escherichia coli), as gram negative bacteria). Ciprofloxacin, ceftriaxone and ampicillin, was used as references for antibacterial activity.
Fluconazole was used as a reference for anti-fungal activity. The synthesized compounds were studied by maximum concentration of (1000 μg/mL) and (250 μg/ mL) in Dimethyl Sulfoxide (DMSO). The zones of inhibitions were measured after 24 h incubation at 37°C.
Anti-inflammatory studies: In vivo acute antiinflammatory effects of the new chemically synthesized compounds (Z1-6) are evaluated by egg white induced paw edema, to study of sulfonamide pyrazoline derivatives with standard diclofenac sodium a well-known COX inhibitor. Eight groups of albino rats (both sex) weighing (160 ± 10 g) were supplied by the animal house of the university of Fallujah, college of veterinary medicine and were put in the same location under standardized conditions.
Animals were fed commercial chaw and had free access to water. Animals divided into eight groups (each group consist of 6 rats).
All rats’ groups were injected intra peritoneally after thirty minutes of Subcutaneous injection (SC) of 0.05 ml undiluted egg white into the planter side of the left hind paw of the rats.
Vernier calliper was used to measure paw thickness at seven time intervals of (0, 30, 60, 120, 180, 240 and 300 min), zero time was the time were tested compounds, standard and control administered by intra peritoneal route. The doses of the newly synthesized compounds were calculated by applying the general formula.

All data collected were manifested as the mean ± SD (standard deviation) and results were analyzed for statistical significance using student T test (two sample assuming equal variances) for comparison between mean values. While comparisons between different groups were made using ANOVA: Two factors without replication. P value (probability) of less than 0.05 was regarded as a significant value.
Results and Discussion
Chemistry
Chalcones (CH1-6) were synthesized by claisen schmitt condensation through reacting aromatic aldehydes with acetophenone in ethanol with NaOH 30% solution. Chalcones were characterized FTIR that shows appearance of C=O stretching at (1658-1647).
First intermediate (S) where synthesized by the reaction of sulfanilamide and chloroacetyl chloride in DMF, amide formation were characterized by FTIR that shows appearance of C=O stretching at (1685), 1HNMR by appearance of 10.63 (1H, s, NH Proton of amide group).
Second intermediate (SH) where synthesized by the reaction of first intermediate (S) with hydrazine hydrate in ethanol, characterized by FTIR by appearance of all bands related hydrazinyl amino groups at (3429), (3305) and 1HNMR by the shielding of the protons of methylene group ∝ to carbonyl from (4.31) ppm to (3.41) ppm as a clue of hydrazinyl intermediate formation.
The final compounds (Z1-6) was synthesized by refluxing chalcones (CH1-6) with Second intermediate (SH) in ethanol using catalytic amount glacial acetic (Figure 6 and Table 1).
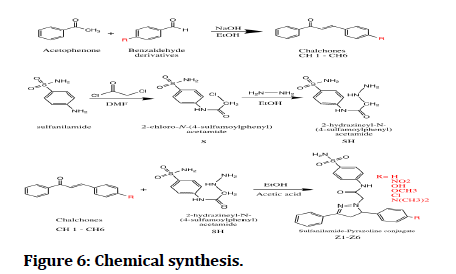
Figure 6: Chemical synthesis.
Anti-microbial evaluation
The antibacterial testing of the newly synthesized compounds (Z1-6) were determined using Mueller Hinton agar medium. All synthesized compounds were evaluated against gram negative bacteria of Escherichia coli and Pseudomonas aeruginosa; Staphylococcus aureus and Streptococcus pyogenes, as a gram positive bacteria and ciprofloxacin, ceftriaxone and ampicillin was used as references for antibacterial activity, Dimethyl Sulfoxide (DMSO) was used as solvent. The zone of inhibition illustrated in Table 1 was measured by millimeter. The tested compound is considered highly active when inhibition zone (more than 15 mm), moderately active when inhibition zone in between (10-15 mm), slightly active when inhibition zone in between (5-10 mm) and inactive when inhibition zone (less than 5 mm) (Figures 7 and 8) [22].
| Compound | Conc. μg/mL | Gram –ve | Gram +ve | ||
|---|---|---|---|---|---|
| E. Coli | Pseudomonas aeruginosa | S. Aureus | Streptococcus pyogenes | ||
| Zone of inhibition | |||||
| Z1 | 1000 | 15 | 18 | ----- | 20 |
| 250 | ----- | 4 | ----- | 9 | |
| Z2 | 1000 | 37 | 35 | 35 | 30 |
| 250 | 18 | 21 | 20 | 12 | |
| Z3 | 1000 | 15 | 10 | ----- | 14 |
| 250 | ---- | 2 | ----- | 5 | |
| Z4 | 1000 | 22 | 24 | 16 | 15 |
| 250 | 10 | 7 | ----- | ------ | |
| Z5 | 1000 | 19 | 21 | 15 | 7 |
| 250 | ----- | ----- | ----- | ----- | |
| Z6 | 1000 | 10 | 12 | 20 | 21 |
| 250 | 5 | 7 | 12 | 8 | |
| Ciprofloxacin | 1000 | 28 | 21 | 15 | 10 |
| 250 | 15 | 10 | 10 | 7 | |
| Ceftriaxone | 1000 | 35 | 20 | 32 | 27 |
| 250 | 30 | 20 | 23 | 25 | |
| Ampicillin | 1000 | 14 | ----- | 12 | 7 |
| 250 | 12 | ----- | 10 | ----- | |
| Tetracycline | 1000 | 12 | 16 | 7 | 4 |
| 250 | 5 | 9 | 2 | ----- | |
Table 1: Anti-bacterial activity of the synthesized compound.
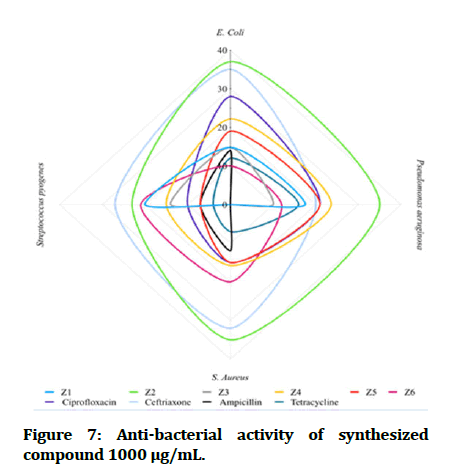
Figure 7: Anti-bacterial activity of synthesized compound 1000 μg/mL.
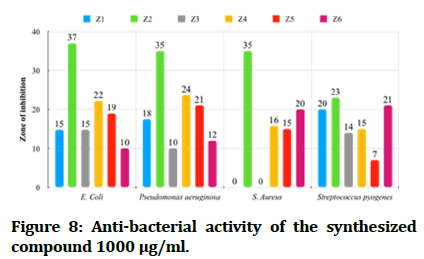
Figure 8: Anti-bacterial activity of the synthesized compound 1000 μg/ml.
In comparisons with standards, the newly synthesized compounds have mostly ranged between high to moderate antibacterial activity in concentration (1000 μg/mL), compound Z2 and compound Z4 have high activity against gram +ve and gram -ve bacteria, while compound Z5 is more effective on gram -ve bacteria and compound Z6 is more effective on gram +ve bacteria. In comparison with dose difference between (250 μg/mL) and (1000 μg/mL), compound Z2 exert a high activity in both concentration. The anti-fungal testing of the newly synthesized compounds (Z1-6) were evaluated against Candida albicans and using fluconazole as reference. The zone of inhibition illustrated in Figure 7 was measured by millimeter, compound Z2 and compound Z4 show a high anti-fungal activity against Candida albicans in dose 1000 μg/mL, compound Z4 has greater inhibition of the fungal growth when compared with fluconazole (reference antifungal) (Figure 9).
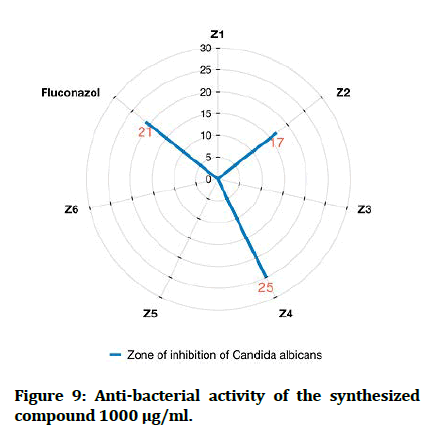
Figure 9: Anti-bacterial activity of the synthesized compound 1000 μg/ml.
Anti-inflammatory evaluation
The effect of newly synthesized compounds on egg white induced edema as an indicator for their anti-inflammatory activity showed in Figure 10 and Table 2.
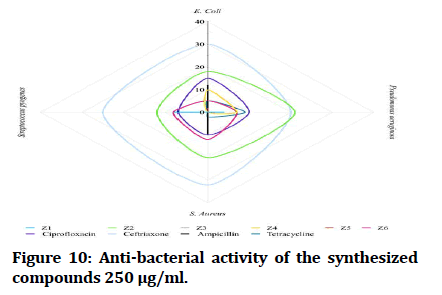
Figure 10: Anti-bacterial activity of the synthesized compounds 250 μg/ml.
| Time | Mean paw thickness (mm) ± SD | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Diclofenac sodium | Z1 | Z2 | Z3 | Z4 | Z5 | Z6 | |
| 0 | 4.51 ± 0.07 | 4.49 ± 0.02 | 4.52 ± 0.01 | 4.49 ± 0.03 | 4.50 ± 0.05 | 4.48 ± 0.01 | 4.46 ± 0.08 | 4.47 ± 0.12 |
| 30 | 4.78 ± 0.03 | 4.81 ± 0.13 | 4.81 ± 0.05 | 4.67 ± 0.05 | 4.69 ± 0.08 | 4.83 ± 0.05 | 4.75 ± 0.05 | 4.78 ± 0.05 |
| 60 | 5.83 ± 0.05 | 5.74 ± 0.04 | 5.78 ± 0.11 | 5.76 ± 0.09 | 5.79 ± 0.02 | 5.75 ± 0.03 | 5.72 ± 0.07 | 5.70 ± 0.02 |
| 120 | 6.81 ± 0.05 | 6.62 ± 0.03* | 6.76 ± 0.08* | 6.71 ± 0.18* | 6.67 ± 0.06* | 6.65 ± 0.09* | 6.61 ± 0.10* | 6.62 ± 0.06* |
| 180 | 7.12 ± 0.03 | 6.45 ± 0.09* | 6.67 ± 0.03* | 6.42 ± 0.02* | 6.51 ± 0.04* | 6.42 ± 0.04* | 6.32 ± 0.03*a | 6.39 ± 0.02* |
| 240 | 6.92 ± 0.02 | 6.27 ± 0.05* | 6.49 ± 0.06* | 6.18 ± 0.07*a | 6.39 ± 0.03* | 6.28 ± 0.03* | 6.08 ± 0.09*b | 6.25 ± 0.02* |
| 300 | 6.74 ± 0.02 | 5.89 ± 0.08* | 6.14 ± 0.02* | 5.76 ±0.01*a | 6.07 ± 0.01* | 5.83 ± 0.04* | 5.72 ± 0.03*b | 5.85 ± 0.04* |
| Non-identical superscripts (a,b) among different tested group are regarded significantly different (p<0.05).(*) significantly different compared to control (p<0.05). Data are expressed in mm paw thickness as mean ± SD. n=number of rats. Time (0) is the time of i.p. injection of diclofenac sodium, tested compounds and propylene glycol. Time (30) is the time of injection of egg white to induce edema. | ||||||||
Table 2: Inflammatory activity of the synthesized compound.
All tested compounds and the reference drug produced significant reduction of paw edema in comparison with the effect of propylene glycol 50%v/v (control group), furthermore compound Z6 and compound Z4, exhibited comparable effect to that of diclofenac (3 mg/kg). While compound Z2 and compound Z5 exhibited superior anti-inflammatory effect when compared to diclofenac (Figure 11).
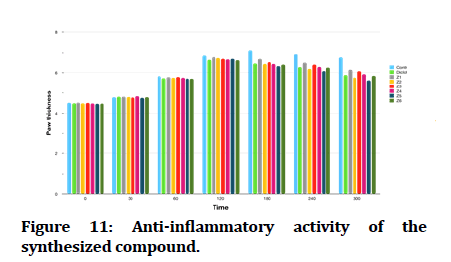
Figure 11: Anti-inflammatory activity of the synthesized compound.
Conclusion
• The chemical synthesis of a new pyrazolines linked with sulfonamide compounds Z1-6 has been achieved successfully.
• Physical properties (melting point and description), FT-IR, 1H-NMR spectra have been checked for the identification and characterization of the synthesized compounds and the results confirm their chemical structure.
• In vivo anti-inflammatory evaluation of all tested compounds and the reference drug produced significant reduction of paw thickness in comparison with the effect of propylene glycol 50% v/v (control group), furthermore compound Z6 and compound Z4, exhibited comparable effect to that of diclofenac sodium (3 mg/kg). While compound Z2 and compound Z5 exhibited superior anti-inflammatory effect when compared to diclofenac sodium (reference drug).
• The anti-microbial assessment of the final compounds with the incorporation of electron withdrawing groups OCH, N(CH3)2 display more activity to gram (+ve) bacteria while the compounds incorporate than the electron withdrawing groups Cl, NO2 display more activity against gram (-ve) bacteria, compound Z2 exert the highest activity even in small concentration. Compound Z4 has greater inhibition of the fungal growth when compared with fluconazole.
References
- Arkawazi B, Mohammed MH. Synthesis and characterization of pyrazoline, dithiocarbamate derivatives with expected biological activity. Biochem Cell Arch 2021; 21:1507–1511. [Crossref][Indexed]
- Alkorta I, Rozas I, Elguero J. Interaction of anions with perfluoro aromatic compounds. J Am Chem Society 2002; 124:8593-8598. [Crossref][Googlescholar][Indexed]
- Panchal AD, Patel PM. Synthesis, anti-bacterial and anti-fungal evaluation of pyrazoline derivatives. E J Chem 2012; 9:1801–1809. [Crossref][Googlescholar][Indexed]
- Jasril J, Teruna HY, Aisyah A, et al. Micro wave assisted synthesis and evaluation of toxicity and antioxidant activity of pyrazoline derivatives. Indones J Chem 2019; 19:583–591. [Crossref][Googlescholar][Indexed]
- Ahn JH, Kim HM, Jung SH, et al. Synthesis and DP-IV inhibition of cyano pyrazoline derivatives as potent anti-diabetic agents. Bioorg Med Chem Lett 2004; 14:4461–4465. [Crossref][Googlescholar][Indexed]
- Bhandari S, Tripathi AC, Saraf SK. Novel 2 pyrazoline derivatives as potential anti-convulsant agents. Med Chem Res 2013; 22:5290–5296. [Crossref][Googlescholar][Indexed]
- Revanasiddappa B, Kumar MV, Kumar H. Synthesis and anti-depressant activity of pyrazoline derivatives. Dhaka Univ J Pharm Sci 2020; 19:179–184. [Crossref][Googlescholar][Indexed]
- Fioravanti R, Bolasco A, Manna F, et al. Synthesis and biological evaluation of N-substituted-3,5-diphenyl-2-pyrazoline derivatives as Cyclooxygenase (COX-2) inhibitors. Eur J Med Chem 2010; 45:6135–6138. [Crossref][Googlescholar][Indexed]
- Matiadis D, Sagnou M. Pyrazoline hybrids as promising anticancer agents: An up to date overview. Int J Mol Sci 2020; 21:1–41. [Crossref][Googlescholar][Indexed]
- Nair AS, Oh JM, Koyiparambath VP, et al. Development of halogenated pyrazolines as selective monoamine oxidase-B inhibitors: Deciphering via molecular dynamics approach. Molecules 2021; 26:3264. [Crossref][Googlescholar][Indexed]
- Salian VV, Narayana B, Sarojini BK, et al. A comprehensive review on recent developments in the field of biological applications of potent pyrazolines derived from chalcone precursors. Lett Drug Des Discov 2018; 15:516–574. [Crossref][Googlescholar][Indexed]
- Ozdemir A, Turan Zitouni G, Kaplancikli ZA, et al. Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives. Eur J Med Chem 2007; 42:403-409. [Crossref][Googlescholar][Indexed]
- Skocovska M, Ferencik M, Svoboda M, et al. Residues of selected sulfonamides, non-steroidal anti-inflammatory drugs and analgesics antipyretics in surface water of the Elbe river basin (czech republic). Vet Med 2021; 66:208–218. [Crossref][Googlescholar][Indexed]
- Sridhar S, Rajendraprasad Y. Synthesis and analgesic studies of some new 2-pyrazolines. E J Chem 2012; 9:476989. [Crossref][Googlescholar][Indexed]
- Gungor T, Ozleyen A, Yilmaz YB, et al. New nimesulide derivatives with amide/sulfonamide moieties: Selective cox-2 inhibition and antitumor effects. Eur J Med Chem 2021; 221:113566. [Crossref][Googlescholar][Indexed]
- Al-Nakeeb MR, Omar TNA. Synthesis, characterization and preliminary study of the anti-inflammatory activity of new pyrazoline containing ibuprofen derivatives. Iraqi J Pharm Sci 2019; 28:133–139. [Crossref][Googlescholar][Indexed]
- Zhou W, Zhang W, Peng Y, et al. Design, synthesis and anti-tumor activity of novel benzimidazole chalcone hybrids as non-intercalative topoisomerase II catalytic inhibitors. Molecules 2020; 25:3180. [Crossref][Googlescholar][Indexed]
- Saglik BN, Cevik UA, Osmaniye D, et al. Synthesis, molecular docking analysis and carbonic anhydrase I-II inhibitory evaluation of new sulfonamide derivatives. Bioorg Chem 2019; 91:103153. [Crossref][Googlescholar][Indexed]
- Mishra D, Singh R, Rout C. A facile amidation of chloroacetyl chloride using DBU. Int J ChemTech Res 2017; 10:365-372.
- Farooq S, Ngaini Z. Chalcone derived pyrazole synthesis via one pot and two pot strategies. Curr Org Chem 2020; 24:1491–1506. [Crossref][Indexed]
- Karabacak M, Altintop MD, Ciftci HI, et al. Synthesis and evaluation of new pyrazoline derivatives as potential anticancer agents. Molecules 2015; 20:19066–19084. [Crossref][Googlescholar][Indexed]
- El Kolli M. Mechanism of antimicrobial activity and antioxidant activities of the essential oil and the methanolic extract of carum montanum from algeria. Ann Agric Sci Moshtohor 2018; 56:253–262. [Crossref][Googlescholar][Indexed]
Author Info
Zeyad D Najmuldeen* and Tagreed NA Omar
Department of Pharmaceutical Chemistry, College of Pharmacy, University of Baghdad, Baghdad, IraqReceived: 07-Nov-2022, Manuscript No. JRMDS-22-62360; , Pre QC No. JRMDS-22-62360; Editor assigned: 11-Nov-2022, Pre QC No. JRMDS-22-62360; Reviewed: 25-Nov-2022, QC No. JRMDS-22-62360; Revised: 24-Dec-2022, Manuscript No. JRMDS-22-62360; Published: 02-Jan-2023
