Research - (2022) Volume 10, Issue 3
The Influence of Chitosan on Adhesion of Silver Oxide Nanoparticles Coating on SS 316L in Biomedical Applications
Dhuha A Al-Ali*, D Al Groosh and Makarim H Abdulkareem
*Correspondence: Dhuha A Al-Ali, Department of Orthodontics, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
The stainless-steel alloys; particularly 316L; are the major metallic alloys to be used in biomedicine due to their superior characteristics. Surface engineering is the process equipped successfully with stainless steel to be used as a functional biomaterial. The purpose of this study is to establish the better parameters (concentration, voltage and time) with electrophoretic deposition (EPD) method of coating to form a layer of silver oxide nanoparticles, to get benefit of its antimicrobial effect in biomedicine; with chitosan as a binder based on microstructure and adhesion of the coated layer; in addition to XRD analysis to evaluate the phases before and after coating. The materials used were stainless steel 316L as a substrate, silver oxide nanoparticles, chitosan and citric acid and ethanol alcohol as a solvent. The EPD coating method was performed using different parameters (concentration, time, and voltage) with various values at room temperature, and the examination of the treated samples under light microscope and adhesion test revealed the best concentration was 7.5 g/L, the voltage was 30 V and the deposition time was 2mins. The coated samples were examined using XRD that showed no phases transformation. The results are promising in medical field since the EPD is a cost effective and easily applied coating procedure.
Keywords
Silver oxide nanoparticles, EPD, Stainless steel, Coating, Chitosan
Introduction
Stainless steel (SS) alloy; predominantly SS 316L; being widely used in medical scope as a biomaterial for fabrication of devices and prosthesis serving the biomedical field because of its properties of durability against fatigue and corrosion and the malleability. Despite of that, it has several cons mainly lacking bifunctionality. To be applied in biomedicine in successful manner, SS have to be treated to be bifunctional by raising its anti-fouling characteristics, restraining the figuration of biofilm (passively modifying the surface), and imparting efficiency for abolishing a particular medicine or targeting specific cells (actively modifying the surface); most of these properties rely on the ultimate surface modification. Several physical and chemical surface engineering procedures; like engagement of special binders to establish subsidiary active groups, plasma vapor deposition, etc; are being utilized to attain SS having enhanced mechanical, biocompatible and antibacterial properties [1].
Nanotechnology nowadays has been correlated with various daily life disciplines and its applications invade many branches of science like medical field [2]. The greater surface area linked to volume (per unit mass) make the nanomaterials to be more effective and reactive when equated with bulk row materials. They also show a prospect in surface chemistry when related to parallel row materials due to its ability to be charged with functional stuffs that has ability to target specific molecules. In addition to that, the physical features of nanomaterials (e.g., morphology, size, chemical composition, and porosity) have a direct influence on the material behavior [3,4]. The superior characteristics of the nanosubstance’s are related to their particles size [5], colloidal stability in water [6], capacity to target living tissues [7] and its antimicrobial behavior [2].
Nano silver (Ag) is extensively studied for its antimicrobial impact that is largely associated with the release of Ag+ ions when comes in contact with aquatic complex [8]. Mechanisms of antibacterial action of nanosilver particles is by targeting the bacterial cell envelope upon contact, then producing reactive oxygen species [9]. Also, its antimicrobial effectiveness has been explored against fungi and viruses [10]. In addition to that, its antibiofilm effect has been investigated as nano silver coating [11].
Surface treatment of biomaterials is a prevalent approach to enhance the substrate's bioactivity and biocompatibility or to improve the antimicrobial characteristics or sustained releasing of drug, whereas maintaining the row material's characteristics [12]. One prospect to attain antimicrobial] surfaces is loading the biomaterial surface with antibiotic agents. Vancomycin could be loaded on the surface of substrate being supported by the porosity of ceramic coatings or incorporated in the sol-gel layer simply by immersion in antibiotic solutions [13]. Different method is the co-deposition of silver with its antibacterial property by various techniques. Teker et al. utilized micro-arc oxidation to incorporate silver with ceramic coating on commercially pure titanium (Cp-Ti) alloy [14], While Chen et al. used the magnetron sputtering to deposit hydroxyapatite coating containing silver on Cp-Ti [15], physical vapor deposition (PVD) being applied to coat Cp- Ti surface with titanium/silver coating [16], electrophoretic deposition (EPD) also employed to deposit bioactive glass/chitosan/nano-silver coating on 316L stainless steel [17] and Zndoped BG/chitosan coatings [18] among others.
EPD is an effective and rapid procedure to fabricate organic/inorganic coating layers on materials, especially metallic substrates [19]. With minor adaptation of the suspension composition and concentration, applied potential and deposition time, it is likely to produce the required thickness and deposited films [20]. EPD applied in many medical aspects by applying surface treatment of biomaterials. For instance, coatings containing hydroxyapatite or bioactive glass (BG) could enhance the bioactivity [21]. In addition to that, the EPD with biopolymer materials; like gelatin, chitosan, etc; offers the possibility of avoiding the sintering step, to increase the coating's mechanical strength [22]. The heating cycle may lead to phase transformation and oxidation, in addition to shrinkage that leads to cracks within the coating [23].
Chitosan is a cationic polysaccharide material that naturally found and possess several crucial characteristics like biocompatibility, the chemical stability, antibacterial effectiveness, and improved mechanical characteristics among others that made it a vivid material in various life aspects, mainly medical field [24].
The aim of this study is to optimize the parameters of electrophoretic deposition coating of silver oxide nanoparticles on stainless steel 316L substrate which is widely used in medical applications by using chitosan (biopolymer material) as a binder between the AgO NPs and the substrate surface instead of sintering step.
Materials and Methods
Materials used
Silver oxide nanoparticles (Purity: 99.9%, Nanoshel company, India) with particle size 50nm. Chitosan (medium molecular weight with a degree of deacetylation of about 85% soluble in 1% acetic acid) (Sigma Aldrich). Ethanol absolute (99.9% purity, Scharlau, Spain) was utilized for the preparation of suspension. 316L stainless steel plate with the chemical components as listed in Table 1, was utilized as the substrate and the counter electrodes in the electrophoretic deposition cell.
| Elements | C | Si | Mn | P | S | Cr | Mo | Ni | Al | Co | Cu | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt.% | 0.015 | 0.578 | 1.35 | 0.043 | 0.008 | 16.8 | 1.97 | 9.35 | 0.003 | 0.221 | 0.244 | 0.005 |
| Elements | Ti | V | W | Ta | N | Sn | Pb | Se | Sb | others | Fe | |
| Wt.% | 0.011 | 0.039 | 0.037 | >0.01 | 0.041 | 0.011 | 0.003 | 0.005 | >0.003 | >0.001 | Remain |
Table 1: Chemical compositions of 316L stainless steel.
Preparation of substrate
The 316L stainless steel plate was divided into pieces with dimensions of 20 × 10 × 2 mm using wire cutting machine, to be used as the electrodes.
To enhance the bonding adhesion between the deposited layer and the surface of substrate material; in preparation to coating with the EPD method; the substrate samples were polished by applying papers of SiC emery with 120, 400, 600, 800, and 1200 μm grits [25]. Before the coating procedure, the samples were washed with ethanol, then acetone in an ultrasonic bath (25 kHz, 100% sweep; Elma, Fisher Bioblock Scientific) for 15 min and then let to dry at room temperature [26].
Preparation of suspension
The suspensions of silver oxide nanoparticles were composed of 0.5 g/L of chitosan dissolved in acetic acid (1 vol.%). After that, ethanol (94 vol.%) and water (5 vol.%) were poured to the mixture. The prepared suspensions were put on a magnetic stirrer for 15 mins to be stirred at room temperature, then 5, 7.5, 10 or 25 g/L concentrations of AgO nanoparticles powder were added to the prepared suspensions. The ethanol–water mixture enhances the colloidal stability of the inorganic particles. The equipped mixtures were put on stirrer for 6 hours. After that, the suspensions were disseminated for 30 mins ultrasonically for particles dispersing to split delicate conglomerates that resulted in homogeneous suspension. Then, the mixtures were magnetically stirred for 10 min to attain a homogeneous mixture in preparation for coating [27]. The pH of the suspensions was measured to be fit 4-4.5. Zeta potential test also accomplished to analyze the stability of suspension, that obtaining a homogeneous solution ensure homogeneous coating layers.
EPD
The EPD cell composed of a glass beaker that was furnished with a direct current (DC) power supply with alternating voltage on demand to gain the optimum deposition result [28]. Two 316L stainless steel pieces were utilized in the cell as the cathode and anode with a 10 mm interspace between the two electrodes. Both electrodes were immersed in the suspension parallel to each other using a 200-ml glass beaker with a 1 cm interspace between them. So as to achieve the superior deposition parameters, procedure was executed at 30, and 60 V utilizing an adjusted DC power supply for a deposition time of 2, 5, and 9 mins at room temperature (Figure 1). The surface area of about 15 mm2 of the substrate was coated. After coating procedure was accomplished, the substrates were let too dry for 24 h at room temperature. Lately, the coated samples were weighted using a calibrated microbalance with an accuracy of ±0.0001% [27].
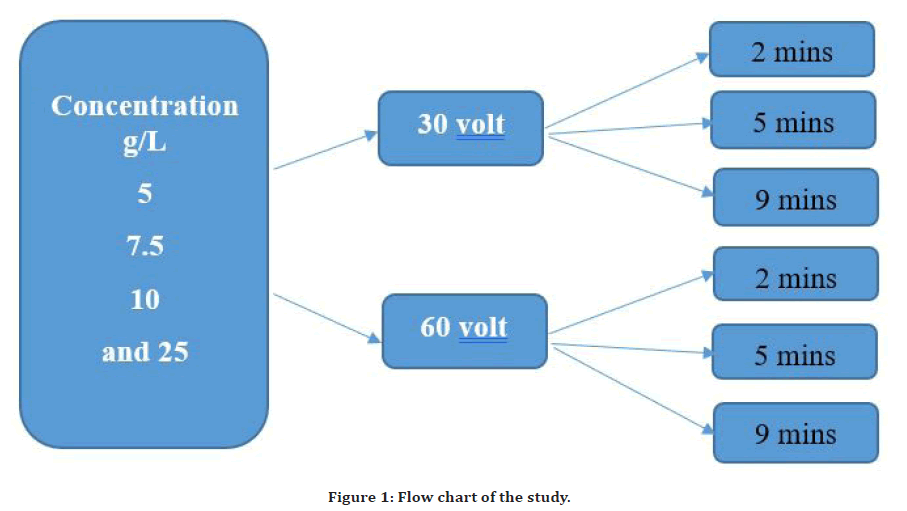
Figure 1. Flow chart of the study.
Results and Discussion
In this study, three variables were checked for their effect on coating characteristics; in the presence of chitosan biomaterial as a binder; which are four concentrations (5, 7.5, 10, 25) g/L, two voltages (30, 60)V and three time of deposition (2, 5, 9)mins. So, we had 24 experiments with triplicate manner.
Cross-cut adhesion test
The evaluation of strength of adhesion of the coated layer on the Stainless steel 316L substrate surface was performed by the cross-cut tape adhesion test [29]. The cross-cut adhesion test, which is adopted as a universal approach to investigate the adhesion of overlay coating layer above the substrate: labeled as the grades 5B, 4B, 3B, 2B, 1B and 0B in a sequence from the best adhesion (5B) to the lowest (0B). According to the ASTM D3359-B standard, the peeling areas were between 4.5-65%. The using of high concentration of NP (25g/L) with high voltage, the deposited layer become nonhomogeneous and easily peeled away (65%) (grade 1B). while using 30V with the low concentration of NP (7.5g/L) and less deposition time results in homogenous coating layer with peeling of 4.5% (grade 4B). This may be explained by the coated layer thickness grew with raising of the applied voltage. This could result in increasing the deposition rate at the expense of the quality of deposited layer but that raising also increase the percentage of porosity. Because the deposit layer installation on an electrode is a kinetic phenomenon so accumulation rate of the deposits influences coating layer behavior. In addition to that, the greater the applied field might result in particles agitation within the suspension. It could produce irregular coating layer due to the perturbation passage of the suspension particles.
It could generate coating layer with high porosity [30, 31]. This came in agreement with Jia et al. in their study about using the ethanol solution with EPD in preparing a high-temperature resistant and electrically insulating h-BN coating [32].
While about the deposition time effect on coating, also there was increased weakening of the adhered layer with prolonged deposition period. Previous studied showed that the deposition rate minimizes with elevated deposition time [33]. At the onset of the deposition procedure, a linear relationship was observed between the deposition rate and deposition time. With raising of the deposition time, the rate of coating may reach zero. This occurrence had happened due to the accumulated thickness that generated isolating deposited layer with time turns into a barrier that debilitated the applied electric field to the suspension particles to reach the substrate [34]. The adhesion results are summarized in Figures 2 and Figure 3.
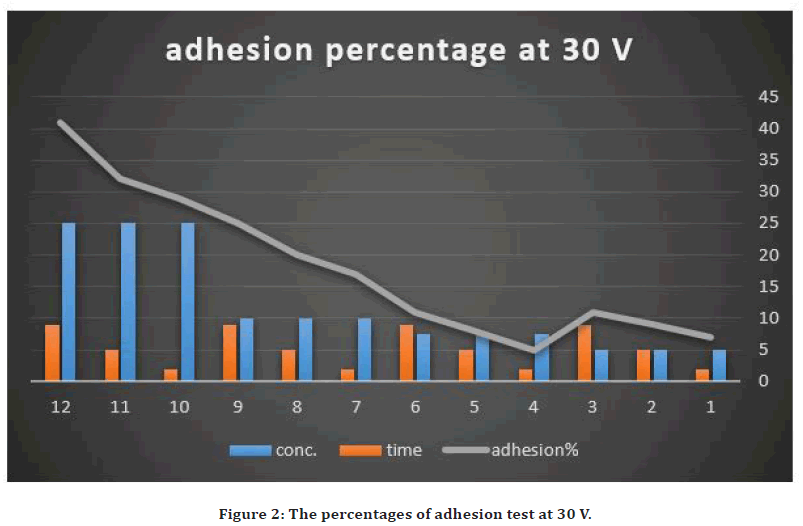
Figure 2. The percentages of adhesion test at 30 V.
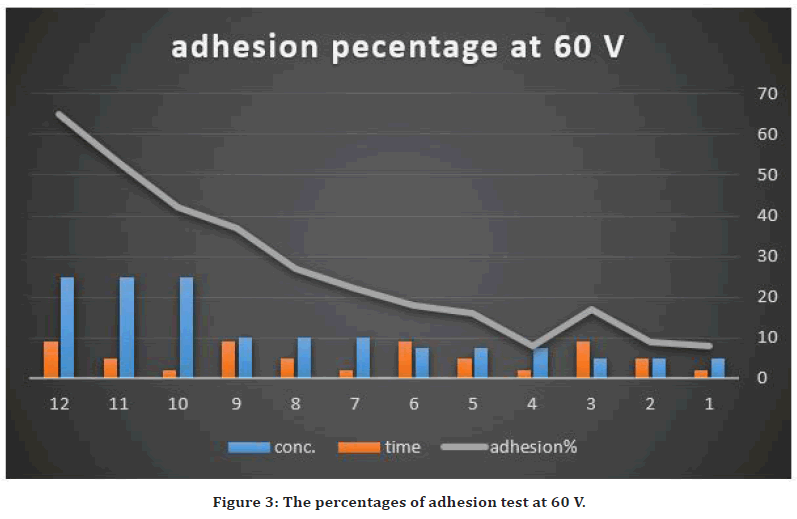
Figure 3. The percentages of adhesion test at 60 V.
So, the excellent adhesion value was obtained by utilizing conc. of 7.5g/L with 30V and 2 min coating time because the removal area was only 5%. In addition to that, the Zeta potential was measured for suspension with 7.5g/L of AgO nanoparticles and the value was +24, which predict the colloidal stability of the suspension.
XRD analysis
Phases of stainless steel 316L substrate before coating, nanoparticles powders, and the coating layer are characterized by XRD profile. Three different 2θ peaks are related for austenitic 316L stainless steel [74.55º (220), 50.57º (200) and 43.58º (111)]. They identified according to standard Joint Committee on Powder Diffraction Standards (JCPDS) card No 33-0397. The XRD pattern of silver oxide nanoparticles showed Three different 2θ peaks [32.99º (111), 38.20º (202), and 55.21º (021)], which is in acceptance with (JCPDS FILE NO. 84–1108) of AgO [35].
While the XRD peaks for silver oxide nanoparticles coating on stainless steel substrate were (44.59º, 55.2 º and 38.31º), which are related to the AgO NPs.
The remaining peaks were included (43.75º, 50.69º, and 74.72º), which were related to the underlying stainless-steel substrate. This agrees with [36]. So, there was no phases transformation mainly due to not using sintering and the procedure was carried out at room temperature.
Conclusion
✓ The best adhesion ratio achieved was by using AgO NPs concentration of 7.5 g/L with 30V DC and deposition time of 2 mins.
✓ The addition of chitosan in coating successfully permitted to avoid the sintering step with its drawbacks.
References
- Bekmurzayeva A, Duncanson WJ, Azevedo HS, et al. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Materials Sci Eng 2018; 93:1073-89.
- Fahmy HM, Mosleh AM, Abd Elghany A, et al. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adva 2019; 9:20118-36.
- Guerra FD, Smith GD, Alexis F, et al. A survey of VOC emissions from rendering plants. Aerosol Air Qual Res 2017; 17:209-217.
- Guerra FD, Campbell ML, Whitehead DC, et al. Tunable properties of functional nanoparticles for efficient capture of VOCs. Chemistry Select 2017; 2:9889-94.
- Zhang S, Ren F, Wu W, et al. Size effects of Ag nanoparticles on plasmon-induced enhancement of photocatalysis of Ag-α-Fe2O3 nanocomposites. J Colloid Interface Sci 2014; 427:29-34.
- Alexander CM, Goodisman J. Size histograms of gold nanoparticles measured by gravitational sedimentation. J Colloid Interface Sci 2014; 418:103-12.
- Wang D, Ye J, Hudson SD, et al. Effects of nanoparticle size and charge on interactions with self-assembled collagen. J Colloid Interface Sci 2014; 417:244-9.
- Sotiriou GA, Meyer A, Knijnenburg JT, et al. Quantifying the origin of released Ag+ ions from nanosilver. Langmuir 2012; 28:15929-36.
- Gunawan C, Faiz MB, Mann R, et al. Nanosilver targets the bacterial cell envelope: the link with generation of reactive oxygen radicals. ACS Appl Mate Interfaces 2020; 12:5557-68.
- Jeremiah SS, Miyakawa K, Morita T, et al. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem Biophy Res Commun 2020; 533:195-200.
- Balagna C, Perero S, Percivalle E, et al. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceramics 2020; 1:100006.
- Heimann RB. Structure, properties, and biomedical performance of osteoconductive bioceramic coatings. Surface Coatings Technol 2013; 233:27-38.
- Zhao L, Chu PK, Zhang Y, et al. Antibacterial coatings on titanium implants. J Biomed Materials Res 2009; 91:470-80.
- Teker D, Muhaffel F, Menekse M, et al. Characteristics of multi-layer coating formed on commercially pure titanium for biomedical applications. Materials Sci Eng 2015; 48:579-85.
- Chen W, Liu Y, Courtney HS, et al. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006; 27:5512-7.
- Ewald A, Glückermann SK, Thull R, et al. Antimicrobial titanium/silver PVD coatings on titanium. Biomed Eng 2006; 5:1-0.
- Shin KR, Yoon SI, Ko YG, et al. Deposition of hydroxyl-apatite on titanium subjected to electrochemical plasma coating. Electrochem Acta 2013; 109:173-80.
- Miola M, Verné E, Ciraldo FE, et al. Electrophoretic deposition of chitosan/45S5 bioactive glass composite coatings doped with Zn and Sr. Frontiers Bioeng Biotechnol 2015; 3:159.
- Seuss S, Lehmann M, Boccaccini AR. Alternating current electrophoretic deposition of antibacterial bioactive glass-chitosan composite coatings. Int J Mol Sci 2014; 15:12231-42.
- Abdulkareem MH, Abdalsalam AH, Bohan AJ. Influence of chitosan on the antibacterial activity of composite coating (PEEK/HAp) fabricated by electrophoretic deposition. Progress Organic Coatings 2019; 130:251-9.
- Moskalewicz T, Kot M, Seuss S, et al. Electrophoretic deposition and characterization of HA/chitosan nanocomposite coatings on Ti6Al7Nb alloy. Metals Materials Int 2015; 21:96-103.
- Javidi M, Javadpour S, Bahrololoom ME, et al. Electrophoretic deposition of natural hydroxyapatite on medical grade 316L stainless steel. Materials Sci Eng 2008; 28:1509-15.
- Boccaccini AR, Keim S, Ma R, et al. Electrophoretic deposition of biomaterials. J Royal Society Interface 2010; 7:S581-613.
- Ishihara MA, Hattori HI, Nakamura SH. A review on biomedical applications of chitosan-based biomaterials. Int J Pharm Biol Sci 2015; 6:162-78.
- Khan AS, Awais M. Low-cost deposition of antibacterial ion-substituted hydroxyapatite coatings onto 316L stainless steel for biomedical and dental applications. Coatings 2020; 10:880.
- Kaya S, Boccaccini AR. Electrophoretic deposition of zein coatings. J Coatings Technol Res 2017; 14:683-9.
- Hammood AS. Biomineralization of 2304 duplex stainless steel with surface modification by electrophoretic deposition. J Applied Biomaterials Functional Materials 2020; 18:2280800019896215.
- Amrollahi P, Krasinski JS, Vaidyanathan R, et al. Electrophoretic deposition (EPD): Fundamentals and applications from nano-to micro-scale structures. Handbook of Nanoelectrochemistry, Springer International Publishing Switzerland. 2015.
- https://www.micomlab.com/micom-testing/astm-d3359/#:~:text=ASTM%20D3359%20is%20a%20standard,as%20the%20Cross%20Hatch%20test.
- Saxena A, Rout A. The study of hydroxyapatite and hydroxyapatite-chitosan composite coatings on stainless steel by electrophoretic deposition method (Doctoral dissertation) 2011.
- Zhang SH, Sorrel CC, Li FY. Electrophoretic deposition of ceramic powders. J Materials Sci Technol 2005; 21:107-10.
- Jia K, Meng X, Wang W. Study on the preparation of high-temperature resistant and electrically insulating h-bn coating in ethanol solution by electrophoretic deposition. Processes 2021; 9:871.
- Lau KT. Controlled surface layer deposition for steel surface hardening, University of New South Wales, PhD Thesis. 2012.
- Maciąg F, Moskalewicz T, Kowalski K, et al. The effect of electrophoretic deposition parameters on the microstructure and adhesion of zein coatings to titanium substrates. Materials 2021; 14:312.
- Pawar O, Deshpande N, Dagade S, et al. Green synthesis of silver nanoparticles from purple acid phosphatase apoenzyme isolated from a new source Limonia acidissima. J Exp Nanosci 2016; 11:28-37.
- Soloviev M, Gedanken A. Coating a stainless-steel plate with silver nanoparticles by the sonochemical method. Ultrasonics Sonochem 2011; 18:356-62.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Dhuha A Al-Ali*, D Al Groosh and Makarim H Abdulkareem
1Department of Orthodontics, College of Dentistry, University of Baghdad, Baghdad, IraqReceived: 04-Mar-2022, Manuscript No. JRMDS-22-57795; , Pre QC No. JRMDS-22-57795 (PQ); Editor assigned: 07-Mar-2022, Pre QC No. JRMDS-22-57795 (PQ); Reviewed: 21-Mar-2022, QC No. JRMDS-22-57795; Revised: 25-Mar-2022, Manuscript No. JRMDS-22-57795 (R); Published: 31-Mar-2022
