Research Article - (2023) Volume 11, Issue 1
The Impact of Calcium Carbonate Nanoparticles Incorporation Into Heat Cured Soft Denture Lining Material On Thermal Conductivity And Some Other Properties
Samir Kamil Abdulmajeed* and Hikmat Jameel Abdulbaqi
*Correspondence: Samir Kamil Abdulmajeed, Department of Prosthodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
The objective of this study was to investigate the impact on thermal conductivity, thermal diffusivity, tensile strength and tear strength in the soft denture lining material by applying Calcium Carbonate Nanoparticles (CaCO3 NPs) to soft lining materials. CaCO3 NPs were applied to the heat cured soft lining material with 1.5% and 2% concentration by weight. Ninety specimens have been prepared according to formed tests. The thermal conductivity and thermal diffusivity of soft liner/CaCO3 NPs composites were measured, the strength of the tensile bond and the tear strength were also measured and the results show that thermal conductivity and thermal diffusivity in the experimental specimens were significantly increased compared to control specimens. The tensile bond strength between the soft liner material and denture base of the acrylic and the tearing strength showed a significant increase. Therefore, the addition of CaCO3 NPS can provide an increased thermal conductivity, thermal diffusivity as well as tensile bonding strength and tear strength for soft liner material.
Keywords
Soft denture liner, Thermal conductivity, Tensile bond strength, Tear strength, Calcium carbonate nanoparticles
Introduction
Soft denture lining materials are placed on a denture tissue surface to provide a more equal distribution of strength, retention and stability for denture, occlusion and the appearance of a patient and a less localized pressure [1,2].
When the residual ridge below has been absorbed, the denture is lost the exact adaptation to the resorbed ridge and the denture must be relined to improve the adaptation of the denture to the underlying supporting tissues [3].
The resilient liner product can be divided into plasticized resins or elastomers of silicone. The acrylic resin consists of acrylic polymers and copolymers, a liquid that includes acrylic monomers and plasticizers to preserve the softness of the material [4,5].
Soft denture liner should have good dimensional stability during and after processing, non-toxic, non-irritant, odorless, stable color, easy to process and use, appropriate esthetics, low absorption and solubility of water, adequate mechanical strength, abrasion and tear resistance, inhabit bacterial and fungal growth and good bonding with denture base material to prevent detachment during use [6-8].
One of the important problems of the soft denture liner is low thermal conductivity between denture base and oral mucosa which effect on the acceptance of the patient to denture. In addition, low thermal conductivity of denture base have main effect on health of underlying supporting tissues and secretion of salivary gland especially parotid gland [9,10].
Several problems with the application of soft dentures, including hard cleaning, science, they are significantly softer and resistant to burning less than polymethacrylate, easy to degrade and microbial growth, loss of bonding between the denture base and the soft liner [11,12].
Several studies had been done to enhancement properties of soft denture liner by addition different types of filler. The physical and mechanical properties of soft dentures liner are drastically improved with inorganic nanoparticles, even if there is less added [13-15].
The improvement of the properties of the inorganic nanoparticles in polymers depends on the form, size and the amount of these components, their concentration and the interaction of the polymer matrix [16].
Calcium Carbonate Nanoparticles (CaCO3 NPs) is most common, biocompatible, available and inexpensive inorganic filler that used in nanocompsite process. Nanoparticles of calcium carbonate have potential to be important functional filler in polymer systems such as polyproplene composite and polyvinyl chloride composite. Using CaCO3 NPs improved the mechanical properties and enhancement thermal conductivity [17-19].
The current study evaluated the impact of CaCO3 nanoparticles incorporated in soft denture material at different concentrations in order to enhance thermal conductivity and some other properties.
Materials and Methods
The pilot study tested the best percentage of CaCO3 NPs by evaluating the effect on thermal conductivity, thermal diffusivity, strength of tensile bonding and strength of tear of the soft denture line. There were six concentrations of CaCO3 NPs used (0.5%, 1%, 1.5%, 2%, 2.5%, 3%).
According to result of pilot study, 1.5% and 2% of CaCO3 NPs were the most suitable concentrations showing a favorable increase in thermal conductivity and thermal diffusivity, tensile bond strength and soft liner tear strength.
Thus, the main study was performed by applying CaCO3 NPs with concentrations of 1.5% and 2% by weight to Polyethyl Methacrylate soft denture liner (PEMA) (Vertex™ Soft, Vertex-Dental, Netherlands). 90 samples were prepared and divided into 3 groups based on the tests to be performed and each group was subdivided by the weight percentages added of CaCO3 NPs into three subgroups, (control group without CaCO3 NPs, experimental groups with 1.5% and 2% CaCO3 NPs). 10 samples for every percentage, totally thirty for each test were fabricated.
For each control (PEMA) and experimental specimens of 1.5% and 2% CaCO3 NPs the scanning electrons (AIS2300C, Angstrom Advanced Inc., USA) were taken to show the degree of CaCO3 NP dispersion in the PEMA matrix.
In order to examine the chemical reaction of CaCO3 NPs and PEMA, Fourier Transform Infrared Spectroscopy (FTIR) (Tensor 27, Bruker, Germany) analysis was performed.
Thermal properties tests
For thermal property tests disk dimensions with diameters of 40 mm and thickness of 2.5 mm, according to instrument specifications have been prepared. The monomer was supplemented with calculated amount of nano CaCO3 and nanoparticles are well dispersed in the monomer using probe sonication apparatus (120 W and 60 KHz) for 3 minutes to make the dough with a specific concentration of nanofiller. To be distributed into single nanoparticles [20]. The powder for the soft lining material has been applied with the proportion of (1.2 g powder/1 ml liquid), then mixed, packaged and cured as instructed by the manufacturer.
After a complete curing, the specimens were polished and submerged in distilling water and kept in the incubator 37°C (48) hours before the examination. The thermal analyzer of the hot disk (Tps 500, Kiteley, Sweden) has tests the thermal conductivity and the diffusivity of these specimens with a double spiral pattern extending from a thick sheet of nickel foil.
After choosing the polymer parameter, readings of the system with a thermal conductivity and thermal diffusive value could be taken in 15 minutes after the hot disk has to be turned on around 1 hour before the tests take place.
Tensile bond strength test
Thirty blocks of acrylic with certain sizes (10 mm × 10 mm × 83 mm width, depth, length respectively) and heat cured acrylic resin blocks were developed in accordance with the instructions of the manufacturer. The central part (3 mm) of the acrylic block has been removed, the mold has replenished two pieces of acrylic block with an area of (3 mm) between them, which is filled with adequate soft liner material, curing was performed on the instructions of the manufacturer [21].
As previously described in thermal properties, 10 specimens were made for each group. Utilizing an instron test machine with a crosshead speed of 5 mm/min (WDW-20, Laryee Technology Co. Ltd., China) [22]. The tested specimens and maximum failure load have been reported to calculate the bonding strength value according to the formula for each specimen:
Bond strength (N/mm2)=F/A
F: The maximum failure load (N)
A: The cross sectional specimen area (mm2)
Tear strength test
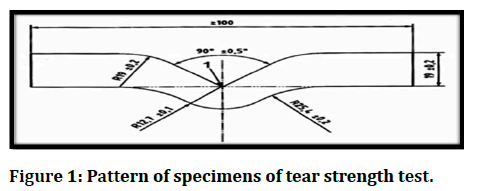
Thirty test specimens of tear strength were manufactured in the measure shown in Figure 1 according to (ASTM specification D-624, 2013). The test method calculates the force per unit thickness required for tear using a universal instron testing machine (WDW-20, Laryee Technology Co., Ltd., China) with cross head speed (500 mm/min) [23]. The following equation has been used to calculate the tear strength:
Tear Strength (Ts)=F/d
Ts: The power of tear (N/mm)
F: Peak failure force (N)
d: Thickness of specimens (mm)
Figure 1: Pattern of specimens of tear strength test.
Statistical analysis
SPSS (Statistical Social Science Package) was used to evaluate study outcomes using computer software (version 21) descriptive statistics and inferential statistics were used. One way ANOVA (Analysis of Variance) test was performed, “P” value of >0.05 was considered statically a non-significant, “d” 0.05 was considered a significant and “d” 0.01 was considered as a highly significant.
Results
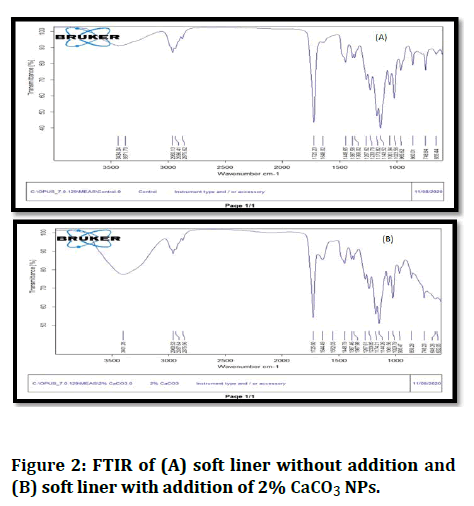
FTIR analysis: FTIR results have shown that soft liner acrylic powder has not chemically reacted to CaCO3 NP, as seen in Figure 2.
Figure 2: FTIR of (A) soft liner without addition and (B) soft liner with addition of 2% CaCO3 NPs.
SEM examination
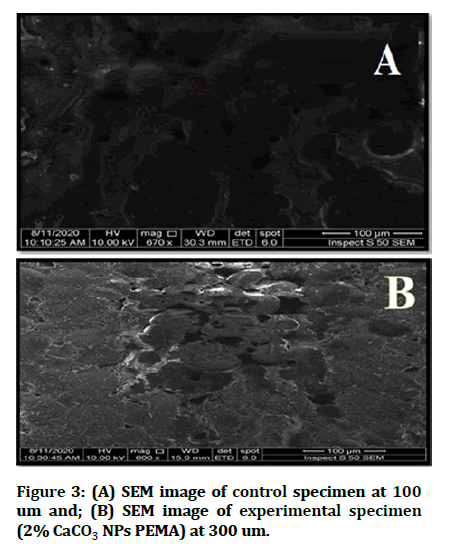
The sample morphology was investigated in the cross section before and after CaCO3 NP was applied as well as samples with PEMA soft liner mapping (Figure 3).
Figure 3: (A) SEM image of control specimen at 100 um and; (B) SEM image of experimental specimen (2% CaCO3 NPs PEMA) at 300 um.
Thermal conductivity test
The results of thermal conductivity of specimens containing 2% wt CaCO3 NPs exhibited the highest mean value of 0.285 W/mK, followed by specimens containing 1.5% wt CaCO3 NPs with 0.281 W/mK, while lowest mean value of 0.242 W/mK was obtained in control group.
The statistically significant difference between all groups in the one way ANOVA for thermal conductivity test as shown in Table 1, while the Post-Hoc Tukey Honestly Significant Difference (Tukey HSD) test used as present in Table 2 to compare the significance of the difference among two independent means showed that significant differences between study groups except for 1.5% and 2% were not significant.
| Groups | N | Mean | ± SD | ± SE | Minimum | Maximum | F | P value |
|---|---|---|---|---|---|---|---|---|
| Control | 10 | 0.242 | 0.009 | 0.003 | 0.231 | 0.255 | 40.604 | 0.000 HS |
| 1.5% CaCO3 NPs | 10 | 0.281 | 0.014 | 0.004 | 0.263 | 0.299 | ||
| 2% CaCO3 NPs | 10 | 0.285 | 0.012 | 0.004 | 0.267 | 0.298 |
Table 1: Means of thermal conductivity and ANOVA test for all studied groups.
| Variables | (I) Groups | (J) Groups | Mean difference (I-J) | P value |
|---|---|---|---|---|
| Thermal conductivity | Control | 1.5% CaCO3 NPs | -0.039 | 0.000 HS |
| 2% CaCO3 NPs | -0.043 | 0.000 HS | ||
| 1.5% CaCO3 NPs | 2% CaCO3 NPs | -0.004 | 0.691 NS | |
| Thermal diffusivity | Control | 1.5% CaCO3 NPs | -0.0398 | 0.000 HS |
| 2% CaCO3 NPs | -0.0439 | 0.000 HS | ||
| 1.5 % CaCO3 NPs | 2% CaCO3 NPs | -0.0041 | 0.725 NS | |
| Tensile bond strength | Control | 1.5% CaCO3 NPs | -0.362 | 0.0014 HS |
| 2% CaCO3 NPs | -0.414 | 0.0003 HS | ||
| 1.5% CaCO3 NPs | 2% CaCO3 NPs | -0.052 | 0.8390 NS | |
| Tear strength | Control | 1.5% CaCO3 NPs | -1.553 | 0.0874 NS |
| 2% CaCO3 NPs | -1.908 | 0.0394 Sig. | ||
| 1.5% CaCO3 NPs | 2% CaCO3 NPs | -0.355 | 0.6646 NS |
Table 2: Multiple comparisons of thermal conductivity, thermal diffusivity, tensile bond strength and tear strength between percentages using Tukey HSD.
Thermal diffusivity test
Thermal diffusivity of specimens containing 2% wt CaCO3 NPs exhibited the highest mean value of 0.255 mm2/sec, followed by specimens containing 1.5% wt CaCO3 NPs with 0.251 mm2/sec, while lowest mean value of 0.221 mm2/sec was obtained in control groups.
The statistically significant difference in one way ANOVA for thermal diffusivity tests between all groups as shown in Table 3, while the Post-Hoc Tukey Honestly Significant Difference (Tukey HSD) test was used as in Table 2 to compare the significance of the difference among two independent means showed that significant differences between study groups except for 1.5% and 2% were not significant.
| Groups | N | Mean | ± SD | ± SE | Minimum | Maximum | F | P value |
|---|---|---|---|---|---|---|---|---|
| Control | 10 | 0.211 | 0.01 | 0.003 | 0.201 | 0.227 | 40.85 | 0.000 HS |
| 1.5% CaCO3 NPs | 10 | 0.251 | 0.014 | 0.004 | 0.234 | 0.268 | ||
| 2% CaCO3 NPs | 10 | 0.255 | 0.012 | 0.004 | 0.234 | 0.265 |
Table 3: Means of thermal diffusivity and ANOVA test for all studied groups.
Tensile bond strength test
The results of tensile bond strength of specimens containing 2% wt CaCO3 NPs exhibited the highest mean value of 1.6450 N/mm2, followed by specimens containing 1.5% wt CaCO3 NPs with 1.5930 N/mm2, while lowest mean value of 1.2310 N/mm2 was obtained in control groups.
One-way ANOVA shows a significant difference between all the groups tested as shown in Table 4 for the strength of tensile bond testing. In the Tukey HSD test, a significant difference among the studied groups was revealed, except that the non-significance as shown in Table 2 appeared between the 1.5% group and 2% group.
| Groups | N | Mean | ± SD | ± SE | Minimum | Maximum | F | P value |
|---|---|---|---|---|---|---|---|---|
| Control | 10 | 1.231 | 0.2208 | 0.0698 | 0.94 | 1.56 | 12.067 | 0.000 HS |
| 1.5% CaCO3 NPs | 10 | 1.593 | 0.245 | 0.0775 | 1.18 | 1.84 | ||
| 2% CaCO3 NPs | 10 | 1.645 | 0.1329 | 0.042 | 1.46 | 1.82 |
Table 4: Means of tensile bond strength and ANOVA test for all studied groups.
Tear strength test
The result of tear strength test of specimens containing 2% wt CaCO3 NPs exhibited the highest mean value of 17.222 N/mm, followed by specimens containing 1.5% wt CaCO3 NPs with 16.867 N/mm, while lowest mean value of 15.314 N/mm was obtained in control group.
The ANOVA test showed a significant difference between all tested groups, shown in Table 5. In order for Tukey HSD test to compare the mean value for both sample groups, as in Table 2 it showed that there was a significant difference between a control group and a 2% group, whereas there was non-significant difference between other studied groups.
| Groups | N | Mean | ± SD | ± SE | Minimum | Maximum | F | P value |
|---|---|---|---|---|---|---|---|---|
| Control | 10 | 15.314 | 1.939 | 0.613 | 12.97 | 18.13 | 5.705 | 0.009 HS |
| 1.5% CaCO3 NPs | 10 | 16.867 | 0.784 | 0.248 | 15.87 | 18.23 | ||
| 2% CaCO3 NPs | 10 | 17.222 | 1.021 | 0.323 | 15.67 | 18.76 |
Table 5: Means of tear strength and ANOVA test for all studied groups.
Discussion
The results of incorporating CaCO3 NP into PEMA have been evaluated in this study. The thermal properties of the soft liner and some other mechanical properties such as tensile bond strength and tear strength were studied.
The peaks of FTIR analysis for control and experimental specimens show no chemical reaction between CaCO3 nanoparticles and soft lining material. Thus no changes seen in the spectral range of soft liner after the additions of the CaCO3 nanoparticles.
SEM images show that the CaCO3 NPs are distributed uniformly throughout the specimen and grouped into groups throughout the polymer matrix.
The EDS of the control and experimental groups study has shown that the difference of two diagrams indicates that CaCO3 NPs are incorporated into the soft liner polymeric matrix.
Thermal conductivity of a material is the ability to assess the rate at which a certain cross sectional region of the material samples may transfer heat over a certain period of time [24].
With the incorporation of CaCO3 NPs into PEMA the thermal conductivity values increased significantly by both 1.5% and 2% (P=0.000). It may occur because particles gradually contact each other and form a network such as a structure called thermal pathways that allow heat to transition from one part of the sample on the other and bridge the polymer's insulating effect. This indicates a high thermal conductivity of the polymer [25].
In addition, the results of SEM showed that the uniform distribution of CaCO3 nanoparticles into the acrylic soft liner together with the high thermal conductivity of CaCO3 nanoparticles may explain the high thermal conductivity of the isolating material.
Thermal diffusivity is the rates at which a body at a uniform temperature reaches equilibrium and is related with the thermal conductivity.
It’s a material capable of responding to changes in temperature, for example during food intake and liquid intake in oral cavity [26].
The results of this research have shown that thermal diffusivity has increased significantly with the rise in calcium carbonate nanoparticle percentage in comparison with the control group. This is interpreted by the addition of CaCO3 NPs as the creation of thermally conducive pathways within PEMA polymer matrix. Atla, et al. agrees with this analysis [27].
The significant increase in the strength of tensile bond after addition of 1.5% wt and 2% wt of CaCO3 NPs into soft lining material due to Van der Waals formation between polymer and nanoparticles the shear strength and stiffness will increase and the critically load transfer time required to contribute to energy dissipation will be reduced [28].
In addition, the soft lining material utilized in this study have highly flow ability meaning the application of CaCO3 NPs into PEMA may influence the flow ability of the polymer which permit the soft liner easily adapt to the bonding surface forming simultaneous inter penetrating network by the molecules of the two chemically similar polymer across the interface with the acrylic resin and provides good contact, as the penetration of soft liner into acrylic resin in inversely proportional to viscosity of liner [29].
The results of tear strength test show a highly significant increased when adding CaCO3 NPs in amounts of 1.5% wt and 2% wt to the PEMA, this can be explained through the creation of the Van der Waals bond between the nanofillers and polymer, which increases polymer chains' resistance to rapture due to the strong shear force between polymer nanoparticles and chains facing tear forces.
This research shows a substantial increase in tear strength directly proportioned to the increased concentration of nanofillers because of the very fine filler dimension, leading to a higher interface bond, effective diffusion and bond homogeneity. The linkage also contributes significantly to enhancing the young's modulus [30].
In the tearing process, nanofillers scatter the energy of the stress in the polymer matrix near the tips of the developing cracks and thus highly tear resistance, which means that a large force is needed to fully break the polymer matrix and that is why the tear strength of the matrix rises [31].
Conclusion
The incorporation of powder from CaCO3 NP in an acrylic soft lining material increases significantly the thermal properties, tensile bond strength and tear strength.
Acknowledgment
Authors thank the dean and the scientific affairs assistant dean of the college of dentistry, Baghdad University for their kind help in making use of the resources and equipment available in order to carry out this work.
References
- Knechtel ME, Loney RW. Improving the outcome of denture relining. J Canadian Dent Assoc 2007; 73:587.
- Kranjcic J, Kostelic-Stunic M, CelebiC A, et al. Denture relining as an indicator of residual ridge resorption. Med Glas 2013; 10:126-132. [Googlescholar][Indexed]
- Kotwal M, Sharma A, Singh R. Evaluation of hardness of silicone and acrylic resin based resilient denture liners over a period of storage in water. Int J Sci Res 2018; 7:18-20. [Crossref]
- Hashem MI. Advances in soft denture liners: An update. J Contemp Dent Pract 2015; 16:314-318 [Crossref][Googlescholar][Indexed]
- Akay C, Tanis MC, Sevim H. Effect of artificial saliva with different pH levels on the cytotoxicity of soft denture lining materials. Int J Artif Organs 2017; 40:581-588. [Crossref][Googlescholar][Indexed]
- Mese A, Guzel KG. Effect of storage duration on the hardness and tensile bond strength of silicone and acrylic resin based resilient denture liners to a processed denture base acrylic resin. J Prosthet Dent 2008; 99:153-159. [Crossref][Googlescholar][Indexed]
- Ozdemir KG, Yilmaz H, Yilmaz S. In vitro evaluation of cytotoxicity of soft lining materials on L929 cells by MTT assay. J Biomed Mater Res B Appl Biomater 2009; 90:82-86. [Crossref][Googlescholar][Indexed]
- Iwasaki N, Yamaki C, Takahashi H, et al. Effect of long time immersion of soft denture liners in water on viscoelastic properties. Dent Mater J 2017; 36:584-589. [Crossref][Googlescholar][Indexed]
- Kapur KK, Fischer EE. Effect of denture base thermal conductivity on gustatory response. J Prosthet Dent 1981; 46:603-609. [Crossref][Googlescholar][Indexed]
- Power JM, Sakaguchi RL. Craig’s restorative dental materials. Elsevier, 12th edition, United State, 2006.
- Farzin M, Bahrani F, Adelpour E. Comparison of the effect of two denture cleansers on tensile bond strength of a denture liner. J Dent 2013; 14:130-135. [Googlescholar][Indexed]
- Sanchez-Aliaga A, Pellissari CVG, Arrais CAG, et al. Peel bond strength of soft lining materials with antifungal to a denture base acrylic resin. Dent Mater J 2016; 35:194-203. [Crossref][Googlescholar][Indexed]
- Avella M, Errico ME, Martuscelli E. Novel PMMA/CaCO3 nano composites abrasion resistant prepared by an in situ polymerization process. Nano Lett 2001; 1:213–217. [Crossref][Googlescholar][Indexed]
- Abdullayev E, Lvov Y. Halloysite clay nanotubes as a ceramic skeleton for functional biopolymer composites with sustained drug release. J Mater Chem B 2013; 1:2894-2900. [Crossref][Googlescholar][Indexed]
- Hasan WY, Ali MM. Evaluation of thermal conductivity and some other properties of heat cured denture soft liner reinforced by halloysite nanotubes. Biomed Pharmacol J 2018; 11:1491-1500. [Crossref][Googlescholar][Indexed]
- Jasim BS, Ismail IJ. The effect of silanized alumina nano-fillers addition on some physical and mechanical properties of heat cured polymethyl methacrylate denture base material. J Baghdad Coll Dent 2014; 26:18-23. [Googlescholar][Indexed]
- Shimpi NG, Verma J, Mishra S. Dispersion of nano CaCO3 on PVC and its influence on mechanical and thermal properties. J Compos Mater 2010; 44:211–219. [Crossref][Googlescholar][Indexed]
- Vakili MH, Ebadi-Dehaghani H, Haghshenas-Fard M. Crystallization and thermal conductivity of CaCO3 nanoparticle filled polypropylene. J Macromol Sci Part B 2011; 50:1637-1645. [Crossref][Googlescholar][Indexed]
- Abdolmohammadi S, Siyamak S, Ibrahim NA, et al. Enhancement of mechanical and thermal properties of polycaprolactone/chitosan blend by calcium carbonate nanoparticles. Int J Mol Sci 2012; 13:4508-4522. [Crossref][Googlescholar][Indexed]
- Taurozzi JS, Hackley VA, Wiesner M. Preparation of nanoparticle dispersions from powdered material using ultrasonic disruption. NIST special publication 2012; 1200:1200-1202. [Googlescholar][Indexed]
- Botega DM, Sanchez JLL, Mesquita MF, et al. Effects of thermo cycling on the tensile bond strength of three permanent soft denture liners. J Prosthodont 2008; 17:550-554. [Crossref][Googlescholar][Indexed]
- ASTM D (American Society of Testing and Materials). Test Standards for the Evaluation of Plastic Tensile and Flexural Properties-ASTM D638 and ASTM D790. ASTM international, Philadelphia, ANSI. 1986.
- ASTM D624-00 (American Society of Testing and Materials). Standard Test Method for Tear Strength of Conventional Vulcanized Rubber and Thermoplastic Elastomers. ASTM International, West Conshohocken, PA, USA. 2012.
- Sakaguchi RL, Powers JM. Craig's restorative dental materials e-book. Elsevier Health Sciences, 13th edition, United State, 2012. [Crossref][Googlescholar][Indexed]
- Yu Z, Wang X, Bian H, et al. Enhancement of the heat conduction performance of boron nitride/cellulosic fiber insulating composites. PloS One 2018; 13:e0200842. [Crossref][Googlescholar][Indexed]
- Ellakwa AE, Morsy MA, El-Sheikh AM. Effect of aluminum oxide addition on the flexural strength and thermal diffusivity of heat polymerized acrylic resin. J Prosthet 2008; 17:439-444. [Crossref][Googlescholar][Indexed]
- Atla J, Manne P, Gopinadh A, et al. The effect of Al2O3 addition on the thermal diffusivity of heat activated acrylic resin. J Clin Diagn Res 2013; 7:1797-1798. [Crossref][Googlescholar][Indexed]
- Sun L, Gibson RF, Gordaninejad F, et al. Energy absorption capability of nanocomposites: A review. Compos Sci Technol 2009; 69:2392-2409. [Crossref][Googlescholar][Indexed]
- Jacobsen NL, Mitchell DL, Johnson DL, et al. Lased and sandblasted denture base surface preparations affecting resilient liner bonding. J Prosthet Dent 1997; 78:153-158. [Crossref][Googlescholar][Indexed]
- Mishra S, Shimpi NG. Studies on mechanical, thermal and flame retarding properties of Polybutadiene Rubber (PBR) nanocomposites. Polym Plast Technol Eng 2007; 47:72-81. [Crossref][Googlescholar][Indexed]
- Shakir DA, Abdul-Ameer FM. Effect of nano titanium oxide addition on some mechanical properties of silicone elastomers for maxillofacial prostheses. J Taibah Univ Med Sci 2018; 13:281-290. [Crossref][Googlescholar][Indexed]
Author Info
Samir Kamil Abdulmajeed* and Hikmat Jameel Abdulbaqi
Department of Prosthodontics, College of Dentistry, University of Baghdad, Baghdad, IraqCitation: Samir Kamil Abdulmajeed, Hikmat Jameel Abdulbaqi, The Impact of Calcium Carbonate Nanoparticles Incorporation into Heat Cured Soft Denture Lining Material on Thermal Conductivity and Some Other Properties, J Res Med Dent Sci, 2023, 11 (01):208-214
Received: 25-Oct-2022, Manuscript No. JRMDS-22-68392; , Pre QC No. JRMDS-22-68392 (PQ); Editor assigned: 28-Oct-2022, Pre QC No. JRMDS-22-68392 (PQ); Reviewed: 11-Nov-2022, QC No. JRMDS-22-68392; Revised: 28-Dec-2022, Manuscript No. JRMDS-22-68392 (R); Published: 25-Jan-2023