Research - (2021) Volume 9, Issue 7
The Effect of Plasma Treatment on the Bonding of a Soft Liner to CAD-CAM Denture Base Resin
Lubna M Qanber*,* and Thekra I Hamad
*Correspondence: Lubna M Qanber*,, Department of Prosthodontics, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Introduction: The primary issue with soft liners is their debonding from the denture base material after a period of usage. Aims: This study aims at evaluating the effect of oxygen and argon plasma treatment on the shear bond strength of soft liner material to CAD-CAM acrylic denture base. Materials and Methods: CAD-CAM acrylic specimens were prepared (n=20 pairs), with the dimensions (75 × 13 × 13 mm) length, width and thickness, respectively, with a 3 mm depth stopper. Ten pairs of specimens were plasma treated for 5 minutes. Then, heat-cured soft liner was added joining each two specimens. Shear bond strength (SBS) was then analyzed using universal testing machine. Vickers microhardness, contact angle, FTIR and AFM were also analyzed. The results were statistically analyzed using T-paired test (α=0.05). Results: The SBS of plasma treated samples were significantly higher than that of the untreated samples (p<0.001). Microhardness was not significantly affected, and contact angle got significantly higher following plasma treatment. Conclusion: Oxygen and argon plasma treatment has efficiently increased SBS between CAD-CAM acrylic and soft liner without affecting the material’s bulk properties.
Keywords
CAD-CAM Acrylic, Bonding strength, Plasma treatment, Soft liner, Microhardness.
Introduction
Patients with edentulous ridges suffer from continuous ridge resorption which results in denture failure, discomfort and pain [1]. These issues can be resolved by the use of resilient denture liners [2]. Soft lining materials, compliant viscoelastic materials, have become commonly used in dental practice for reshaping denture surfaces that are in contact with the soft tissue of the oral cavity [3]. They provide cushioning effect which aids in the distribution and reduction of the functional forces, and help the tissue recover from trauma giving comfort to the patient [4]. However, the main shortcoming of soft lining materials is their debonding from the denture base after a period of usage, which may create an environment for potential bacterial growth, thereby expediting the breakdown of the material [5, 6].
Along with conventional heat polymerization and autopolymerization procedures, dentures can currently be created using computer-aided design and computer-aided manufacturing (CAD-CAM) [7]. Not only do they demand less chair time and appointments, but they also exhibit minimal shrinkage due to the absence of a polymerization process. Duplicate dentures are simple to create due to the fact that documents are stored digitally [8].
The literature, on the other hand, has shown a paucity of information regarding the bonding of CAD-CAM resins to soft liners.
To overcome the problem of debonding between acrylic resins and soft liners, researchers have studied various methods of denture surface modification prior to applying soft lining material. One of the tested methods is plasma treatment, a gaseous mixture of free radicals, high-energy electrons and ions [9].
Plasma treatment has been found to increase the bonding strength between denture acrylic material and soft lining material in a significant manner [10]. Plasma treatment with oxygen has been found to increase hydrophilicity of the polymer surfaces and thereby increasing its surface energy [11], on the other hand plasma treatment of polymers with argon gas has been stated to induce cross linking properties of polymers [12].
There are widely accepted tests for evaluating soft liners mechanical properties, like tensile, peel and shear bond strength testing. Al-Athel and Jagger had concluded that shear bond strength best replicated how a liner functions in the oral cavity [13].
The purpose of this in-vitro study was to evaluate the effect of oxygen and argon plasma treatment on shear bond strength of soft liner to CAD-CAM denture resin. The null hypothesis was that plasma treatment would not improve the shear bond strength.
Materials and Methods
Preparation of specimens
Twenty CAD-CAM (XT-Cera, China) specimens for testing shear bond strength were prepared. Each specimen consisted of two blocks with dimensions of (75 × 13 × 13 mm) length, width, thickness, respectively, with a 3 mm depth stopper [14]. One block is placed over the other, leaving a space in between for the soft liner material application. The shape of these specimens was created using AutoCad software. The design was then sent to a lab technician to mill the CAD-CAM blocks into the designed shape using a dental CAD-CAM machine (Figure 1). Specimens for testing microhardness (n=20) were cut in the same way (12 × 12 × 3 mm) [15]. Specimens for testing wettability (n=20) were also prepared (20 × 15 × 2 mm) [16]. All of the specimens were then cleaned by washing with a 1% detergent solution (liquid soap and water) and then with distilled water in an ultrasonic cleaner for 15 minutes. After that, they were dried in the air, then immediately placed in the sample holder inside the plasma chamber [17].
Figure 1: Preparing CAD-CAM specimens: A: Milling with CAD-CAM machine; B-D: The final shape produced by milling.
Plasma treatment
Ten specimens for each test were treated with plasma. A mixture of argon and oxygen gases was used in a plasma jet for surface treatment of the specimens. Plasma treatment was done by using DC-glow discharge plasma device (homemade at Ministry of Science and Technology, Iraq); the apparatus was equipped with a direct current (DC). Bonding surfaces of the SBS specimens and the other tests specimens were placed on the center of the cathode surface at a right angle to the gas flow with a 4 cm distance. Gas pressure was maintained at 4 × 10-2 mbar. The plasma was excited by a combination of direct current (DC) voltage supply working up to 650 V and a maximum DC of 30 mA. A uniform glow could be seen directed on the samples. All of the specimens were exposed to plasma for 5 min.
At the end of plasma exposure time, the chamber was kept locked for an extra 15 min for the gas to be evacuated; the specimens were then repurchased and covered with a cling film for isolation.
Preparing final SBS samples
For each SBS test sample, two acrylic specimens were mounted facing each other; forming a space between them measuring (13 × 13 × 3 mm) width, length, and depth, respectively. The two specimens were secured with clear tape and then submerged completely in laboratory putty (Zetalabor, Italy) and left to set completely. To facilitate flasking, a custom-made flask was designed to match the samples size. The silicone containing the sample was then invested inside stone and allowed to set. Following that, the samples were extracted from the silicone to remove the tape and reinserted into the silicone.
Heat cured soft liner (Vertex-soft, Netherlands) was prepared according to manufacturer’s instructions. Once the material has reached its dough stage, it was incrementally inserted and condensed into the space between each two blocks using a metal mixing spatula; the space was overfilled, the flask was then covered under pressure (1kg) and firmly screwed until edge-toedge contact was achieved.
The flask was then put in a digital water bath for curing. According to the manufacturer’s instructions, the curing cycle was performed by heating water up to 70°C for 90 minutes, then elevated up to 100°C for 30 minutes. After curing, the flask was removed from the water bath, and bench cooled for 30 minutes, followed by 15 minutes of cooling under tap water to ensure complete cooling [18]. The flask was opened and the samples were extracted and finished using a sharp blade to cut any excess and then stored in a container filled with distilled water.
Shear bond strength test
Shear bond strength test was carried out using a universal testing machine at a load cell capacity of 100 kg and cross head speed of 0.5 mm/min. The machine readings represent the maximum load of failure. Bond strength was calculated by dividing the maximum load of failure by the cross-section area of each sample (13 mm × 13 mm = 169 mm).
Vickers micro hardness testing
The Vickers microhardness test was performed using a calibrated Vickers microhardness tester. It involved the use of diamond pyramid indenters. Microhardness of CAD-CAM acrylic samples was determined with the application of a 30 g load for 30 s. The final value of was measured by the average microhardness among four points measurements of the indentation for each sample.
Wettability testing
Contact angle reflects the wetting of the substrate by a liquid. It is an essential parameter in measuring the wettability and the free surface energy of surfaces—the greater the contact angle, the lower the wettability value. Contact angle of the treated and untreated CAD-CAM acrylic surfaces was captured using an optical tensiometer (TL 1000, Theta Lite, OneAttension, Biolin Scientific, Lichfield, UK). A drop of distilled water was used at room temperature. In this technique, a graduated syringe with hydrophobic needle deposits a drop; after 5 sec the contact angle is calculated with 60 images per second over 10 seconds. The images captured were analyzed using the special software of the microscope. This software automatically drew a tangent; the angle located at the three-phase-lines air/solid/liquid was measured to give the contact angle value.
Chemical surface analysis (FTIR Analysis)
To understand the chemical surface changes of CAD-CAM denture base material after plasma treatment, the specimens’ surfaces were investigated using FTIR analyzer (Fourier Transform Infra-Red Spectrophotometer, Bruker, Germany). Specimens were prepared with the exact dimensions of wettability test specimens (20 × 15 × 2 mm). The FTIR spectra were obtained using a potassium bromide disk and attenuated total reflection (ATR) techniques of FTIR spectroscopy with a resolution of 4 cm−1 and 32 scans in the range of 4000 to 400 cm-1.
Atomic force microscopy or AFM analysis
Atomic force microscopy was used to study and compare the surface topography/morphology of untreated and plasma-treated acrylic polymer specimens. Specimens with the same measurements of wettability test specimens (20 × 15 × 2 mm) were prepared.
Statistical analysis
Analyses are conducted with the assistance of statistical analysis software (IBM SPSS Statistics 26). The mean values obtained were evaluated using paired sample Ttest. Statistical significance was declared for all comparisons when the p value was less than 0.05.
Results
SBS, micro hardness and contact angle findings were analyzed for the untreated and treated samples (Table 1). The results indicated a highly significant difference between plasma-treated and untreated SBS mean values (p<0.001).
Table 1: Comparative analyses of the mean values of SBS, microhardness and contact angle tests.
| Test | Group | N | Mean | Standard deviation | Min. | Max. | T-test | df | Sig. |
|---|---|---|---|---|---|---|---|---|---|
| SBS (N/mm2) | Control | 10 | 0.8122 | 0.01919 | 0.6917 | 0.9024 | -10.167 | 18 | 0 |
| Treated | 10 | 1.6156 | 0.07369 | 1.2116 | 1.9342 | ||||
| Microhardness (HV) | Control | 10 | 24.342 | 0.55904 | 23.73 | 25.33 | -2.113 | 18 | 0.064 |
| Treated | 10 | 25.067 | 0. 82240 | 23.89 | 26.74 | ||||
| Contact angle (°) | Control | 10 | 74.735 | 1.79123 | 72.12 | 77.06 | 17.564 | 18 | 0 |
| Treated | 10 | 64.427 | 1.78 | 61.2 | 67.22 |
The mean values of the microhardness of the CAD-CAM acrylic surface had a non-significant change (p>0.05). Table 1 also shows a significant increase of contact angle mean values following plasma treatment (p<0.001), implying that the wettability has improved significantly.
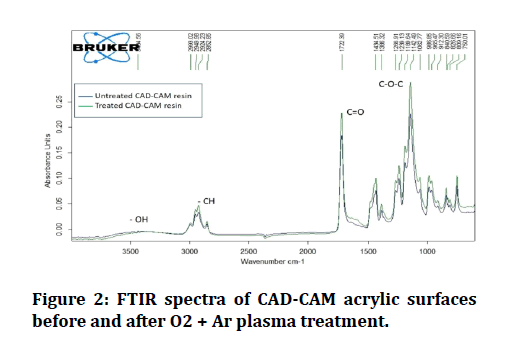
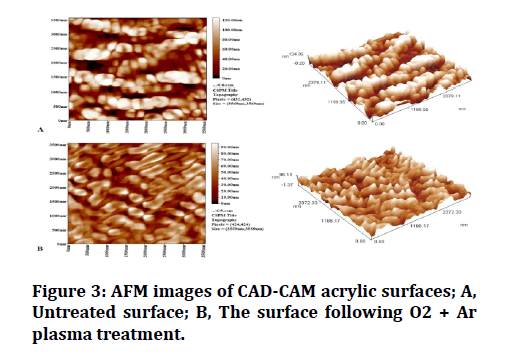
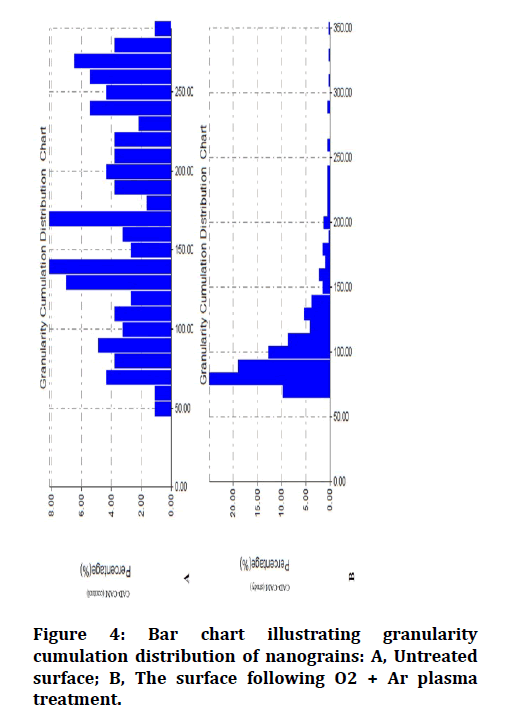
Analyses of the chemical composition of the surfaces of each of the control and treated groups were performed using an FTIR analyzer (Figure 2). The two- and threedimensional images obtained by the AFM analysis are shown in (Figure 3). AFM analysis of plasma treated CADCAM acrylic surface has shown a more uniformly distributed granular film when compared to that of the untreated surface (Figure 4).
Figure 2: FTIR spectra of CAD-CAM acrylic surfaces before and after O2 + Ar plasma treatment.
Figure 3: AFM images of CAD-CAM acrylic surfaces; A, Untreated surface; B, The surface following O2 + Ar plasma treatment.
Figure 4: Bar chart illustrating granularity cumulation distribution of nanograins: A, Untreated surface; B, The surface following O2 + Ar plasma treatment.
There was an increase in the mean values of average surface roughness following plasma treatment (55.1 nm), when compared to the control group (22.7 nm).
Discussion
Discussion
In clinical practice, soft lining materials are subjected to shear and tearing forces. The shear strength test has been deemed suitable for in vitro studies [13]. Using CAD-CAM machines for fabricating acrylic dentures is a relatively new technology, and there are limited studies regarding the bonding of this type of resin to soft liners [7]. As such, in the current study, the effect of oxygen + argon plasma treatment on SBS between CAD-CAM acrylic and soft liner was evaluated.
SBS was significantly enhanced following plasma treatment. Consequently, the null hypothesis that plasma treatment would not improve the SBS was rejected.
This enhancement may be attributed to the fact that oxygen gas in plasma treatment induces an etching process by chemically removing particles from the surface material. Furthermore, the chemical oxidation reaction generates new chemical functional groups on the treated surface, such as hydroxyl group.
It also increases the surface energy, thereby enhancing the penetration of soft liner into the irregularities and strengthening the adhesion between the two materials [10, 19]. Although argon is an inert gas and inert gas plasma treatments do not induce any new reactive functionality onto the polymer surface, treatment with inert gases could induce free radicals formation on the polymer surface through ion bombardment and ultraviolet radiation [12].
Additionally, adding inert gas, argon, to an active gas like oxygen in plasma increases oxygen functionality [20].
The results of SBS are supported by the contact angle measurements, which revealed that the treated group's contact angle was significantly lower than that of the control group. This substantial drop in the contact angle may indicate the grafting of new polar functionalities onto the surface, reactive groups open bonds on the surface, increasing surface energy and consequently the wettability [21].
There was no significant change in microhardness, which coincides with the conclusion of Dos Santos et al [22], that plasma treatment did not affect the microhardness of acrylic denture resin, making it a suitable method for surface modification when compared to other treatments [22].
Microhardness being preserved can be supported by the findings of FTIR surface chemical analysis that had shown no change in peak positions, indicating that the hybridization state and electron distribution within the molecular bond have remained constant. This means that plasma treatment did not affect the chemical structure of these acrylic materials, which coincides with the FTIR results of Mustafa’s study [14].
However, the peak intensities of all functional groups (C=O, C-O, and C-H) have increased, implying an increase in the amount (per unit volume) of these functional groups [23]. As explained earlier, oxygen plasma treatment induces the formation of functional groups on the polymer surface, increasing the surface hydrophilicity.
AFM of the control group of the CAD-CAM acrylic material revealed uneven distribution of granular film surface. In comparison, the AFM of plasma treated specimens had shown a more uniform granular film distribution, with an increase in average surface roughness producing new nanograins with smaller diameters.
These findings coincide with the AFM findings of Dorranian et al, confirming that plasma treatment degrades the surface irregularities to bear polar groups and removes the materials with less attachment energy to the surface; i.e physical sputtering [24].
This change in surface morphology is assumed to be caused primarily by the surface being bombarded by high-energy ions present in the plasma. It indicates that plasma treatment improved the cross-linking phenomenon [25].
The test settings may not represent the clinical scenario because the specimens designed for the study contained several adhesive surfaces, yet in clinical cases, dentures have only one adhesive surface. Thus, in vivo investigations should also be carried out. Meanwhile, the results of this study may serve as a baseline for future research into novel materials and other variables affecting bond strength.
Conclusion
Within the limits of this study, it was concluded that 5- minutes oxygen + argon plasma treatment was effective in increasing the shear bond strength of heat cure soft liner material to CAD-CAM acrylic, without impairing chemical structure and microhardness of CAD-CAM acrylic material.
References
- Sánchez-Aliaga A, Pellissari CVG, Arrais CAG, et al. Peel bond strength of soft lining materials with antifungal to a denture base acrylic resin. Dent Mater J 2016; 35:194–203.
- Kitagawa Y, Yoshida K, Takase K, et al. Evaluation of viscoelastic properties, hardness, and glass transition temperature of soft denture liners and tissue conditioner. Odontol 2020; 108:366–375.
- Shanmuganathan N, Padamanabhan TV, Subramaniam R, et al. The compliance of temporary soft lining materials-An in vivo & vitro study. Int J Sci Res Publ 2012; 2:1–7.
- Tayebi L. Applications of biomedical engineering in dentistry. 1st Edn. Springer 2020.
- Chladek G, Zmudzki J, Kasperski J. Long-term soft denture lining materials. Mater 2014; 7:5816–5842.
- Xiaoqing M, Qiao C, Zhang X, et al. Improvement of the adhesive strength between silicone-based soft liner and thermocycled denture base with plasma treatment. Dent 2015; 5:5-12.
- Choi JE, Ng TE, Leong CKY, et al. Adhesive evaluation of three types of resilient denture liners bonded to heat-polymerized, autopolymerized, or CAD-CAM acrylic resin denture bases. J Prosthet Dent 2018; 120:699–705.
- Manappallil JJ. Basic dental materials. 4th Edn. JP Medical Ltd. 2016.
- Gherardi M, Tonini R, Colombo V. Plasma in dentistry: brief history and current status. Trends Biotechnol 2018; 36:583-585.
- Zhang H, Fang J, Hu Z, et al. Effect of oxygen plasma treatment on the bonding of a soft liner to an acrylic resin denture material. Dent Mater J 2010; 29:398–402.
- Aljudy HJ. Effect of plasma treatment of acrylic denture teeth and thermocycling on the bonding strength to heat cured acrylic denture base material. J Baghdad Coll Dent 2013; 25:6–11.
- Bicer AZY, Dogan A, Keskin S, et al. Effect of argon plasma pretreatment on tensile bond strength of a silicone soft Liner to denture base polymers. J Adhes 2013; 89:594–610.
- Al-Athel MS, Jagger RG. Effect of test method on the bond strength of a silicone resilient denture lining material. J Prosthet Dent 1996; 76:535–40.
- Mustafa SB, Hamad TI. The effect of plasma treatment on shear bond strength of high impact acrylic resin denture base lined with two types of soft lining materials after immersion in distilled water and denture cleanser. J Baghdad Coll Dent 2015; 27:44-51.
- Machado AL, Breeding LC, Vergani CE, et al. Hardness and surface roughness of reline and denture base acrylic resins after repeated disinfection procedures. J Prosthet Dent 2009; 102:115–212.
- Ramanna PK. Wettability of three denture base materials to human saliva, saliva substitute, and distilled water: A comparative in vitro study. J Indian Prosthodont Soc 2018; 18:248–256.
- Zamperini CA, Carneiro HDL, Rangel EC, et al. In vitro adhesion of Candida glabrata to denture base acrylic resin modified by glow-discharge plasma treatment. Mycoses 2013; 56:134-144.
- Alamen BM, Naji GA. The effect of adding coconut oil on candida albicans activity and shear bond strength of acrylic based denture soft lining material. J Res Med Dent Sci 2018; 6:310-318.
- Abdullah ZS, Mahmood WS, Ibrahem RA. The effect of plasma treatment on shear bond strength of soft denture liner with two different types of denture base material (heat cure and light cure). J Baghdad Coll Denti 2014; 26:44–49.
- Sparavigna A. Plasma treatment advantages for textiles. Man-Made Text 2006; 9:85–89.
- Lee MH, Min BK, Son JS, et al. Influence of different post-plasma treatment storage conditions on the shear bond strength of veneering porcelain to zirconia. Mater 2016; 9:43.
- Dos Santos DM, Vechiato-Filho AJ, Pesqueira AA, et al. Effect of nonthermal plasma treatment on the surface of dental resins immersed in artificial saliva. J Polym Eng 2016; 36:785–793.
- Munajad A, Subroto C, Suwarno. Fourier transform infrared (FTIR) spectroscopy analysis of transformer paper in mineral oil-paper composite insulation under accelerated thermal aging. Energies 2018; 11:364.
- Dorranian D, Abedini Z, Hojabri A, et al. Structural and optical characterization of PMMA surface treated in low power nitrogen and oxygen RF plasmas. J Non-Oxide Glasses 2009; 1:217–229.
- Hassouba M, Dawood N. Comparison of surface modification of CR-39 polymer film using RF and DC glow discharges plasma. J Mod Phys 2017; 8:2021–2033.
Author Info
Lubna M Qanber*,* and Thekra I Hamad
Department of Prosthodontics, College of Dentistry, University of Baghdad, IraqCitation: Lubna M Qanber, Thekra I Hamad,The Effect of Plasma Treatment on the Bonding of a Soft Liner to CAD-CAM Denture Base Resin, J Res Med Dent Sci, 2021, 9(7): 90-95
Received: 05-Jun-2021 Accepted: 06-Jul-2021