Research - (2020) Volume 8, Issue 2
Usage of Synthetic Nanoparticles in Masking of White Spot Dental Lesion
Bushra Habeeb Al-Maula1*, Suhad Jabbar Hamed Al-Nasrawi2, Wijdan Abdulameer Kadhim3, Abtesam Imhemed Aljdaimi4 and Abdullatif Alfutaimi5
*Correspondence: Bushra Habeeb Al-Maula, Research Center for Laser and Photonics, Al-Hamdaniya University, Iraq, Email:
Abstract
Aim: This research aimed to estimate the potential impact of CaO nanoparticles at different concentrations on the remineralization of enamel undergoing cariogenic attack.
Materials and methods: Eighteen enamel samples were prepared from sound molars to measure its calcium (Ca) and phosphorus (P) contents by scanning electron microscope equipped with energy dispersive X-ray spectroscopy (SEM-EDS) before and after exposure to cariogenic attack and treatment with saturated gum of 100% CaO nanoparticles (Group 1), 50% CaO nanoparticles (Group 2), and artificial saliva (Group 3). The research data were statistically analysed using the one-way ANOVA and Bonferroni tests.
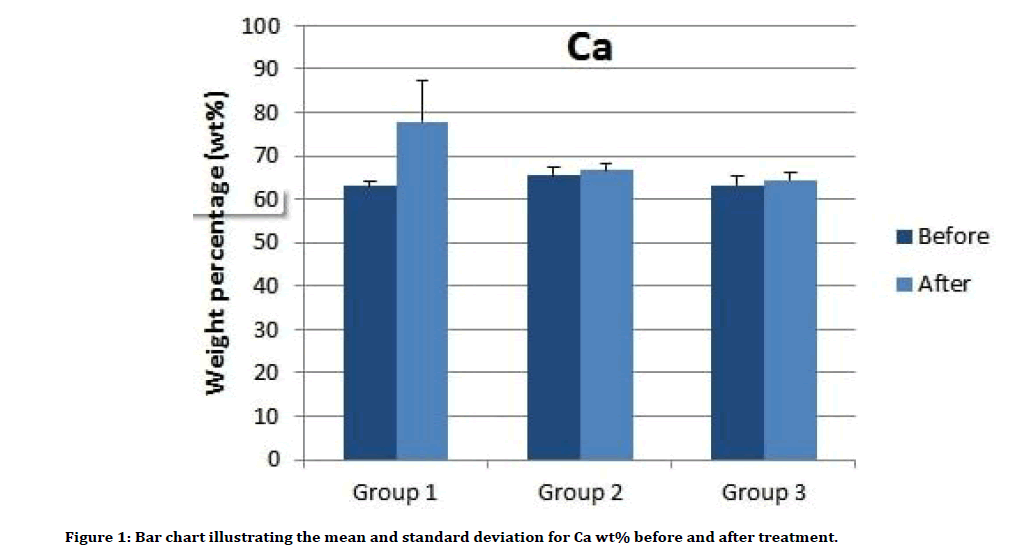
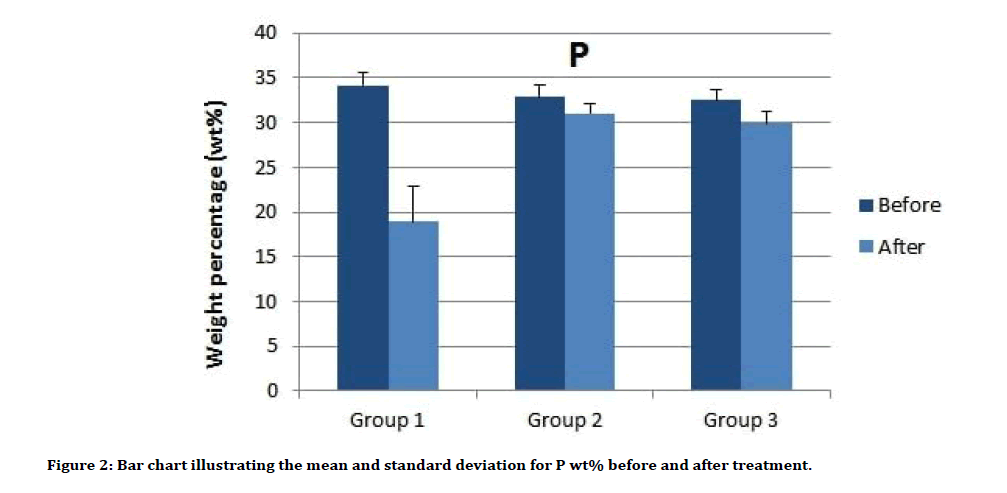
Results and discussion: Group 1 of treatment with 100% CaO nanoparticles demonstrated the largest content of Ca (77.8 wt % ± 9.6) followed by Group 2 and 3 (66.6 wt% ± 1.5, 64.4 wt% ± 1.9, respectively). Conversely, Group 1 had the lowest amount of P (18.9 wt% ± 3.9) followed by Group 3 and 2 (29.9 wt% ± 1.4, 31.0 wt% ± 1.1, respectively). The demineralization is a reversible process which starts with Ca loss before P loss, so it is logical to use a recalcifying agent (Ca containing agent) to enhance the recalcification and regrowth of hydroxyapatite (HA) crystals where the P could be driven from the saliva. CaO is rich with bioavailable Ca contents, favouring the remineralization of caries lesions. Furthermore, as the CaO applied in a nano-size particle, it has the ability to diffuse actively through the tiny spaces of the etched enamel.
Conclusion: The use of saturated gum with CaO nanoparticles could overcome the cariogenic challenge. The Ca uptake increased with the increased concentration of applied CaO nanoparticles.
Keywords
CaO nanoparticles, Demineralization, Remineralization
Introduction
Dental enamel is acellular hypermineralized tissue where the mineral part represents about 96-98% of its weight, while the rest consists of water and organic constituents [1]. The mineral constituents of enamel are mainly composed of calcium phosphate salts that are arranged in the form of nanoscale hexagonal hydroxyapatite (HA) (Ca1 (PO4)(OH)2) crystals. The enamel crystals are elongated and packed, forming enamel rods or prisms. The length of enamel rods are tens of microns (up to 100 μm), but sometimes just 50 nm wide [2]. The basic structural units of enamel (Enamel rods or enamel prisms) run merely parallel to each other, and project in a perpendicular direction from the dentino-enamel junction (DEJ) toward the tooth surface [3].
The differences in chemical composition are systematic and are attributed to both developmental stages and position within the tissue [4]. Various ions could incorporate into the enamel structure as a substitution to Ca, hydroxyl and phosphate ions. Subsequently, this is likely to alter the mineral structure with a possibility of a critical effect on the mineral properties, such as hardness, solubility, brittleness, thermal stability, strain, and the optical properties [5]. The great substitution activity that takes place is the calcium-deficient carbonated HA. Furthermore, a small amount of Ca ions, 1%, could be replaced by other metal ions, including sodium, potassium, and magnesium [6]. However, during demineralization and remineralization processes, calcium, phosphate, and fluoride ions play a significant role and accordingly modify the susceptibility of the tooth to the progression of caries [7]. During the demineralization process, releasing of Ca precedes the release of phosphate ions from enamel, dentin, and cementum. Thus, utilizing Ca instead of phosphate to defeat the demineralization would be effective [8].
Chemical demineralization of teeth structure is produced by an acidic challenge exerted by two primary means: one is the dietary acid consumed through drink or food; second is the microbial attack from bacteria that occupied the oral cavity [9]. Demineralization is a reversible process may appear as a white spot; subsequently, the partially demineralized HA crystals have the ability to grow to restore their original size if they are subjected to environments that favour process of remineralization [10]. The remineralization term refers to both prevention and curing of demineralization that can be reversed or arrested, especially in its early stage. Fluoride therapy, saliva, diet control, and probiotic bacteria are described as preventive measures for tooth demineralization. Dental composites containing various forms of calcium phosphates (CaPs) are produced as a potential curative regime for dental demineralization [11].
The nanotechnology received progressive attention because of the advantage of nanoparticles as multifunctional properties with unique optical and catalytically properties [12]. The unique physical and chemical properties of the nanoparticles refer to their Nano scale size, high surface area and structure. However, their activities might differ from their bulk material requiring further investigation. Another drawback is the possible toxicity and environmental risk [13]. Calcium oxide (CaO) is an important inorganic compound that is employed across a wide range of industries [14]. In dentistry, it is enthusiastic material for its biocompatibility [15], good mechanical property [16], low solubility with good adherence to tooth structure which results in acceptable sealing property [17]. In comparison to traditional CaO particles, CaO nanoparticles showed excellent antimicrobial activity and ability to inactivate the endotoxins [18].
To date, no studies have been carried out to analyse the effects of remineralization potential of synthetic CaO nanoparticles. Thus, the aim of the current research was to define the impact of CaO nanoparticles with different concentrations on demineralized enamel. The null hypothesis was stated as: there would be no significant effect for CaO nanoparticles application on the surface elemental composition of enamel lesion.
Materials and Methods
Preparation of CaO nanoparticles
The preparation procedure of CaO nanoparticles started by dissolving of 1.5 g of CaCl2.2H2O (calcium chloride dihydrate) (BDH Chemicals Ltd Pool England) in 50 ml of redistilled water. The solution was mixed with 15 ml of NaOH. A suspension of nano-powder was produced, which kept at 75°C for 1 h. After cooling, particles were segregated with the aids of centrifugation, and then washed with distilled water to reduce any contamination. Dryness was achieved using an oven at 80 °C for 3h. A 0.0075 g CaO nanoparticle were placed in 30 ml of distilled water to be sonicated for 5h. This was to separate the nanoparticles, overcoming the agglomeration. Then, the drop-casting method was used to deposit a CaO layer on a glass surface to test the properties of nanoparticles using an X-ray diffractometer (XRD-6000, Shimadzu, Japan) with CuKa radiation at 0.154056 nm. A UV-VisNIR Split-beam Optics with Dual detectors spectrophotometer (100 Conc plus, Cary, US) was applied to test the optical absorption of the colloid of the nanoparticles. Double-distilled water was used throughout the preparation.
Calcium oxide suspension preparation
100% CaO suspension prepared by adding 10 ml distal water to 10 mg CaO nanoparticles powder.
50% CaO suspension prepared by adding 10 ml distal water to 5 mg CaO nanoparticles powder.
The two suspensions were shaken before use.
Artificial cariogenic solution preparation
A cariogenic solution was prepared by dissolving of soluble compounds in 1000 mL distilled water. The compounds were: 0.2442 g NaCl (sodium chloride), 0.2992 g KH2PO4 (potassium dihydrogen phosphate), 1.56 × 103 g KCl (potassium chloride), 3 g HAc (Hydrogen acetate), and 0.56 g KOH. The pH was adjusted to be 4.6 [19].
Artificial saliva preparation
Artificial saliva containing 0.33g KH2PO4, 0.34g NaH2PO4 (sodium dihydrogen phosphate), 1.27 g KCl, 0.16 g CNNaS (Sodium sulphocyanide), 0.58 g NaCl, 0.17g CaCl2 (calcium chloride, 0.16 g NH4Cl (ammonium chloride), 0.2g urea, 0.03 g glucose, 2.7g mucin, and 0.002g vitamin C was prepared. The above components were dissolved in 1000 mL of distilled water, and the pH was adjusted to be 6.8 [19].
Sample preparation
Eighteen extracted human teeth were extracted for reasons other than for this project. They were collected under Human Tissue Authorization and with university ethical approval (The University of Kufa, ref 7 in 05/03/2018), in accordance with the Helsinki Declaration of 1975, as revised in 2000. The teeth inclusion criteria were: (1) Freshly extracted, (2) Sound, (3) Molar, (4) from young age patients. The exclusion criteria were teeth with: (1) caries, (2) white spot, (3) fracture, (4) Abnormalities, (5) Endodontically treated teeth. The selected teeth were disinfected by 5 min immersion in 5% sodium hypochlorite, and then kept in distilled water at 4°C before the experiment. Each tooth was assessed under criteria. Each tooth was transversally embedded in an acrylic resin (Metrodent Ltd., Huddersfield, UK) block, then polished to expose a buccal enamel area of about 2 mm × 2 mm of flat surface to be treated with the tested reagents. The samples were randomly divided into three groups:
Group 1 (6 samples): covered with gum saturated by 0.5 ml of 100% CaO.
Group 2 (6 samples): covered with gum saturated by 0.5 ml of 50% CaO.
Group 3 (6 samples): covered with gum saturated by 0.5 ml artificial saliva.
Each sample was exposed to continuous cycles of demineralization/remineralization for 15 days. In each cycle the sample was immersed in artificial saliva for 10 h, then 1 h immersion in artificial cariogenic solution, followed by 1 h covering with gum. During the whole process, the samples were incubated in 100% relative humidity at 37°C, the two solutions and the gum were changed with each use.
Minerals analysis
Calcium and P contents of enamel were measured as wt% before and after the treatment. Prior to the examination, each sample was dehydrated with ascending concentrations of ethanol solutions; 50%, 75%, 90% and 100% respectively, for 20 minutes for each concentration. The treated samples surfaces were coated with 24 nm gold layer using Sputter Coater (Quorum technologies Ltd, Laughton, East Sussex, England) to reduce charging from the electron beam. The surface elemental analysis was accomplished using SEM-EDS (Inspect S50, FEI Eindhoven, Netherlands) at 12.5 kV accelerating voltage, 9.5 mm working distance. A spot size of 2.5, and magnification of 5000×.
Statistical analysis
The obtained data were statistically analysed using the SPSS software (SPSS version 22.0, IBM Corp., Armonk, US). The results were processed by one-way ANOVA, and multiple comparisons between groups were achieved using LSD test. The data was statistically analysed at a confidence level of 95%, and a p-value ≤ 0.05.
Results
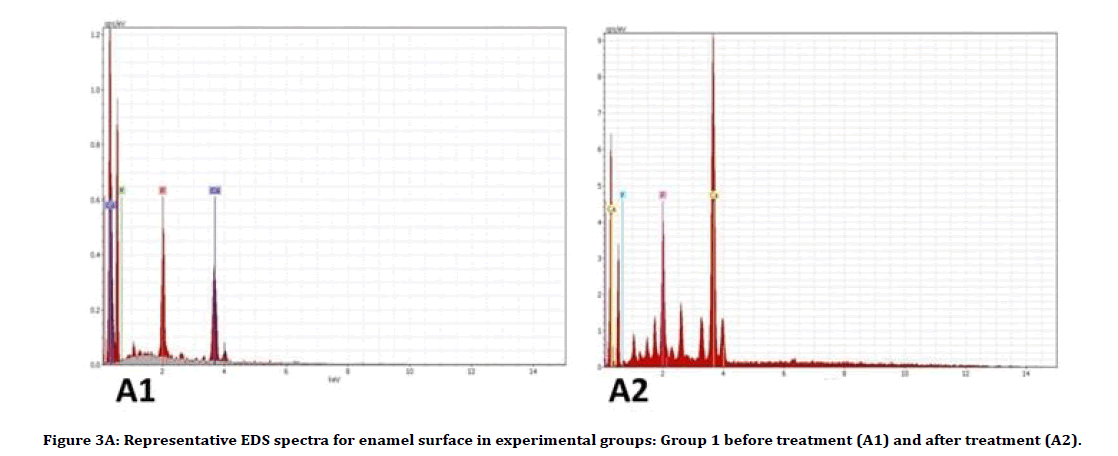
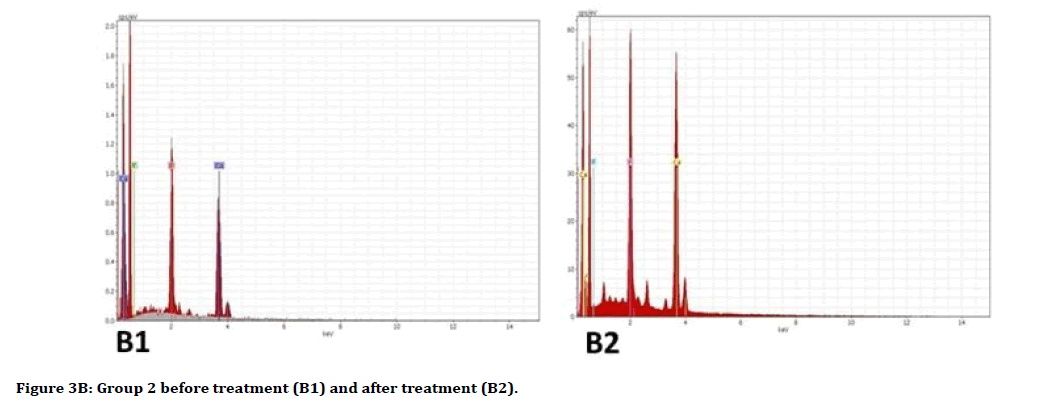
Descriptive analysis of Ca wt% and P wt% before and after treatment showed an increase in the Ca wt% means and a decrease in P wt% means in all groups posttreatment (Table 1). The multiple comparisons by ANOVA test showed in Table 2, there was no significant difference between all groups in pretreatment analysis for both Ca and P contents. In post-treatment analysis for Ca wt%, there is a significant difference between group 1 and 3, and a significant difference between group 1 with both groups 2 and 3 for P wt%. The Ca increase, and P loss in groups as a result of treatment were determined to take the following order: Group 1>Group 2>Group 3. Figures 1 and 2 show the means difference of Ca wt% and P wt% in all groups as pre and post-treatment, while Figure 3A-C illustrates the EDS spectra for representative samples.
Figure 1: Bar chart illustrating the mean and standard deviation for Ca wt% before and after treatment.
Figure 2: Bar chart illustrating the mean and standard deviation for P wt% before and after treatment.
| Ca wt% | P wt% | |||
|---|---|---|---|---|
| Group (n = 6) | Before | After | Before | After |
| Group 1 | 63.0 (1.0) | 77.8 (9.6) | 34.1 (1.4) | 18.9 (3.9) |
| Group 2 | 65.5 (1.8) | 66.6 (1.5) | 32.8 (1.3) | 31.0 (1.1) |
| Group 3 | 63.2 (2.0) | 64.4 (1.9) | 32.5 (1.2) | 29.9(1.4) |
| The elemental composition was presented in Mean (SD) | ||||
Table 1: The surface elemental composition (wt%) of enamel samples measured by EDS.
| Element | Groups | P value | |
|---|---|---|---|
| Ca wt% pre | 1 | 2 | 0.117 |
| 3 | 0.931 | ||
| 2 | 3 | 0.132 | |
| Ca wt% post | 1 | 2 | 0.052 |
| 3 | 0.028 | ||
| 2 | 3 | 0.659 | |
| P wt% pre | 1 | 2 | 0.253 |
| 3 | 0.166 | ||
| 2 | 3 | 0.767 | |
| P wt% post | 1 | 2 | 0.001 |
| 3 | 0.002 | ||
| 2 | 3 | 0.606 | |
Table 2: P values of multiple comparisons by LSD test for Ca and P as pre and post treatment.
Figure 3A: Representative EDS spectra for enamel surface in experimental groups: Group 1 before treatment (A1) and after treatment (A2).
Figure 3B:Group 2 before treatment (B1) and after treatment (B2).
Figure 3c:Group 3 before treatment (C1) and after treatment (C2).
Discussion
A traumatic conservative approach has been the preferred method of treating early caries (white spot). It could be achieved by using of remineralizing agents. Scientific literature suggests that the clinical management of tooth demineralization should concentrate on the early detection and prevention prior to the application of a restorative approach. The recent development and advancement in technologies can promote enamel remineralization or downscale demineralization, thereby aiding and reinforcing oral health [20]. Considerable efforts and research have been undertaken to limit the progression of carious lesions. However, although the clinical studies are considered the gold standard, in cariology research, the standardized in vitro models are the most applicable design of studies and can work as a valuable tool for evaluating anticaries efficiency of remineralizing agents [21]. The progressive utilization of gums that neutralizes mouth pH and induces salivary release is also explored and would aid toward building an adequate enamel layer for acid resistance, particularly when it has remineralizing materials [22].
The EDs elemental analysis illustrates the availability of Ca and P in all the analysed sections, where the Ca enamel uptake increased with the increased Ca concentration of the applied agent. That was combined with a decrease in P content in experimental groups. This part of the result agrees with an elemental analysis achieved by Raghu, et al. [23] who demonstrated a significant increase in Ca/P ratio after application of a remineralizing agent. Furthermore, it was stated that CaO is rich with bioavailable Ca contents, favouring the remineralization of caries lesions [24]. In fact, the demineralization is a reversible process which starts with Ca loss before P loss [8], so it is logical to use a recalcifying agent (Ca containing agent) to enhance the recalcification and regrowth of HA crystals where the P could be driven from the saliva. In addition, as the CaO applied in a nano-size particle, it has the ability to diffuse actively through the tiny spaces of the etched enamel. Based on the result of this study, CaO nanoparticles demonstrated to be efficient for recalcification of hard tissue lesions. Therefore, the test hypothesis was rejected.
One of the limitations in the current study is that the result might be affected by individual differences between samples as they were taken from different teeth; therefore, each sample was examined before and after the treatment. The SEM was previously used to evaluate the demineralization/ remineralization and impact of fluoride-containing products [25]. The majority of studies, applying a SEM for non-conductive specimens must first be coated with a thin layer of conductive material. Samples are coated with carbon or metals such as gold or palladium to improve image quality [26]. In addition, the samples examined under the SEM must have the ability to withstand the intensity of the electron beam. Specimens that do not conduct electricity can charge up, resulting in distortion of images and signals. The coating is nanometer thickness, and was standardized for all samples, and it was removed when the samples subjected to the cariogenic challenge, so it was expected to have no effect on the EDS results [27].
Conclusion
The use of saturated gum with CaO nanoparticles could overcome the effect of the cariogenic challenge. The Ca enamel uptake increased with the increased concentration of applied CaO nanoparticles. Due to the consumption of more fizzy, sugary drinks and different types of sugary gum foods especially by children, so the challenge is to either change or promote the population habits or development of new products such as the combinations in our results that might counter the demineralization that results from these habits.
Acknowledgement
The authors are grateful to Dr. Nawfal H Aldujaili from the faculty of Sciences at the University of Kufa for his help in samples analysis. My thanks are also extended to Mr. Michael Faulkner School of Materials at the University of Manchester for his helpful and scientific advice.
Conflicts of Interest
There is no conflict of interest.
References
- Cuy JL, Mann AB, Livi KJ, et al. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch Oral Biol 2002; 47:281-291.
- Nanci A, Ten Cate AR. Ten cate's oral histology development, structure, and function. Elsevier 2013.
- Anderson P, Elliott J. Rates of mineral loss in human enamel during in vitro demineralization perpendicular and parallel to the natural surface. Caries Res 2000; 34:33-40.
- Robinson C, Connell S, Kirkham J, et al. Dental enamel-A biological ceramic: Regular substructures in enamel hydroxyapatite crystals revealed by atomic force microscopy. J Mater Chem 2004; 14:2242-2248.
- Elliott JC. Calcium phosphate biominerals. Rev Mineral Geochem 2002; 48:427-453.
- Le Geros R. Calcium phosphates in enamel, dentin and bone, in calcium phosphates in oral biology and medicine. Karger Publishers. 1991; 15:108-129.
- Hara AT, Zero DT. The caries environment: Saliva, pellicle, diet, and hard tissue ultrastructure. Dental Clin 2010; 54:455-467.
- Cummins D. The development and validation of a new technology, based upon 1.5% arginine, an insoluble calcium compound and fluoride, for everyday use in the prevention and treatment of dental caries. J Dent 2013; 41:1-11.
- Scaramucci T, Carvalho JC, Hara A, et al. Causes of dental erosion: Intrinsic factors. Dental Erosion Clin Man 2015; 35-67.
- https://www.dentalcare.com/en-us
- Abou Neel EA, Bozec L, Perez RA, et al. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int J Nanomedicine 2015; 10:6371.
- Das SK, Marsili E. A green chemical approach for the synthesis of gold nanoparticles: Characterization and mechanistic aspect. Rev Environ Sci Biotechnol 2010; 9:199-204.
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arabian J Chem 2019; 12:908-931.
- Madhusudhana N, Yogendra K, Mahadevan K. A comparative study on photocatalytic degradation of violet GL2B azo dye using CaO and TiO2 nanoparticles. Int J Eng Res Appl 2012; 2:1300-1307.
- AL-Jubori SH, Alsalman TH, Taqa AA. Evaluation of the biocompatibility of a newly prepared endodontic biosealer using subcutaneous implant on rabbits. Int JEnhanced Res Med Dent Care 2017; 4:5-14.
- Alkhalidi EF, Alsalman TH, Taqa AA. Mechanical properties of new calcium based cement prepared from egg Shell. Int J of Enhanced Res Sci Technol Eng 2014; 3:70-76.
- Chalbi AS, Chakmakchi M, Taqa MA, et al. Effect of nano calcium oxide intra canal medicaments on the sealing integrity of root canal treated teeth. EC Dent Sci 2019; 18: 1369-1377.
- Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J Microbiol Methods 2003; 54:177-182.
- Ou XY, Zhao YH, Ci XK, et al. Masking white spots of enamel in caries lesions with a non-invasive infiltration technique in vitro. Genet Mol Res 2014; 13:6912-6919.
- Li X, Wang J, Joiner A, et al. The remineralisation of enamel: A review of the literature. J Dent 2014; 42:12-20.
- Neto FR, Maeda FA, Serra MC. Potential agents to control enamel caries-like lesions. J Dent 2009; 37:786-790.
- Abou Neel EA, Aljabo A, Strange A, et al. Demineralization–remineralization dynamics in teeth and bone. Int J Nanomedicine 2016; 11:4743-4763.
- Raghu T, Ananthakrishna S. Remineralization potential of calcium sucrose phosphate on demineralized enamel: Results of an in vitro study. J Int Oral Health 2016; 8:704-708.
- Haghgoo R. Mehran M, Ahmadvand M, et al. Remineralization effect of eggshell versus nano-hydroxyapatite on caries-like lesions in permanent teeth (in vitro). J Int Oral Health 2016; 8:435-439.
- Duschner H, Götz H, Øgaard B. Fluoride-induced precipitates on enamel surface and subsurface areas visualised by electron microscopy and confocal laser scanning microscopy. Euro J Oral Sci 1997; 105:466-472.
- Nelson D, Jongebloed W, Arends J, Morphology of enamel surfaces treated with topical fluoride agents: SEM considerations. J Dent Res 1983; 62:1201-1208.
- Hashem DF, Foxton R, Manoharan A, et al. The physical characteristics of resin composite–calcium silicate interface as part of a layered/laminate adhesive restoration. Dent Mater 2014; 30:343-349.
Author Info
Bushra Habeeb Al-Maula1*, Suhad Jabbar Hamed Al-Nasrawi2, Wijdan Abdulameer Kadhim3, Abtesam Imhemed Aljdaimi4 and Abdullatif Alfutaimi5
1Research Center for Laser and Photonics, Al-Hamdaniya University, Iraq2Department of Conservative Dentistry, University of Kufa, Iraq
3Department of Conservative Dentistry, University of Karbala, Iraq
4College of Dentistry and Oral Surgery, Alasmarya University, Libya
5School of Chemical Engineering and Analytical Sciences, The University of Manchester, United Kingdom
Citation: Bushra Habeeb Al-Maula, Suhad Jabbar Hamed Al-Nasrawi, Wijdan Abdulameer Kadhim, Abtesam Imhemed Aljdaimi, Abdullatif Alfutaimi, Usage of Synthetic Nanoparticles in Masking of White Spot Dental Lesion, J Res Med Dent Sci, 2020, 8(2): 20-26
Received: 14-Feb-2020 Accepted: 02-Mar-2020