Research - (2022) Volume 10, Issue 9
Titanium Ions Release from Commercially Pure Titanium used in Dentistry after Exposure to Hydrogen Peroxide (In vitro Study)
*Correspondence: Amrah Y Al-Jmmal, Department of Prosthodontic, College of Dentistry, University of Mosul, Iraq, Email:
Abstract
Background and Methods:: Evaluate the effects of antiviral hydrogen peroxide in different percentage (1%, 2%, 3%) applied at different time intervals on commercially pure titanium surfaces in prostheses and calculate the amount of titanium ions release, eighty of commercially pure titanium samples were divided in to (4) groups according the concentration of hydrogen peroxide that exposed to different time interval (5 ,10,15,30) mint as aging time, cpTi samples were coded, the quantities of ions released of (Ti) were measured for each solutions, after the immersion time was finished. Result: After immersion time, (Ti) ion release in solution with (1% H2 O2) present in small amount in of all exposure time, while 3% H2O2 solution (Ti) ion release in large amount when compare with control (immersed in deionize water) when compare between four immersion solutions after various immersion times, a statistically significant difference occur at (p ≤ 0.05). Conclusion: Antiviral hydrogen peroxide usage as a therapeutically agent in different concentration and immersion in different time lead to release of ions (toxic ions), release of (Ti) ions increase with the more in the (H2O2) concentration and increase in the time of exposure to it.
Keywords
Corrosion, Ti alloy, Ion release
Introduction
Materials of implants are commonly made of titanium. Success of commercially pure titanium in osseointegration of implants this payable more attention usage of titanium in dentistry [1]. Different therapeutic agents used in the mouth, titanium alloys properties affected by these various materials. Hydrogen peroxide (H2O2) and fluoride frequently used in dentifrices, mouthwashes, chewing gums [2-5]. Numerous studies said after exposure to therapeutic agent for example hydrogen peroxide. Titanium and its alloys subjected to corrosion, degradation process of titanium lead to (Ti) ions release from titanium alloys to the around area occurring inflammation of implant [6]. Through corrosion, release elements from casting alloys through the body for little times, days and more times for months [7]. A suitable substance for titanium ions determination by spectrophotometric the ascorbic acid is [8]. Hydrogen peroxide antiviral mouthwash used against viral infections, presented in various studies as (1, 1.5) percentage daily rinse follow up after two years. In vitro study originate that (3%) mouth wash with H2O2 rinse within 1-30 mint before oral surgery this lead to reduce the number of microorganisms in the oral cavity excellently inactivated adenovirus [9,10].
Aim
We aimed to assess the effects of antiviral hydrogen peroxide in different percentage (1%, 2%, 3%) applied at different time intervals on commercially pure titanium surfaces in prostheses and calculate the amount of titanium ions released.
Material and Methods
Eighty samples of commercially pure titanium (grade2, Orotic, lot 3754) was used in this study that are generally used as dental casting alloy and material of implant, dimensional of samples (20* 10 * 0.6) mm were designed according to ADA(ADA, 2002) [11]. After samples preparation and polishing, isopropyl alcohol should use for cleaning the sample for fifteen mint and then for ten mint wash within distilled water.
Immersion test
Total samples of (cpTi) used in this study divided to five specimens immersed in each of four types of solution (1%, 2% and 3%) concentration of hydrogen peroxide:
Samples immersed in deionize water (control group).
Samples immersed in 1% hydrogen peroxide (H2O).
Samples immersed in 2% hydrogen peroxide (H2O).
Samples immersed in 3% hydrogen peroxide (H2O).
Samples was divided in to (4) groups according the concentration of hydrogen peroxide that exposed to different time interval (5 mint,10 mint,15 mint,30mint, as aging time) according to recommended used hydrogen peroxide mouth wash for one mint up to four times daily, (cpTi) specimens were coded, the quantities of ions released of (Ti) were measured for each solutions, after the immersion time was finished.
Analysis of titanium ions release with spectrophotometric determination with (Ascorbic acid)
Analyzed titanium metallic ions release in solution by spectrophotometer, to determination a small amounts of (Ti) with ascorbic acid reaction made (yellow complex) formed for the spectrophotometric The ascorbic acid substance prepared by dissolving (L-ascorbic acid) two and half gram in water and (1g) of sodium bisulfite, diluting to (hundred ml). Then addition of (sodium bisulfite) to form (dehydro ascorbic acid). From (potassium titanium oxalate) was prepared a standard solution of (Ti). After immersion, time completed of samples collected (2 mL) of hydrogen peroxide of (1%, 2%, 3%) of each time exposure, in sterile recipients at four °C and then mixed with ascorbic acid to form yellow complex, then analyzed with spectrophotometric.
Statistical analysis
One-way analysis of variance (ANOVA p<0.05), multiple analysis rang test of control, and four immersion solutions were statistically analyzed metallic ion release.
Results
Hydrogen peroxide is one of the materials that present in mouthwash in deferent concentration that can affect the metal surface. Behavior of titanium and its alloys effects by (H2O2) submitted the corrosion [5].
Titanium ion releases
The value of mean of (Ti ion release), of control, and four immersion solutions with different concentration of hydrogen peroxide (1%,2% and 3%) after exposure to various time interval are shown in Figure 1. After (5, 10, 15 and 30) mint of immersion, Ti ion release in small amount in solution with (1% H2O2) of all exposure time, while in (3% H2O2) Ti ion release in large amount when compare with control (immersed in deionize water) as shown in Figure 1. Ti ion release, test of multiple analyses rang of four immersion and control solutions with different concentration of hydrogen peroxide (1%, 2% and 3%). Table 1 shown statistically significant difference (at p≤0.05) for four immersion solutions after various immersion times.
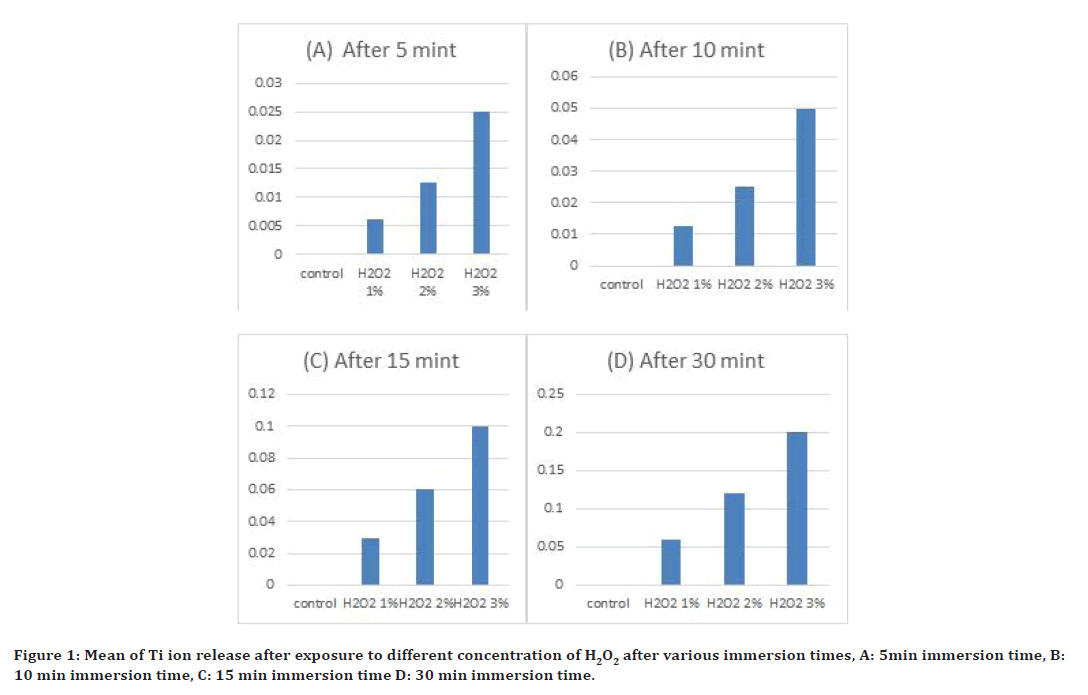
Figure 1. Mean of Ti ion release after exposure to different concentration of H2O2 after various immersion times, A: 5min immersion time, B: 10 min immersion time, C: 15 min immersion time D: 30 min immersion time.
| Sum of Squares | df | Mean Square | F | Sig | |
| Ti 5 min | |||||
| Between Groups | 0.002 | 3 | 0.001 | 1.73E+33 | 0 |
| With Groups | 0 | 16 | 0 | ||
| Total | 0.002 | 19 | |||
| Ti 10 min | |||||
| Between Groups | 0.007 | 3 | 0.002 | 1.73E+33 | 0 |
| With Groups | 0 | 16 | 0 | ||
| Total | 0.007 | 19 | |||
| Ti 15 min | |||||
| Between Groups | 0.27 | 3 | 0.009 | 2.28E+33 | 0 |
| With Groups | 0 | 16 | 0 | ||
| Total | 0.27 | 19 | |||
| Ti 30 min | |||||
| Between Groups | 0.11 | 3 | 0.037 | 2.28E+33 | 0 |
| With Groups | 0 | 16 | 0 | ||
| Total | 0.11 | 19 | |||
Table 1: Anova of Ti ion release after different immersion times.
Discussion
The usage of dental gels and mouthwashes having hydrogen peroxide as a preventive agent has improved in recent years. However high (H2O2) concentration affects the resistance of titanium and its alloys corrosion, causing in release of metal ions from dental appliances and prosthesis [12,13].
The objective of my study was to investigated the hydrogen peroxide agents effectively with different concentration and with different time exposure on the titanium surface, results was showed that the higher content of ion release of (Ti) was found in the solution of H2O2 of concentration 3% of all time interval, when compare with control and among groups, When titanium exposure to (H2O2), phenomenon will take place at the metal interface, a thin poorly crystalline oxide layer (2-6 nm) were formed by oxidation reaction. This oxide film is denser than when the metal immersion in the saline solutions for likes time. Although the mechanism of titanium alloys corrosion faster by the presence of hydrogen peroxides containing solution [14].
This vitro studies suggested that peroxide produced by inflammatory cells and therapeutically substances caused discoloration in titanium alloys and signs of pitting corrosion [15].
After exposure titanium surfaces to hydrogen peroxide, lactic acid and fluorides, when analyzed the morphological aspects of samples showed changes of titanium surfaces with (0.1-10) % H2O2 [15,16].
Implants fixed prosthesis, orthodontics and removable dentures made from commercially pure titanium, when exposed to any intraoral immersion should prevent products with more acidic pH, attendance of reactive material, O2 concentration, and temperature. Many studies were investigated, the corrosion of titanium potential in electrolytic, after immersion every (day or every (2) weeks) titanium oxide film was damage and lead to release of (Ti) ions [17,18].
Faverani, et al. [19], said “cola soft drink and artificial saliva did not alter the surface topography of the (cpTi and Ti-6Al-4V) alloy but bleaching agents (35% carbamide peroxide and 35% hydrogen peroxide) caused significant changes in the surfaces topography of both materials tested and can affect the long term success of the implant”.
This study suggested that the release of metallic (Ti) ions from titanium-based structures around the areas of peri implant, stimulate the attraction of macrophages and (T lymphocytes) from the immune system and corrosion occurrences, this is agreement with the findings of the present study [12,20,21]. Gölz, et al. [22] said "Any metal in human body is a potential source of toxicity. Corrosion has observed in hip implants, bone screws, and plates. Some experiments conducted revealed release of metallic ions from dental implants that lead to alterations in the passive layer due to changes in the oral environment causes corrosion".
Corrosive material that present in saliva for example (chloride ions, H2, free radicals, sulfide, and dissolved O2. Acidic foods and attendance of infection will reduction salivary pH about (two to three), which has a harmful effect on Ti alloy prostheses when present in high concentrations that exposed sign of corrosion [23].
This is agreement with the findings of the our study, this study was evaluated the effect carbamide act as bleaching agents has affect to corrosion resistance of titanium, (35% H2O2) at (pH 7) was exposure to the implant and supporting structure changes the surfaces of implant and abutment, after exposure to hydrogen peroxide at 4 min [24-26]. Sam, et al. [27] said “Metal ions released can pass in the bloodstream and can health affect, this study show there is a significant increase in releasing (Ni ions), while there was no significant increase in release (Ti) ions” . This study disagreement with the findings of the present study.
Zhu, et al. [28] said “In vivo study, ten ppm of (Ti) ions release in the peri-implant tissues may affect the process of osteogenesis, induced cytotoxicity in osteoblasts, where the mechanism of JNK signaling involved in regulating bone formation after Ti ion exposure could be explored further”. Lidia, et al. [29] said "The inflammatory compound of hydrogen peroxide added to saline solution affect also the passivation state of titanium alloy, the passive potential domain becomes narrower having with a higher passive current density".
Conclusion
Hydrogen peroxide (H2O2) use as antiviral therapeutically agent in different concentration and immersion in different time, this lead to degradation of commercial pure titanium (cp) material of prostheses and implant lead to release of (Ti) ions release of (Ti) ions increase with the more in the (H2O2) concentration and increase in the time of exposure to it.
References
- Bijella B, Brighenti F, Bijella M, et al. Fluoride kinetics in saliva after the use of a fluoride-containing chewing gum. Br Oral Res 2005; 19:256-260.
- Souza J, Henriques M, Teughels W, et al. Wear and corrosion interactions on titanium in oral environment: Literature review. J Bio Tribo Corrosion 2015; 1:13.
- Souza J, Henriques M, Oliveira R, et al. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling 2010; 26:471‐478.
- Souza J, Ponthiaux P, Henriques M. Corrosion behavior of titanium in the presence of Streptococcus mutans. J Dent 2013; 41:528-534.
- Sampaio M, Buciumeanu M, Henriques B, et al. Tribocorrosion behavior of veneering biomedical PEEK to Ti6Al4V structures. J Mech Behav Biomed Mater 2016; 54:123-130.
- Fais L, Carmello J, Spolidorio D, et al. Streptococcus mutans adhesion to titanium after brushing with fluoride and fluoride-free toothpaste simulating 10 years of use. Int J Oral Maxillofac Implants 2013; 28:463-469.
- Adya N, Alam M, Ravindranath T, Mubeen A, Saluja B. Corrosion in titanium dental implants: literature review. The Journal of Indian Prosthodontic Society. 2005 Jul 1;5(3):126.
- Katherine M, Joseph M, Ann M. Titanium (IV) and vitamin C: Aqueous complexes of a bioactive form of Ti (IV). Am Chemi Soci 2012; 51:11030-11039.
- Vergara-Buenaventura A, Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg 2020; 58:924-927.
- Herb M, Michelle M. Comparative analysis of antiviral efficacy of four different mouthwashes against severe acute respiratory syndrome coronavirus 2: An in vitro study. Int J Exp Dent Sci 2020; 9:1-3.
- https://www.ada.org/resources/practice/dental-standards
- Souza JC, Barbosa SL, Ariza EA, et al. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater Sci Eng 2015; 47:384-393.
- Ghazal A, Hajeer M, Al-Sabbagh R, et al. An evaluation of two types of nickel-titanium wires in terms of micromorphology and nickel ions release following oral environment exposure. Progress Orthod 2015; 16:9.
- Ramazanzadeh BA, Ahrari F, Sabzevari B, et al. Nickel ion release from three types of nickel-titanium-based orthodontic archwires in the as-received state and after oral simulation. J Dent Res Dent Clin Dent Prospects 2014; 71.
- Mabilleau G, Bourdon S, Joly-Guillou ML, et al. Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium. Acta Biomater 2006; 2:121-129.
- Noguchi T, Takemoto S, Hattori M, et al. Discoloration and dissolution of titanium and titanium alloys with immersion in peroxide-or fluoride-containing solutions. Dent Mater J 2008; 27:117-123.
- Toniollo MB, Galo R, Macedo AP, et al. Effect of fluoride sodium mouthwash solutions on cpTi: Evaluation of physicochemical properties. Br Dent J 2012; 23:496-501.
- Souza JCM, Barbosa SL, Ariza E, et al. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear 2012; 292:82-88.
- Faverani LP, Barao VA, Ramalho‐Ferreira G, et al. Effect of bleaching agents and soft drink on titanium surface topography. J Biomed Mater Res 2014; 102:22-30.
- Wachi T, Shuto T, Shinohara Y, et al. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology 2015; 327:1-9.
- Juanito GM, Morsch CS, Benfatti CA, et al. Effect of fluoride and bleaching agents on the degradation of titanium: Literature review. Dent 2015; 5:1.
- Gölz L, Knickenberg AC, Keilig L, et al. Nickel ion concentrations in the saliva of patients treated with self-ligating fixed appliances: A prospective cohort study. J Orofac Orthop 2016; 77:85-93.
- Fage SW, Muris J, Jakobsen SS, et al. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Derm 2016; 74:323-345.
- Noronha Oliveira M, Schunemann WVH, Mathew MT, et al. Can degradation products released from dental implants affect periimplant tissues? J Periodontal Res 2017; 53:1‐11.
- Apaza-Bedoya K, Tarce M, Benfatti CAM, et al. Synergistic interactions between corrosion and wear at titanium-based dental implant connections: a scoping review. J Periodontal Res 2017; 52:946‐950.
- Peñarrieta‐Juanito G, Sordi MB, Henriques B, et al. Surface damage of dental implant systems and ions release after exposure to fluoride and hydrogen peroxide. J Periodontal Res 2019; 54:46-52.
- Sam P, Dhanraj M, Jain AR. Release of titanium ions in titanium alloys used in dentistry-A systematic review. Drug Invention Today 2018; 10:536-541.
- Zhu WQ, Ming PP, Zhang SM, et al. Role of MAPK/JNK signaling pathway on the regulation of biological behaviors of MC3T3‑E1 osteoblasts under titanium ion exposure. Mol Med Reports 2020; 22:4792-4800.
- Lidia B, Nicoleta S. Impact of hydrogen peroxide and albumin on the corrosion behavior of titanium alloy (Ti6Al4V) in saline solution. Int J Electrochem Sci 2021; 16:2021.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Department of Prosthodontic, College of Dentistry, University of Mosul, IraqReceived: 03-Sep-2022, Manuscript No. jrmds-22-74395; , Pre QC No. jrmds-22-74395(PQ); Editor assigned: 05-Sep-2022, Pre QC No. jrmds-22-74395(PQ); Reviewed: 19-Sep-2022, QC No. jrmds-22-74395(Q); Revised: 23-Sep-2022, Manuscript No. jrmds-22-74395(R); Published: 30-Sep-2022
