Research Article - (2022) Volume 10, Issue 9
The Gas Chromatography Mass Spectroscopy Analysis of One Unani Drug, "Muffarah Ahmedi"
Hassan Mohammad M1, Janaki CS2, Rao MRK3*, Prabhu K4, Deepa K5, Franklin6 and Vijayalakshmi N7
*Correspondence: Rao MRK, Department of Anatomy, Amritha University, Thiruporur, Tamil Nadu, India, Email:
Abstract
The Unani medicine, Muffarah Ahmedi, which is prescribed for duodenal and peptic ulcers, was subjected to gas chromatography mass spectroscopic analysis. The medicine was bought procured from Unani medicine supplier and was processed suitably for analysis. The profile results indicated the availability of many compounds, namely, trans-2-methyl-4-n-pentylthiane, S,Sdioxide,PhenylethylAlcohol,trans-3-Methyl-2-n-propylthiophane,5-Hydroxymethylfurfural, Tetradecanedioic acid, 6-Octadecenoic acid, trans-13-Octadecenoic acid, Methyl 2-hydroxy-octadeca-9,12,15-trienoate, which correspond well with the medicinal role of this medicine.Keywords
GCMS, Unani, Muffarah Ahmedi, Phenylethyl alcohol, Trans-3-Methyl-2-n-propylthiophane, 5-HydroxymethylfurfuralIntroduction
Muffarah Ahmedi is a Unani medicine used to treat duodenal and gastric ulcers and supposed to work as coolant. Its ingredients constitute of Thabasheer (Bamboo extracts), Sandal-e-sufeed (White sandal wood), Amila (Phyllanthus embelica), Gul e-surkh (Rose flower), Kishneez (Coriandrum sativum L.) and Khande sufeed (White sugar). It is imperative to establish the authenticity of alternative medicines such as Ayurveda, Sidhha and Unani systems as they are time tested and in use for centuries. The present workers have worked to scientifically evaluate the veracity of these medicine systems by latest techniques so that deeper knowledge of the mechanism of action of these medicines could be gained [1-19].The present study in one step further in this endeavour. Not much work in this direction is reported on the medicinal role of this medicine.
Materials and Methods
Muffarah Ahmedi was bought from a Unani medicine vendor at chennai. The medicine was suitably processed by standard procedures and the GC-MS analysis was performed.
Results
Muffarah Ahmedi gas chromatography mass spectroscopy profile and possible medicinal role of each molecule indicated in the GC MS profile is tabulated in Table 1.
| Ret. Time | Molecule | Mol. Formula | Mol. Mass | % peak Area | Possible Medicinal Roles |
|---|---|---|---|---|---|
| 4.41 | trans-2-methyl-4-n-pentylthiane, S,S-dioxide | C11H22O2S | 218.1 | 1.8 | Nitric oxide synthase inhibitor, Glutathione-S-Transferase-Inhibitor, Myo-neuro stimulant, Nitric Oxide scavenger, stimulates norepinephrine production, Stimulates Sympathetic nervous system, Catechol-O- Methyl transferase inhibitor, known as smart drug, Adrenocortical stimulant, Decreases Glutamate Oxaloacetate Transaminase, Decreases Glutamate Pyruvate Transaminase, Glucosyl-Transferase-Inhibitor, Glutathione-S-Transferase-Inhibitor, Increases Glyoxalate Transamination, Reverse-Transcriptase-Inhibitor, Transdermal |
| 4.64 | Phenylethyl Alcohol | C8H10O | 122.1 | 6.17 | Alcohol dehydrogenase inhibitor, Alcohol detoxicant |
| 4.94 | trans-3-Methyl-2-n-propylthiophane | C8H16S | 144.1 | 0.95 | Catechol-O-Methyl-Transferase-Inhibitor, increases Glutathione-S-Transferase (GST) activity, decreases glutamate oxaloacetate transaminase, decreases glutamate pyruvate transaminase, glucosyl-transferase-inhibitor, glutathione-S-transferase-inhibitor, increases glyoxalate transamination, reverse-transcriptase-Inhibitor, GABAergic, increased NK cell activity, inhibits production of tumour necrosis factor, Myo-Neuro-stimulator |
| 5.26 | Dodecane, 1-fluoro- | C12H25F | 188.2 | 5.48 | Not known |
| 6.31 | 5-Hydroxymethylfurfural | C6H6O3 | 126 | 12.72 | It is reported to stop neuron apoptosis |
| 6.39 | Triallylmethylsilane | C10H18Si | 166.1 | 0.76 | Not known |
| 8.03 | Methylparaben | C8H8O3 | 152 | 20.26 | Not known |
| 9.28 | Isobutyl 4-hydroxybenzoate | C11H14O3 | 194.1 | 11.6 | Not known |
| 13.78 | Palmitoyl chloride | C16H31ClO | 274.2 | 0.68 | Not known |
| 14.3 | Tetradecanedioic acid | C14H26O4 | 258.2 | 1.77 | acidifier, acidulant, arachidonic acid-inhibitor, increases aromatic amino acid decarboxylase activity, inhibits Production of Uric Acid |
| 16.22 | 6-Octadecenoic acid | C18H34O2 | 282.3 | 5.26 | acidifier, acidulant, arachidonic acid-inhibitor, increases aromatic amino acid decarboxylase activity, inhibits production of uric Acid |
| 16.82 | trans-13-Octadecenoic acid | C18H34O2 | 282.3 | 10.52 | Catechol-O-methyl-transferase-Inhibitor, increases Glutathione-S-Transferase (GST) activity, decreases glutamate oxaloacetate transaminase, decreases glutamate pyruvate transaminase, tlucosyl-transferase-Inhibitor, glutathione-S-transferase-Inhibitor, increases glyoxalate transamination, Reverse-transcriptase-inhibitor, transdermal, acidifier, arachidonic acid Inhibitor, increases aromatic amino acid decarboxylase activity, inhibits production of uric acid, urine acidifier |
| 17.07 | Methyl 2-hydroxy-octadeca-9,12,15-trienoate | C19H32O3 | 308.2 | 10.63 | 17 beta hydroxyl steroid dehydrogenase inhibitor, aryl hydrocarbon hydroxylase inhibitor, testosterone hydroxylase inducer, Catechol-O-methyl-transferase-inhibitor, methyl donar, methyl guanidine inhibitor |
Table 1: Indicates the retentions values, types of possible compound, their molecular formulae, molecular mass, peak area and their medicinal roles of each compound as shown in the GC MS profile of Muffarah Ahmedi.
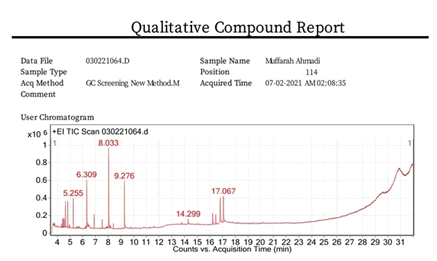
Figure 1 shows gas chromatography mass spectroscopy profile of Muffarah Ahmedi. The identification of metabolites was compared with NIST spectral library and the possible pharmaceutical roles of each bio molecule was referred with National Agriculture Library, USA and others as shown in Table 1 [20].
Figure 1: Indicates the gas chromatography mass spectroscopic profile of Muffarah Ahmedi.
Discussion
Muffarah Ahmedi contained some compounds, namely, trans-2-methyl-4-n-pentylthiane, S,S-dioxide, phenylethyl alcohol, trans-3-Methyl-2-n-propylthiophane, 5-hydroxymethylfurfural, tetradecanedioic acid, 6-octadecenoic acid, trans-13-octadecenoic acid, methyl 2-hydroxy-octadeca-9,12,15-trienoate etc. which were reported to have far reaching medicinal roles that could support the role of Muffarah Ahmedi as a good medicine for duodenal and peptic ulcers.
Conclusion
It could be summarized from the results and discussion that Muffarah Ahmedi does contain important biomolecules which provides a clue to its prescription for the ailments it is given. It will be of interest to probe into the medicinal roles of many compound present in Muffarah Ahmedi for which reports are not available.
Acknowledgements
The authors thankfully acknowledge the support of all the people and organizations.
References
- Rao MRK, S Philip, Kumar MH, et al. GC-MS analysis, antimicrobial, antioxidant activity of an Ayurvedic medicine, Salmali Niryasa. J Chem Pharm Res 2015; 7:131-139.
- Sivakumaran G, Prabhu K, Rao MRK, et al. Gas chromatography–mass spectrometry analysis of one ayurvedic oil, Anu thailam. DIT 2019; 11:2675-2678.
- Sivakumaran G, Prabhu K, Rao MRK, et al. Gas chromatography–mass spectrometry analysis of one ayurvedic oil, Ksheerabala Thailam. DIT 2019; 11: 2661-2665.
- Sivakumaran G, Prabhu K, Rao MRK, et al. Gas chromatography–mass spectrometry analysis of one Ayurvedic oil, Triphaladi Thailam. DIT 2019; 11:2679-2683.
- Narayanan G, Prabhu K, Rao MRK, et al. Gas chromatography–mass spectrometry analysis of one Ayurvedic medicine, Drakshadi Kashayam. DIT 2019; 11: 2652-2656.
- Narayanan G, Prabhu K, Rao MRK, et al. Gas chromatography–mass spectrometry analysis of one ayurvedic medicine, Kutajarishtam. DIT 2019; 11:2666-2669.
- Narayanan G, Prabhu K, Rao MRK, et al. Gas chromatography–mass spectrometry analysis of one Ayurvedic antiobesity medicine, Lohasava. DIT 2019; 11:2670-2674.
- Kumar MH, Prabhu K, Rao MRK, et al. Gas chromatography/mass spectrometry analysis of one Ayurvedic skin oil, Eladi Kera Thailam. DIT 2019; 11:2657-2660.
- Mohammad H, Prabhu K, Rao MRK, et al. The GC MS study of one Ayurvedic Pain relieving OIL "Mahamasha thailam". Drug Discov Today 2019; 12:1524-1527.
- Mohammad H, Prabhu K, Rao MRK, et al. The GC MS study of one Ayurvedic Pain relieving oil "Karpooradi thailam", Drug Invention Today, 2019; 12:1542-1546.
- Prabhu J, Prabhu K, Chaudhury A, et al. Neuro protective role of Saraswatharishtam on Scopolamine induced memory impairment in animal model. Pharmacogn J 2020; 12:465-472.
- Prabhu K, Rao MRK, AK Bharath, et al. The GC MS study of one Ayurvedic Rasayana formulation Narasimha Rasayanam. DIT 2020; 13:658-662.
- Prabhu K, Rao MRK, Vishal S K, et al. GC MS study of one Ayurvedic Rasayana drug, Dhanwantari Rasayanam. DIT 2020; 14:783-786.
- Sharmila D, Poovarasan A, E Pradeep, et al. GC MS analysis of one Ayurvedic formulation, Sitopaladi. RJPT 2021; 14:911-915.
- Narayanan G, Prabhu K, Chaudhuri A, et al. Cardio protective role of Partharishtam on isoproterenol induced myocardial infarction in animal model. Pharmacogn J 2021; 13:591-595.
- Kalivannan J, Janaki CS, Rao MRK, et al. The GC MS astudy of one ayurvedic formulation, Chandanasavam. Ind J of Nat Sci 2021; 12:33671-33676.
- Akshaya SR, Kalaivani S, Prabhu K, et al. The GC MS study of one Ayurvedic churnam, Avalgujabijadi churnam. Ind J Nat Sci 2021; 12:34395-34402.
- Subbiah AJ, Kavimani M, Rao MRK, et al. The GC MS study of one Ayurvedic. Formulation, Pushyanuga churnam. Ind J Nat Sci 2021; 12:35757-357-366.
- Yuvaraj R, Vijayakumar S, Rao M R K et al. The GC MS study of one Ayurvedic medicine Pippalyasavam'. Ind J Nat Sci 2021; 12:35612-35618.
- Duke, James A. Duke's Phytochemcial and Ehnobotanical Databases. U.S. Department of Agriculture, Agricultural Research Service. Ag Data Commons, U.S, 2021.
Author Info
Hassan Mohammad M1, Janaki CS2, Rao MRK3*, Prabhu K4, Deepa K5, Franklin6 and Vijayalakshmi N7
1Department of Anatomy, Northern Borders University, Arar, Saudi Arabia2Department of Anatomy, Bhaarath Medical College, Chennai, Tamilnadu, India
3Department of Anatomy, Amritha University, Thiruporur, Tamil Nadu, India
4Department of Anatomy, Sree Balaji Medical College and Hospital, Chennai, Tamil Nadu, India
5Department of Anatomy, Quest International University, IPOH Perak, Malaysia
6Deparmentt of Microbiology, CEO Anna Medical College, Mauritius, Montagne Blanche, Island, Mauritius
7Department of Chemical and Biotechnology, SASTRA (Deemed to be University), Thanjavur, Tamil Nadu, India
Citation: Hassan mohammad M, Janaki CS, Rao MRK, Prabhu K, Deepa K, Franklin, Vijayalakshmi N,The Gas Chromatography Mass Spectroscopy Analysis of One Unani Drug, “Muffarah Ahmedi”, J Res Med Dent Sci, 2022, 10 (9): 000-000.
Received: 01-Jul-2022, Manuscript No. JRMDS-22-57365; , Pre QC No. JRMDS-22-57365; Editor assigned: 04-Jul-2022, Pre QC No. JRMDS-22-57365; Reviewed: 18-Jul-2022, QC No. JRMDS-22-57365; Revised: 01-Sep-2022, Manuscript No. JRMDS-22-57365; Published: 07-Sep-2022