Review Article - (2022) Volume 10, Issue 9
A Revolutionary Journey of Platelet Concentrates in Dentistry: A Review
Kushal P Bhuskute*, Anuja Ikhar, Pradnya Nikhade, Akshay Jaiswal and Kajol Relan
*Correspondence: Kushal P Bhuskute, Department of Conservative Dentistry and Endodontics, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences (Deemed to be University) Sawangi (Meghe) Wardha, Maharastra, India, Email:
Abstract
Platelet concentrates seem to be blood derivatives made from the blood of patient, inside which activated platelets have been captured within the fibrin matrix scaffold and cytokines, release growth factors included in tissue regeneration processes like cell proliferation and differentiation, extracellular matrix synthesis, chemo taxis, and angiogenesis. Over the last twenty years, these procedures have been found to enhance healing in soft as well as hard tissues, leading to beneficial therapeutic applications in dental professions. Numerous preparations were produced from various leukocyte content, fibrin network structures, and growth factors, and consequently different physical and physiological properties and applications, related to different centrifugation speeds and times, introducing chemicals, as well as the choice of supernatants and precipitates. A biological reason behind the method of preparation on certain conditions and scientific evidence applications of various platelet concentrates, into several domains in dentistry shall be discussed.Keywords
Platelet concentrates, Regeneration, Platelet rich fibrin, Platelet rich plasma
Introduction
Tissue regeneration itself a multi-step healing and tissue growth process including a variety of biological factors and techniques. Bone grafts, biomaterials, growth hormones, natural/synthetic scaffolds, and more lately stem cells are all used in this procedure. In medical domains, including dentistry, autologous platelet concentrates represent an auspicious and advanced therapeutic approach [1]. Many different biomaterials, both natural and manmade, have been explored to expedite wound healing in soft and hard tissue for many years. Highly specialised tissue that is blood which accounts for 7-8% of body's weight of human and is made up of over 4,000 diverse components. Red blood cells, white blood cells, platelets, and plasma are most significant blood elements. Platelets have irregularly shaped tiny a nuclear cells that form when progenitor megakaryocytes are fragmented. To persuade fibrin polymerization prior to application to the surgical site, chemical additives like anticoagulants, thrombin, and calcium chloride are required. A surgical haemostatic agent that encourages epithelial, endothelial, and epidermal regeneration while also reducing cutaneous scarring. In the dentistry practise, platelet aggregate has been employed for fast tissue regeneration and healing. Platelet concentrates have progressed significantly since introduced, a source of blood proteins loaded along growth factors to induce healing of wound, as well as A-PRF, PRF lysates, t-PRF, platelet rich plasma, PRF, I-PRF, CGF (concentrated growth factors) and fibrin glue, have all been introduced in recent years [2]. A natural blood clot is composed of 95% RBCs, platelets of 5%, less than 1% of WBCs, and a substantial number of fibrin strands. The PRP (Plasma Rich Platelet) blood clot is composed of 4% RBCs, 95% of platelets, and 1% WBCs. Because of the absence of suitable categorization of these many different products, the literature on Platelet Concentrates (PC) is highly confused and contentious [3]. Platelets have a wide range of applications due to their increased role in regular haemostasis, which has sparked clinical interest in recent platelet resultant products, leading towards development of PC classified as 1st generation platelet concentrate like a platelet rich plasma and 2nd generation platelet concentrates like a platelet rich fibrin. Hard and soft tissue repair has been aided by signalling proteins that regulate a vast range of intracellular and extracellular functions. Platelets are renowned neither for their importance nor only in haemostasis even in wound healing [4]. PRP stands as an autologous compilation of the platelets derived from less volume of plasma gained from centrifugation. The clinical applications of PRP in dentistry include cosmetic surgery, neck surgery, burns, head surgery and otolaryngology [5]. Platelet Rich Plasma (PRP) are not the same as fibrin glue. Surgical adjuncts such as glue and sealants have been used to aid in the development of haemostasis of incision sites in surgery. Glues may be made through platelet deficient plasma that is mostly made up of fibrinogen. PRP, on the other hand, have a greater concentration of platelets containing growth factors, which aids in regeneration. These are concentrated components decreases during the course of seven days. The rate at which a clot in a graft or wound heals is proportional to the platelet count, and PRP increases that initial platelet count. This is the coagulation cascade’s deadly state, that fibrin clot formation is platelet rich plasma. Then a few growth factors are released through granules [6]. Collagen synthesis is boosted by angiogenesis, which leads towards improving primary wound strength. Glycoproteins are the growth factors. PRP “jump starts” the regeneration cascade for high-quality tissue recovery after damage.
Literature Review
Historical aspects of platelet concentration
During blood coagulation research in 1954, Kingsley [7] coined the term PRP for the first time. In 1970, Matras presented "Fibrin glue" as a potential regeneration property of platelets [8]. Choukroun, et al. [9] created Platelet rich fibrin in France that preparation has a strong fibrin gel polymerization, which was discovered in 2000. Because it differed greatly from prior PRPs, it was dubbed a "second generation" platelet concentrate. Sacco utilized a Medifuge centrifuge to manufacture an innovative notion of growth factors that are concentrated in 2006, which is comparable with PRF, however at a variable speed of centrifugation, allowing for the breaking of the fibrin matrix along a higher concentration of growth factors [10]. CGF was later discovered for having more adaptability and regenerative capacity [11]. Dohanet, et al. [12] given the first classification of platelet concentrates, which consisted of four primary categories based on product separation utilising two important parameters: The fibrin architecture and the cellular content (mainly leukocytes):
- Pure platelet rich plasma/leukocyte that are poor platelet rich plasma
- Leukocyte and platelet rich plasma
- Pure plasma rich fibrin/leukocyte-poor platelet rich fibrin
- Leukocyte and PRF.
Sticky bones (autologous fibrin glue combined into bone transplant) were first proposed by Sohn et al. [13]. Then, at 2012, Mishra et al. [14] created a new categorization that was limited to PRP. They defined four categories of All four forms of PRP may be divided in two sub-types that depends on its either presence or absence of the leukocytes, as well as whether PRP was not activated or activated, and all four types may be separated into 2 of sub types: "Platelets>5 baseline" and "Platelets 5 baseline (A or B)". Non-activated PRP is called "solution," while activated PRP is called "gel."
- Type I: LPRP solution,
- Type II: L-PRP gel,
- Type III: P-PRP solution,
- Type IV: P-PRP gel.
DeLong et al [15] did another classification around the same time termed PAW (Platelet number, Activation approach, WBCs present), that resembled Mishra et al. [14] in that it had been confined for PRP families alone. Tunal, et al. [16] presented T-PRF (Titanium-prepared PRF) was introduced in 2013, with titanium tubes replacing glass tubes to centrifuged, premised on the idea that titanium activates platelets more successful than silica, resulting in L-PRF in a much denser form. Then Mouro, et al. [17] developed i-PRF form which was injectable, in which an orange coloured fluid was achieved by centrifuging for 2 minutes at 3300 rpm, resulting in the sticky form which might be managed conveniently within the bone defect.
Histology of platelet
Platelets range in size from 1 to 4 m in diameter, are colourless, non-nucleated, and have mildly refractive bodies. Megakaryocytes, that are exceptionally massive hematopoietic cells found inside bone marrow, are platelet precursor cells. These megakaryocytes break down into small disc-shaped formations known as platelets in the bloodstream either in bone marrow, squeezing their way from capillaries. The concentration of platelets in the blood must be in 150,000-300,000 per mm. A platelet's usual lifespan is a few days, perhaps 5 to 10 days. Platelets are kept in the spleen and regulated whenever required via splenic muscle spasms that are triggered sympathetically [18].
1st generation=platelet concentrates-platelet rich plasma: Platelet rich plasma, platelet concentrates, and platelet gel have been developed for combining platelets' fibrin sealing properties and effects of growth factor, leading to an ideal growth factor delivering mechanism at site of injury. Growth factors have recognised to have a vital part for hard and soft tissue healing procedure [19]. Hence their usage is scientifically warranted. The growth factors, chemotactic and mitogenic activities promote and change cellular functions associated with tissue repair, regeneration, and cell proliferation [20].
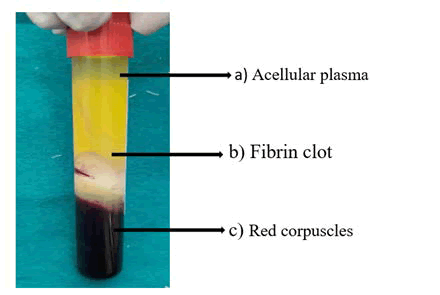
2nd generation=platelet concentrate-platelet rich fibrin: In 2001, Choukroun et al. became the 1st to synthesise platelet rich fibrin at France. The use of bovine thrombin is no longer necessary with this 2nd generation platelet concentrate [20]. The PRF procedure is straightforward: A sample of blood is obtained into a 10 ml tube without anticoagulant and centrifuged for 10 minutes at 3,000 rpm (about 400 g) in a table centrifuge [21]. Anticoagulants are not present, many of the platelets in sample of blood in touch with the inside walls of the glass tube are activated in a few minutes, resulting in the release of coagulation cascades. Fibrinogens are concentrated in upper half of a tube unless circulating thrombin converts it to fibrin. The fibrin clot forms inside middle of that tube, in between of red corpuscles in the bottom and the cellular plasma on the top Figure 1.
Figure 1: Test tube showing, A) A cellular plasma; B) Fibrin clot; C) Red corpuscles.
Preparation of platelet concentration
- To avoid platelet activation and degranulation, venous blood is obtained with an anticoagulant.
- The initial centrifugation ("soft spin") in a centrifugation machine Figure 2 permits the blood to be separated into three distinct layers.
Figure 2: Centrifugation machine.
- The clinician aspirates Platelet Poor Plasma (PPP), Platelet Rich Plasma (PRP), and few red blood corpuscles by the sterile syringes, (that were systematically involved throughout an operation). The substance used to then they are moved to the tube that does not contains anticoagulant.
- The 2nd tube should be centrifuged again, this time for a longer and faster period of time ("hard spin"). This allows platelets to cluster towards the tube's bottom, resulting in the formation of three different layers once more.
- Collecting PRP becomes simple at this point. The clinician may eliminate a majority of PPP with a syringe, parting only adequate serum for suspending the platelets concentration. After that, a device is slightly shaken to acquire a CPRP that is prepared for using (platelet rich plasma’s concentration).
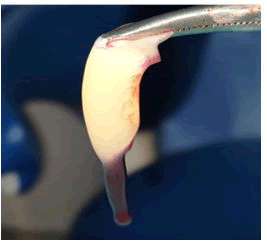
- When to apply, CPRP is collectively taken with bovine thrombin and calcium chloride using a mixing syringe. The platelet concentration will gel shortly after that: Fibrinogen is concentrated while CPRP preparation, and when it polymerizes, it forms a fibrin matrix along unique haemostatic and adhesive properties Figure 3 [21,22].
Figure 3: Platelet rich fibrin.
Recent advances in platelet concentrates
Despite the fact that Choukran's L-PRF has been widely acknowledged to be effective, clinicians such as O'Connell [23] have expressed worry about the silica particles in the glass tubes posing a health risk. Although the silica particles are thick enough to settle with the RBCs,
They're sufficiently small that the proportion of them shall be colloidally trapped into platelet-poor plasma layers, buffy coat, and fibrin throughout treatment, and may finally approach patient.
In relation to the, Dohan Ehrenfest, et al. [21] had studied in 2010 to evaluate the cell composition and 3D organisation of L-PRF as a result of various kinds of the collection tubes like glass wrapped plastic tubes, dry glass, and compression techniques (soft/forced) at finalized L-PRF-membrane architecture. The authors established the type of testing tube had no effect on architecture of 2nd generation PC. In 2014, Tunal, et al. released an innovative product named Titanium Prepared PRF (T-PRF). For making L-PRF, titanium tubes had been used in place of glass tubes for collection and centrifugation, On the basis of titanium is thought to be a more efficient platelet activator even more than the silica. Tunal, et al. [22] determined the T-PRF had a well-structured network through constant integrity, and there was thicker fibrin network and enclosed a wider zone based on light, scanning electron, and fluorescence microscopy studies. In an in-vitro investigation, Anitua, et al. [24] looked at the effects of several ozone treatments on PRGF biologic characteristics.
They discovered that by employing the "continuous flow procedure" after ozone therapy for PRGF fibrin scaffold synthesis, growth factor levels, and proliferation capacity have all been significantly lowered. A biological results of the autologous therapy were unaffected by ozone treatment using the "syringe method," indicating that ozone therapy with combination of PRGF may be employed successfully.
Applications of regenerative dentistry
In the dentistry and medical fields, platelet concentrates are being frequently employed for the expedite tissue regeneration and tissue repair [25]. In terms of the use of PRP preparations, a collection of latest systematic reviews and meta-analyses found such as there was not at all a significant difference at bone augmentation favouring supplemental utilization of PRP into development of bone surrounding implant [26], and there was inadequate number of human trials to fully support the advantages of utilizing PRP at sinus augmentation process [27]. Regenerative infra-bony treatment, however, a “systematic review” and “meta-analysis” on fourteen Root Canal Treatments was published in a year 2018 that determined, PRP and bone grafts have constantly produced improved clinical outcomes when combined in relations to Clinical Attachment Loss gaining and reducing pocket then Irrespective of a flap surgery or the considerable heterogeneity across research, adjunctive membrane usage, following short-term and longer term reassessment [28]. In 2008, the potential advantages from platelet-rich plasma at soft tissue root covering procedures include better aesthetics, reduced patient morbidity, as well as increased healing of wounds, according to an overview an involvement of Platelet Rich Plasma at soft tissue root coverage treatments. However, the impacts of PRP on improving root-coverage cannot be concluded because of a lack of clinical evidence. Some of clinical investigations later described conflicting outcomes, claiming about combining PRP with connective tissue grafts dramatically increases the breadth of keratinized gingiva and increases CAL [29]. Other research has found that PRP can only promote early soft tissue repair with no clinically significant improvements in final treatment results [30,31]. The literature on the usage of autologous platelet concentrates at implant and reconstructive surgery are extremely condensed as in different variants of platelet rich plasma that are pure platelet rich plasma and leukocyte and platelet rich plasma and yet to confined in Platelet-Rich Fibrin figure 3 and its subfamilies [32,33]. Variety of plasma derivative was used in treating peri-implant bone deficiencies (following peri-implantitis, after implantation into low bone volume, and throughout immediate post-extraction as well as post-avulsion implantation), sinus lift surgeries, and other difficult implant-supported procedures.
Discussion
To speed up tissue regeneration and healing, platelet concentrates are routinely used [22]. When progenitor megakaryocytes are broken up, tiny, irregularly shaped nuclear cells called platelet precursors are produced. Chemical additives such as anticoagulants, thrombin, and calcium chloride are necessary to induce fibrin polymerization before application to the surgical site. A surgical haemostatic that lessens cutaneous scarring and promotes epithelial, endothelial, and epidermal regeneration. For quick tissue regeneration and healing, platelet aggregate has been used in dentistry. In regards to Clinical Attachment Loss gaining and reducing pocket, adjunctive membrane usage, following short-term and longer term reassessment, and regardless of flap surgery or the considerable heterogeneity across research, PRP and bone grafts have consistently produced improved clinical outcomes [28]. According to an overview of platelet-rich plasma use in soft tissue root-coverage operations, the possible benefits include improved aesthetics, decreased patient morbidity, and accelerated wound healing. The information on the use of autologous platelet concentrates in implant and reconstructive surgery is extremely condensed, as is the information on the different types of platelet rich plasma, including pure platelet-rich plasma, leukocyte and platelet rich plasma, and platelet-rich fibrin and its subfamilies [33]. The platelet concentrates used into regeneration has shown to be a promising outcome as a result of modern advancements in dentistry [34-36].
Conclusion
Platelet concentrations have been utilized in dentistry for a long time in a variety of applications. The platelet concentrates used into regeneration has shown to be a promising outcome as a result of modern advancements in dentistry. Several studies has performed to determine the use of PRP and PRF in different treatments such as oral surgery, endodontic periodontology, implant dentistry, with promising findings of hard tissue and soft tissue regeneration. Integrity about a fibrin scaffold is influenced by a number of parameters such as centrifuge speed, time, temperature, and blood haematocrit. Finally, the importance of leukocytes as well as fibrin within PRF scaffolds is explored as a prospective research topic for the upcoming time. Despite the obvious therapeutic benefits of Platelet Rich Plasma and fibrin sealant's evidences of their beneficial effects are still missing, justification for their widespread use is awaited. To assess the long-term advantages and eventual surgical results related with Plasma Rich Platelet and Fibrin Sealants, many of controlled investigation are required. Similarly, to Plasma Rich Fibrin, despite the fact that this biomaterial seems to speed up physiologic recovery, the many perspectives about PRF are yet to be practically tested.
References
- Anitua E, Pelacho B, Prado R, et al. Infiltration of plasma rich in growth factors enhances in vivo angiogenesis and improves reperfusion and tissue remodelling after severe hind limb ischemia. J Control Release 2015; 202:31-39.
- Dohan Ehrenfest DM, Bielecki T, Del Corso M, et al. Shedding light in the controversial terminology for platelet-rich products: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Platelet-Leukocyte Gel (PLG), Preparation Rich In Growth Factors (PRGF), classification and commercialism. J Biomed Mater Res A 2010; 95: 1280-1282.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T, et al. Classification of platelet concentrates: from Pure Platelet-Rich Plasma (P-PRP) to Leucocyte-Platelet-Rich Fibrin (L-PRF). Trends Biotechnol 2009; 27:158-167.
- Agarwal AA. Evolution current status and evidences in application of platelet Concentrates in periodontology and implantology. World J Clin Cases 2017; 5:159-71.
- Gasling VLW, Acil Y, Springer IN, et al. Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108:45-48.
- Smith RG, Gassmann CJ, Campbell MS, et al. Platelet-rich plasma: Properties and clinical applications. J Lanc Gen Hosp 2007; 2:73-77.
- Kingsley CS. Blood coagulation; evidence of an antagonist to factor VI in platelet-rich human plasma. Nature 1954; 173:723-724.
- Matras H. Effect of various fibrin preparations on reimplantations in the rat skin. Osterr Z Stomatol 1970; 67:338-359.
- Choukroun J, Adda F, Schoeffer C, et al. PRF: An opportunity in perio implantology. Implantodontie 2000; 42:55-62.
- Bernardi S, Mummolo S, Tecco S, et al. Histological characterization of Sacco has concentrated growth factors membrane. Int J Morphol 2017; 35:114-119.
- Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants 2007; 22:49-70.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte and platelet-rich fibrin (L-PRF). Trends Biotechnol 2009 27:158–167
- Sohn DS, Moon JW, Moon YS, et al. The use of concentrated growth factors (CGF) for sinus augmentation. J Oral Implant 2009; 38:25–38.
- Mishra A, Harmon K, Woodall J, et al. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol 2012; 13:1185-1195.
- DeLong JM, Russell RP, Mazzocca AD, et al. Platelet-rich plasma: the PAW classification system. Arthroscopy 2012; 28:998-1009.
- Tunali M, Ozdemir H, Kucukodaci Z, et al. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): a new platelet concentrate. Br J Oral Maxillofac Surg 2013; 51:438
- Mourao CF, Valiense H, Melo ER, et al. Obtentionof injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note. Rev Col Bras Cir 2015; 42:421-423.
- Guyton AC, Hall JE. Textbook of medical physiology. Philadelphia: Saunders, 1986.
- Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 85:638-646.
- Anitua E, Sanchez M, Orive G, et al. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007; 28:4551-4560.
- Dohan Ehrenfest DM, Del Corso M, Diss A, et al. Three dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol 2010; 81: 546-555.
- Tunali M, Ozdemir H, Kucukodaci Z, et al. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): a new platelet concentrate. Br J Oral Maxillofac Surg 2013; 51:438-443.
- O'Connell SM. Safety issues associated with platelet-rich fibrin method. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103:587:587-593.
- Anitua E, Zalduendo MM, Troya M, et al. Ozone dosing alters the biological potential and therapeutic outcomes of plasma rich in growth factors. J Periodontal Res 2015; 50:240-247.
- Simonpieri A, Del Corso M, Vervelle A, et al. Current knowledge and perspectives for the use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol 2012; 13:1231-1256.
- Pocaterra A, Caruso S, Bernardi S, et al. Effectiveness of platelet-rich plasma as an adjunctive material to bone graft: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Oral Maxillofac Surg 2016; 45:1027-1034.
- Arora NS, Ramanayake T, Ren YF, et al. Platelet-Rich Plasma in Sinus Augmentation Procedures: A Systematic Literature Review: Part II. Implant Dent 2010; 19:145-157.
- Saleem M, Pisani F, Zahid FM, et al. Adjunctive Platelet-Rich Plasma (PRP) in Infrabony Regenerative Treatment: A Systematic Review and RCT's Meta-Analysis. Stem Cells Int 2018: 9594235.
- Naik A, Ramesh A, Dwarkanath C, et al. Use of autologous platelet rich plasma to treat gingival recession in esthetic periodontal surgery. J Indian Soc Periodontol 2013; 17:345.
- Huang LH, Neiva REF, Soehren SE, et al. The Effect of Platelet-Rich Plasma on the Coronally Advanced Flap Root Coverage Procedure: A Pilot Human Trial. J Periodontol 2005; 76:1768-1777.
- Biradar SM, Satyanarayan A, Kulkarni AJ, et al. Clinical evaluation of the effect of platelet rich plasma on the coronally advanced flap root coverage procedure. Dent Res J 2006; 12:469-475.
- H Sugita, Y Maeda, H Alam, et al. A randomized controlled clinical study of autologous platelet rich fibrin (PRF) in combination with HA and beta-TCP or HA and beta-TCP alone for treatment of furcation defects. J Hard Tissue Biol 2019; 28:185-190.
- Dhadse P, Ragit G, Kale B, et al. Autologous platelet rich fibrin as a sole grafting material in regeneration of large periapical (palatal) defects: Report of four consecutive cases. J Datta Meghe Inst Med Sci Univ 2020; 15:108-113.
- Pundkar A, Date S, Shrivastava S, et al. Role of biologics-Platelet-rich plasma in treatment of moderate osteoarthritis of the knee. J Datta Meghe Inst Med Sci Univ 2020; 15:521-525.
- Sharma R, Sharma P, Sharma SD, et al. Platelet-Rich Fibrin as an Aid to Soft-and Hard-Tissue Healing. J Oral Maxillofac Surg 2021; 20:496-501.
- Jagati A, Chaudhary R, Rathod S, et al. Preparation of platelet-rich fibrin membrane over scaffold of collagen sheet, its advantages over compression method: A novel and simple technique. J Cutan Aesthet Surg 2009; 12:174-178.
Author Info
Kushal P Bhuskute*, Anuja Ikhar, Pradnya Nikhade, Akshay Jaiswal and Kajol Relan
Department of Conservative Dentistry and Endodontics, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences (Deemed to be University) Sawangi (Meghe) Wardha, Maharastra, IndiaCitation: Kushal P Bhuskute, Anuja Ikhar, Pradnya Nikhade, Akshay Jaiswal, Kajol Relan, A Revolutionary Journey of Platelet Concentrates in Dentistry: A Review, J Res Med Dent Sci, 2022, 10 (9): 000-000.
Received: 13-Jun-2022, Manuscript No. JRMDS-22-47336; , Pre QC No. JRMDS-22-47336; Editor assigned: 16-Jun-2022, Pre QC No. JRMDS-22-47336; Reviewed: 30-Jun-2022, QC No. JRMDS-22-47336; Revised: 16-Aug-2022, Manuscript No. JRMDS-22-47336; Published: 01-Sep-2022