Research - (2021) Volume 9, Issue 4
Albumin, pH, PaCO2 and Alveolar-Arterial Gradient Difference Can Predict 30Day Mortality in COPD Exacerbation
Sema Avci1 and Gokhan Perincek2
Abstract
Introduction: Early treatment, prognosis and short-term mortality prediction of COPD exacerbation are substantial for physicians in the ED. To compare the A-a O2 gradient (together with expected A-a O2 gradient, A-a O2 gradient difference), inflammatory markers (CRP, CAR, procalcitonin, NLR, PLR), and arterial blood gas (pH, pCO2, lactate clearance) in predicting COPD exacerbation patients’ 30-day mortality.
Methods: A questionnaire was designed for each patient, including detailed demographic profile, smoking status, comorbidities, vital signs, laboratory results and outcomes (discharge, hospitalization, ICU, 30-day mortality).
Results: 135 (60.3%) of the cases were male and 89 (39.7%) were female. Their ages ranged from 48 to 95 years, with an average of 72.22 ± 10.09. The LOS of cases varied between 1 and 28 days, with an average of 7.00 ± 3.83, ICU admission rate was 8% and the overall mortality rate was 5.4%. Albumin (AUC 0.81) and pH (AUC 0.68) showed the highest 30-day mortality prediction. While A-a O2 gradient difference (AUC 0.68) showed the highest 30-day mortality prediction, expected A-a O2 gradient (AUC 0.53) indicated a statistically lower 30-day mortality prediction. While PaCO2 (AUC 0.71) showed the highest 30-day mortality prediction, lactate clearance (AUC 0.54) indicated a statistically lower mortality estimation.
Conclusion: Albumin is a strong predictor of 30-day mortality in COPD exacerbation patients in the ED. In addition, an arterial blood gas sampling measurement including pH, PaCO2 and A-a O2 gradient difference are simple, precise and practical measurements for estimating 30-day mortality in these patients.
Keywords
Albumin, pH, PaCO2, Alveolar-arterial gradient, Chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory progressive condition which causes serious morbidity and mortality, constitutes approximately 1.5 million emergency department (ED) visits and is the fourth cause of all deaths [1,2]. COPD exacerbation is defined as the acute worsening of respiratory symptoms including dyspnea, cough and purulent sputum, and periods of exacerbation are the most common causes of ED admissions, hospitalization and mortality in COPD patients.3 The increasing number of COPD exacerbations leads to decreased quality of life, increased COPDrelated mortality rates and high healthcare costs [2,3]. Obviously, early treatment, prognosis and short-term mortality prediction are essential for physicians in the EDs.
Several laboratory results including well-known inflammatory markers such as neutrophil-tolymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), c-reactive protein (CRP)/albumin ratio (CAR), procalcitonin and arterial blood gas analysis including (pH, PaCO2, lactate clearance) have been used to predict severity, outcomes and short-long term mortality of patients with COPD exacerbation [4-8]. In addition to these laboratory parameters, the alveolar-arterial oxygen (A-a O2) gradient which is a measure of difference between the alveolar and arterial concentration of oxygen, has also been used to evaluate the outcomes (mortality, length of stay, severity of the disease) of the patients with pneumonia and pulmonary embolism [9,10].
The aims of the study were to compare the A-a O2 gradient (together with expected A-a O2 gradient, A-a O2 gradient difference), inflammatory markers (CRP, CAR, procalcitonin, NLR, PLR) and arterial blood gas (pH, pCO2, lactate clearance) in predicting COPD exacerbation patients’ 30-day mortality in the ED.
Materials and Methods
Study design and population
This prospective cross-sectional study was conducted with the approval of Kafkas University Medical Faculty Ethics Commitee between January and April 2020. The study included 224 (89 female, 135 male) patients with COPD exacerbation who admitted to ED, stages I-IV, for all patients. The diagnosis of COPD exacerbation was in accordance with the criteria established by Global Initiative for Chronic Obstructive Lung Disease 2019 [11]. Patients with a diagnosis of COPD, who were admitted to ED due to exacerbation and hospitalized were included in the study. A questionnaire was designed for each patient, including detailed demographic profile, smoking status, comorbidities, vital signs, laboratory results and outcomes (discharge, hospitalization, intensive care unit, 30-day mortality).
The laboratory findings were analyzed within 3 hours after admission to ED including arterial blood gas analysis, serum electrolytes, liver and kidney function tests, complete blood count, NLR, PLR, CRP, CAR, and procalcitonin.
Arterial blood gas samples were drawn from the radial artery in all patients while they were breathing room air to prevent any intervention caused by the maintenance of supplementary oxygen. Atmospheric pressure (mmHg), partial oxygen pressure (PaO2, mmHg), fraction of inspired oxygen (FiO2, 21% for room air), partial carbon dioxide pressure (PaCO2, mmHg) and age (for expected A-a O2 gradient) were recorded for all patients and calculated with https://www.mdcalc.com/a-a-O2-gradient [12]. After calculation process, the A-a O2 gradient and expected A-a O2 gradient for age were obtained. A-a O2 difference was calculated as A-a O2 gradient-expected A-a O2 gradient. Daily atmospheric pressure (mmHg) changes of the region where the study was carried out were obtained from the Turkish State Meteorological Service. Lactate clearance of the patients was calculated as ([initial lactate – second lactate (6 hr later)] /initial lactate) × 100 [13]. After one-month follow up, 30-day mortality after ED admission was evaluated.
Statistical reviews
All statistical calculations were performed with IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY. The continuous variables were expressed as mean ± standard deviation; categoric variables were defined as percentages (%). The normal distribution was determined by histogram and Kolmogorov-Smirnov test. Mean values of continuous variables were compared between the groups using Mann- Whitney U test. Prediction accuracy was assessed using the area under the receiver operating characteristic (ROC) curve. The results were evaluated as 95% confidence interval and p value <0.05, which was considered statistically significant.
Results
Table 1 lists sociodemographic and clinical characteristic of the patients. Accordingly, 135 (60.3%) of the cases were male and 89 (39.7%) were female. Their ages ranged from 48 to 95 years, with an average of 72.22 ± 10.09. 104 (49.3%) of the cases were ex-smoker, 25 (11.8%) were current smoker and 81 (38.4%) were nonsmoker. The respiratory rate of the patients ranged between 13 and 29 per minute with an average of 19.99 ± 1.90. The systolic blood pressure of cases varied between 50 and 160 mmHg, with an average of 120.01 ± 11.64. The fever of the cases ranged from 36 to 37.2oC, with an average of 36.41 ± 0.23. The LOS of cases varied between 1 and 28 days, with an average of 7.00 ± 3.83, (intensive care unit) ICU admission rate was 8% and the overall mortality rate was 5.4%. Coexisting diseases were not detected 43 (19.2%) of the patients and anyone was living in nursing home resident. The frequency of comorbidities seen among the patients were: Hypertension (n=53), diabetes mellitus (n=31), congestive heart failure (n=19), asthma (n=8), hyperlipidemia (n=3), chronic liver disease (n=1), coronary artery disease (n=4), chronic renal disease (n=4).
| n | % | ||
| Gender | Female | 89 | 39.7 |
| Male | 135 | 60.3 | |
| Monthly income | 0-150 USD | 99 | 45.8 |
| 151-450 USD | 102 | 47.2 | |
| 451-750 USD | 13 | 6 | |
| 751-1100 USD | 2 | 0.9 | |
| Oxygen concentrator | (+) | 80 | 36.7 |
| (-) | 138 | 63.3 | |
| Home nebulizer | (+) | 122 | 56 |
| (-) | 96 | 44 | |
| BiPAP machine | (+) | 20 | 9.2 |
| (-) | 198 | 90.8 | |
| Education status | Illiterate | 113 | 52.3 |
| Primary School | 82 | 38 | |
| Middle School | 9 | 4.2 | |
| High School | 6 | 2.8 | |
| University | 2 | 0.9 | |
| Literate | 4 | 1.9 | |
| Marital Status | Single | 6 | 2.8 |
| Married | 148 | 68.5 | |
| Divorced | 3 | 1.4 | |
| Death of partner | 59 | 27.3 | |
| Social condition | Living with family | 197 | 93.4 |
| Alone | 12 | 5.7 | |
| Support of distant relative or neighbour | 1 | 0.5 | |
| Support of civil society organization | 1 | 0.5 | |
| Place of residence | Village | 137 | 64.3 |
| County | 18 | 8.5 | |
| City | 58 | 27.2 | |
| Smoking status | Ex-smoker | 104 | 49.3 |
| Current smoker | 25 | 11.8 | |
| Never smoker | 81 | 38.4 | |
| Passive smoker | 1 | 0.5 | |
| COPD drugs | Short-acting beta agonists | 1 | 0.6 |
| Inhaled corticosteroids | 49 | 29.9 | |
| Combination inhalers | 114 | 69.5 | |
| Result of hospitalization | Discharge | 215 | 96 |
| Death | 9 | 4 | |
| Hospitalization unit | Respiratory medicine unit | 206 | 92 |
| ICU | 18 | 8 | |
Table 1: Sociodemographic properties and clinical findings of the cases.
Table 2 lists comparisons of vital signs and age with Mann Whitney U test between survivors and non-survivors. The mean age of the non-survivors was significantly higher than the survivors (z=-2.153; p=0.031). Oxygen saturation, respiratory rate and systolic blood pressure did not show significantly difference between two groups) (p>0.05).
| Groups | Xsıra | Σ sira | U | z | p | |
|---|---|---|---|---|---|---|
| Oxygen saturation | Deaths | 68 | 680 | 625 | -1.412 | 0.158 |
| Survivors | 91.82 | 15610 | ||||
| Respiratory rate | Deaths | 117.14 | 1288.5 | 801.5 | -1.192 | 0.233 |
| Survivors | 96.86 | 17821.5 | ||||
| Temperature | Deaths | 100.59 | 1106.5 | 1016.5 | -0.066 | 0.948 |
| Survivors | 99.44 | 18594.5 | ||||
| Systolic blood pressure | Deaths | 109.09 | 1200 | 923 | -0.599 | 0.549 |
| Survivors | 98.94 | 18501 | ||||
| Age | Deaths | 145.54 | 1746.5 | 767.5 | -2.153 | 0.031* |
| Survivors | 105.78 | 21473.5 |
Table 2: Comparison of oxygen saturation, respiration, fever, systolic blood pressure and age values by groups.
Table 3 lists analysis of blood parameters with Mann Whitney U test between survivors and nonsurvivors. Blood urea nitrogen (BUN) (p=0.009) and creatinine (p=0.028) were significantly lower and hemoglobine (p=0.048) was higher in non-survivors.
| Groups | Xsıra | Σ sira | U | z | p | |
|---|---|---|---|---|---|---|
| White blood cell | Non-survivors | 107.58 | 21516.5 | 983.5 | -1.049 | 0.294 |
| Survivors | 88.46 | 1061.5 | ||||
| Monocyte | Non-survivors | 105.99 | 20986 | 1091 | -0.475 | 0.635 |
| Survivors | 97.42 | 1169 | ||||
| Neutrophil | Non-survivors | 106.24 | 21036.5 | 1040.5 | -0.722 | 0.471 |
| Survivors | 93.21 | 1118.5 | ||||
| Lymphocyte | Non-survivors | 108.5 | 21699.5 | 800.5 | -1.936 | 0.053 |
| Survivors | 73.21 | 878.5 | ||||
| Eosinophil | Non-survivors | 104.68 | 20727.5 | 1026.5 | -0.792 | 0.428 |
| Survivors | 118.96 | 1427.5 | ||||
| Basophil | Non-survivors | 106.59 | 21104.5 | 972.5 | -1.072 | 0.284 |
| Survivors | 87.54 | 1050.5 | ||||
| Hemoglobine | Non-survivors | 108.54 | 21707.5 | 792.5 | -1.975 | 0.048 |
| Survivors | 72.54 | 870.5 | ||||
| Hematocrit | Non-survivors | 108.04 | 21608 | 892 | -1.492 | 0.136 |
| Survivors | 80.83 | 970 | ||||
| Platelet | Non-survivors | 107.51 | 21502.5 | 997.5 | -0.981 | 0.327 |
| Survivors | 89.63 | 1075.5 | ||||
| Red cell distribution width | Non-survivors | 103.98 | 20691.5 | 791.5 | -1.96 | 0.051 |
| Survivors | 139.54 | 1674.5 | ||||
| Glucose | Non-survivors | 106.54 | 21308.5 | 1191.5 | -0.041 | 0.967 |
| Survivors | 105.79 | 1269.5 | ||||
| BUN | Non-survivors | 104.29 | 20963 | 662 | -2.623 | 0.009 |
| Survivors | 152.33 | 1828 | ||||
| Creatinine | Non-survivors | 105.74 | 21464.5 | 758.5 | -2.195 | 0.028 |
| Survivors | 146.29 | 1755.5 | ||||
| Uric acid | Non-survivors | 86.83 | 14413.5 | 552.5 | -1.774 | 0.076 |
| Survivors | 116.25 | 1162.5 | ||||
| Phosphor | Non-survivors | 83.84 | 13331 | 611 | -0.187 | 0.851 |
| Survivors | 87.13 | 697 | ||||
| Aspartate amino transferase | Non-survivors | 109.89 | 22307.5 | 834.5 | -1.832 | 0.067 |
| Survivors | 76.04 | 912.5 | ||||
| Alanine aminotransaminase | Non-survivors | 108.51 | 21919 | 806 | -1.533 | 0.125 |
| Survivors | 79.27 | 872 | ||||
| Protein | Non-survivors | 106.26 | 20826 | 832 | -1.7 | -0.089 |
| Survivors | 75.83 | 910 | ||||
| Calcium | Non-survivors | 106.42 | 21178.5 | 910.5 | -0.94 | 0.347 |
| Survivors | 88.77 | 976.5 | ||||
| Sodium | Non-survivors | 105.38 | 21076 | 976 | -1.09 | 0.276 |
| Survivors | 125.17 | 1502 | ||||
| Magnesium | Non-survivors | 85.49 | 13763.5 | 565.5 | -0.581 | 0.561 |
| Survivors | 75.19 | 601.5 | ||||
| Bicarbonate | Non-survivors | 106.72 | 21451.5 | 1150.5 | -0.268 | 0.789 |
| Survivors | 111.63 | 1339.5 | ||||
| Lactate (initial) | Non-survivors | 107.27 | 21453 | 1047 | -0.743 | 0.458 |
| Survivors | 93.75 | 1125 | ||||
| Lactate (second) | Non-survivors | 106.64 | 21115 | 962 | -1.107 | 0.268 |
| Survivors | 86.67 | 1040 |
Table 3: Comparison of blood parameters between survivors and non-survivors.
Table 4 and Figure 1 demonstrate the accuracy of pH, pCO2, NLR, PLR, CRP, albumin, CAR, procalcitonin, A-a O2 gradient, A-a O2 gradientexpected, A-a O2 gradient difference and lactate clearance in predicting 30-day mortality. Albumin (AUC 0.81, 95% CI: 0.69-0.92) and pH (AUC 0.68, 95% CI: 0.49-0.88) showed highest 30-day mortality prediction. pCO2 (AUC 0.25, 95% CI: 0.09-0.41) and A-a O2 gradient-expected (AUC 0.29, 95% CI: 0.12-0.46) indicated statistically lower 30-day mortality prediction.
| AUC | SE | 95% CI | |
|---|---|---|---|
| pH | 0.684 | 0.1 | 0.487 to 0.881 |
| PaCO2 | 0.25 | 0.08 | 0.094 to 0.407 |
| NLR | 0.415 | 0.087 | 0.244 to 0.586 |
| PLR | 0.36 | 0.089 | 0.186 to 0.535 |
| CRP | 0.506 | 0.084 | 0.342 to 0.670 |
| Albumin | 0.811 | 0.061 | 0.692 to 0.929 |
| CAR | 0.484 | 0.085 | 0.317 to 0.652 |
| Procalcitonin | 0.37 | 0.087 | 0.199 to 0.541 |
| A-a O2 gradient | 0.364 | 0.109 | 0.149 to 0.578 |
| A-a O2 gradient-expected | 0.288 | 0.086 | 0.120 to 0.456 |
| A-a O2 gradient difference | 0.456 | 0.105 | 0.250 to 0.662 |
| Lactate clearance | 0.425 | 0.099 | 0.232 to 0.619 |
Table 4: Investigation of measurements effective in estimating 30-day mortality.
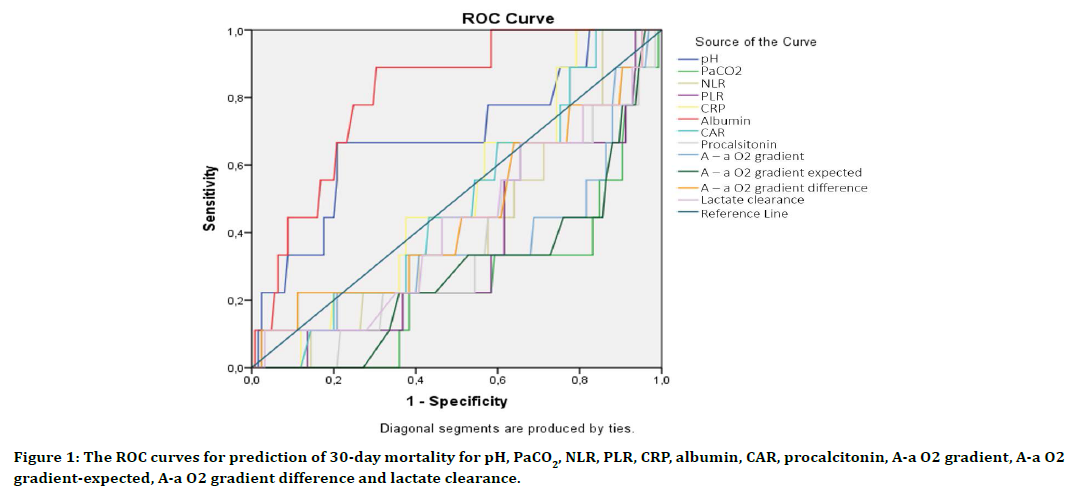
Figure 1. The ROC curves for prediction of 30-day mortality for pH, PaCO2, NLR, PLR, CRP, albumin, CAR, procalcitonin, A-a O2 gradient, A-a O2 gradient-expected, A-a O2 gradient difference and lactate clearance.
Table 5 and Figure 2 demonstrate the accuracy of A-a O2 gradient, expected A-a O2 and A-a O2 gradient difference in predicting 30-day mortality. While A-a O2 gradient difference (AUC 0.68, 95% CI: 0.62-0.75) showed highest 30-day mortality prediction, expected A-a O2 gradient (AUC 0.53, 95% CI: 0.46-0.60) indicated statistically lower 30-day mortality prediction.
| AUC | SE | 95% CI | |
|---|---|---|---|
| A-a O2 gradient | 0.598 | 0.102 | 0.528 to 0.664 |
| A-a O2 gradient-expected | 0.53 | 0.0956 | 0.460 to 0.598 |
| A-a O2 gradient difference | 0.684 | 0.0775 | 0.617 to 0.746 |
Table 5: Investigation of measurements effective in estimating 30-day mortality.
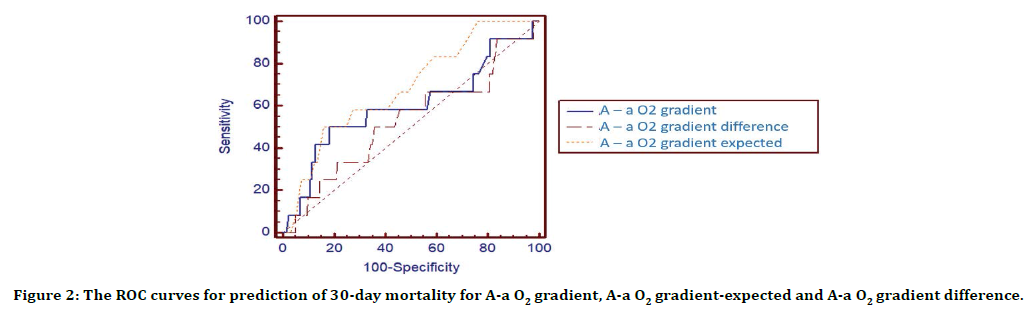
Figure 2. The ROC curves for prediction of 30-day mortality for A-a O2 gradient, A-a O2 gradient-expected and A-a O2 gradient difference.
Table 6 and Figure 3 demonstrate the accuracy of pH, PaCO2 and lactate clearance in predicting 30- day mortality. While PaCO2 (AUC 0.71, 95% CI: 0.64-0.77) showed the highest 30-day mortality prediction, lactate clearance (AUC 0.54, 95% CI: 0.47-0.61) indicated statistically lower mortality estimation.
| AUC | SE | 95 % CI | |
|---|---|---|---|
| pH | 0.658 | 0.102 | 0.590 to 0.721 |
| PaCO2 | 0.71 | 0.0874 | 0.644 to 0.770 |
| Lactate clearance | 0.54 | 0.0802 | 0.470 to 0.608 |
Table 6: Investigation of measurements effective in estimating 30-day mortality.
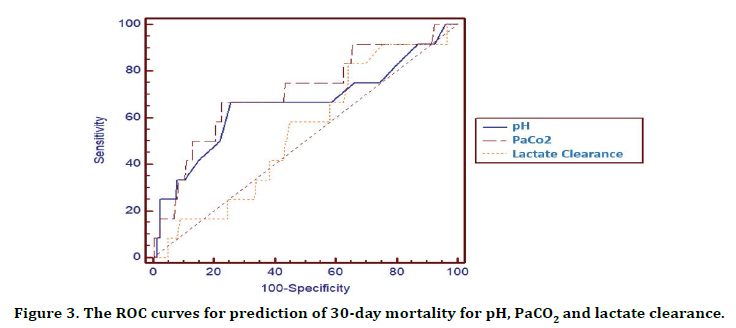
Figure 3. The ROC curves for prediction of 30-day mortality for pH, PaCO2 and lactate clearance.
Table 7 and Figure 4 demonstrate the accuracy of procalcitonine, CRP, CAR, albumin and NLR in predicting 30-day mortality. While albumin (AUC 0.81, 95% CI: 0.73-0.87) showed highest 30-day mortality prediction, CRP (AUC 0.51, 95% CI: 0.42-0.59) and CAR (AUC 0.52, 95% CI: 0.43-0.60) indicated statistically lower mortality estimation.
| AUC | SE | 95% CI | |
|---|---|---|---|
| Procalcitonin | 0.63 | 0.0924 | 0.543 to 0.712 |
| CRP | 0.506 | 0.0876 | 0.418 to 0.593 |
| CAR | 0.516 | 0.0895 | 0.428 to 0.603 |
| Albumin | 0.811 | 0.0632 | 0.734 to 0.873 |
| NLR | 0.585 | 0.0916 | 0.497 to 0.670 |
Table 7: Investigation of measurements effective in estimating 30-day mortality.
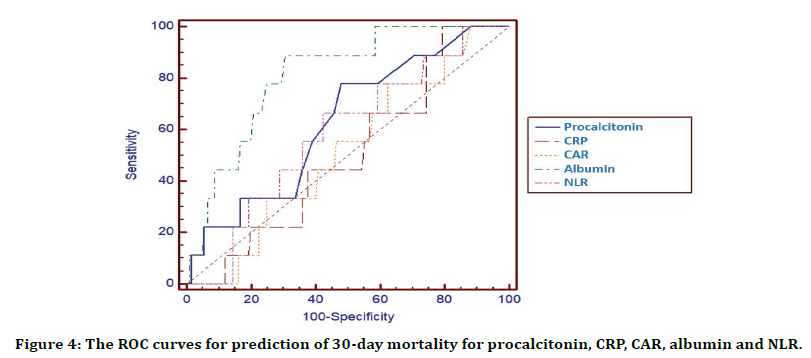
Figure 4. The ROC curves for prediction of 30-day mortality for procalcitonin, CRP, CAR, albumin and NLR.
Discussion
Mortality prediction in patients with COPD exacerbation in the ED is important for patient management. The study aimed to compare arterial blood gas (pH, PaCO2, lactate clearance), A-a O2 gradient and various inflammatory markers (NLR, PLR, CRP, CAR, procalcitonin). In the study, the 30-day mortality rate and ICU admission rate were 5.4% and 8%, respectively. The mean age of the non-survivors was higher than the survivors. The long and short term mortality rates of COPD exacerbation are quite different and the portion of this study is lower compared to these studies [14-17]. Although non-survivors in our study were elderly and the number of comorbidities was high, the lower mortality rate may be due to early admission to the ED and the high rate of hospitalization.
In the study, lower BUN, creatinine and higher hemoglobine were related to 30-day mortality among COPD exacerbation patients. Dehydration due to high fever, acute inflammatory reaction, decreased oral intake in the elderly population, smoking, increased number of comorbidities, hypoperfusion caused by hypoxia and hypercapnia may lead to impaired renal functions in COPD exacerbation patients [18,19]. Moreover, the impairment of renal functions is a factor which increases the severity of COPD and acute renal failure may increase mortality and hospital admissions.20 Serum creatinine level provides indirect information about the total muscle mass in the body [20]. Furthermore, acute serum creatine elevation is an indicator of muscle breakdown, and this increase is not seen patients with body muscle atrophy or wasting such as severe COPD [21,22]. In addition, BUN increases with the catabolism of body mass, low BUN level indicates cessation of catabolism [21]. Low creatinine and BUN levels in non-survivors may be result from severe COPD stage and muscle atrophy. Hemoglobin abnormalities such as anemia and polycythemia are common in patients with COPD and hypoxiainduced erythropoiesis leads to secondary polycythemia in these patients [23]. So, cor pulmonale and pulmonary hypertension are based on polycythemia in COPD [24]. In addition, high hemoglobin level concentration increases mortality rate and leads to poor outcomes as it predisposes to hypertension, stroke, thrombosis and cardiovascular events [23,24].
In this study, albumin (0.81) and pH (0.68) were the strongest predictor of 30-day mortality in COPD exacerbation patients among pH, pCO2, NLR, PLR, CRP, albumin, CAR, procalcitonin, A-a O2 gradient, A-a O2 gradient-expected, A-a O2 gradient difference and lactate clearance. Albumin (0.81) was also superior in predicting mortality compared to the other inflammatory markers. The mortality predictive power of PaCO2 (0.71) was higher than pH and lactate clearance. A-a O2 gradient difference (AUC 0.68) was better than expected A-a O2 gradient and A-a O2 gradient in predicting mortality. Besides albumin, pH, PaCO2 and A-a O2 gradient difference can also be used mortality prediction for COPD exacerbation in the ED. Trauma, critical conditions such as sepsis, organ failure, chronic inflammatory diseases cause increased vascular permeability and lower serum albumin level [25,26]. Moreover, albumin is an acute plasma protein responsible for microvascular permeability, acid-bas equilibrium and prevention of platelet aggregation. So, decreased serum level of albumin is associated with poor outcomes including morbidity, mortality and ICU admission in particularly critically ill patients [26-29]. COPD is a chronic and inflammatory disease and the worsening of the disease severity during exacerbation may explain the role of albumin as a predictor of mortality. Additionally, albumin is an inflammatory marker which can be obtained more easily and practically than arterial blood gas in the ED. Although the blood gas sampling procedure is difficult, pH, PaCO2 and A-a O2 gradient difference are substantial in terms of showing respiratory acidosis, compensation status and deep hypoxia [30]. The effects of pH and PaCO2 in predicting mortality, hospital admission rate, long-term changes, and non-invasive ventilation duration in COPD patients have been shown in several studies [31- 34]. In addition, we could not find any study in the literature on the relationship between A-a O2 gradient difference and COPD exacerbation.
This single-center study had some limitations. To begin with, the patient population was comparatively small and we did not evaluate a coexisting pneumonia for arterial blood gas analysis including A-a O2 gradient, expected A-a O2 gradient and A-a O2 gradient difference. A detailed effect of comordid diseases to mortality was unknown. 30-day follow-up period after ED admission, merely mortality was evaluated and the treatment protocol was not recorded. Furthermore, local hematoma, aneurysm, air or thrombus embolism, infection, laceration, hemorrhage, needle stick injuries and pain may ocur after arterial blood gas sampling [35,36].
Conclusion
Albumin is strong predictor of 30-day mortality in COPD exacerbation patients in the ED. In addition, an arterial blood gas sampling measurement including pH, PaCO2 and A-a O2 gradient difference are simple, precise and practical measurements for estimating 30-day mortality in these patients.
Acknowledgements
We thank to Murat Yasar Capan for extraction of data.
Author Contributions
S.A. and G.P. helped in the concept analysis, data collection, design, literature search, interpretation of data, preparation of initial and final draft. All the authors read and approved the final draft.
Funding
None declared.
Availability of Data and Material
The authors agree to the conditions of publication including the availability of data and materials in our manuscript.
Conflict of Interest
None declared.
Informed Consent
Informed consent was obtained from the participants or their legally authorized representatives.
Ethical Approval
This study was approved by the local ethics committee of Kafkas University Medical Faculty.
Human Rights
The principles outlined in the Declaration of Helsinki have been followed.
References
- Khialani B, Sivakumaran P, Keijzers G, et al. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease and factors associated with hospitalization. J Res Med Sci 2014; 19:297-303.
- Suau SJ, DeBlieux PMC. Management of acute exacerbation of asthma and chronic obstructive pulmonary disease in the emergency department. Emerg Med Clin N Am 2016; 34:15–37.
- Bartels W, Adamson SL, Leung L, et al. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease: factors predicting readmission. Int J Chron Obstruct Pulmon Dis 2018; 13:1647-1654.
- Yao CY, Liu X, Tang Z. Prognostic role of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis 2017; 12:2285–2290.
- Atalay E, Erdogdu HI, Tur BK, et al. The relationship between c reactive protein /albumin ratio and 1-year mortality in hospitalized elderly copd patients with acute exacerbation. Turk J Geriatr 2019; 22:9-17.
- Kurt NG, Orak M, Ustundag M. The role of lactate clearance on deciding discharge in exacerbation of chronic obstructive pulmonary disease: Retrospective cohort study. J Surg Med 2018; 2:96-98.
- Pantzarisa NK, Spiliotia DX, Psaromyaloua A, et al. The use of serum procalcitonin as a diagnostic and prognostic biomarker in chronic obstructive pulmonary disease exacerbations: a literature review update. J Clin Med Res 2018; 10:545-551.
- Ringbaek TJ, Terkelsen J, Lange P. Outcomes of acute exacerbations in COPD in relation to pre-hospital oxygen therapy. Eur Clin Respir J 2015; 2:27283.
- Avci S, Perincek G. The alveolar-arterial gradient, pneumonia severity scores and inflammatory markers to predict 30-day mortality in pneumonia. Am J Emerg Med 2020; 38:1796-1801.
- Ince O, Altintas N, Findik S, et al. Risk stratification in submassive pulmonary embolism via alveolar-arterial oxygen gradient. Hippokratia 2014; 18:333–339.
- Global initiative for chronic obstructive lung disease. pocket guide to copd diagnosis, management, and prevention a guide for health care professionals 2019 report.
- https://www.mdcalc.com/a-a-O2-gradient
- Ryoo MS, Lee JB, Lee YS, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med 2018; 46:489-495.
- Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur Respir J 2016; 47:113-121.
- Walker PP, Thompson E, Crone H, et al. Use of mortality within 30 days of a COPD hospitalisation as a measure of COPD care in UK hospitals. Thorax 2013; 68:968–970.
- Aburto M, Esteban C, Moraza FJ, et al. COPD Exacerbation: Mortality prognosis factors in a respiratory care unit. Arch Bronconeumol 2011; 47:79-84.
- Soler-Cataluna JJ, Martinez-Garcia MA, Sanchez PR, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Baha A, Ogan N, Akpinar EE, et al. The course of renal functions in copd. two statition: exacerbation and stable period. Eurasian J Pulmon 2019; 21:63-68.
- Li S, Zhang L, Zhang Y, et al. Renal impairment in patients with chronic obstructive pulmonary disease: a retrospective observational study. Int J Clin Exp Med 2018; 11:1285-1290.
- Barakat MF, McDonald HI, Collier TJ, et al. Acute kidney injury in stable COPD and at exacerbation. Int J COPD 2015; 10:2067–2077.
- Mocin OY, Karakurt Z, Sen E, et al. Serum creatinine and weaning in patients with chronic obstructive pulmonary disease: Multicenter pilot study. J Palliative Care Med 2013; 3:143.
- Barreiro E, Ariel Jaitovich. Muscle atrophy in chronic obstructive pulmonary disease: molecular basis and potential therapeutic targets. J Thorac Dis 2018; 10:1415-1424.
- Xu L, Chen Y, Xie Z, et al. High hemoglobin is associated with increased in-hospital death in patients with chronic obstructive pulmonary disease and chronic kidney disease: a retrospective multicenter population-based study. BMC Pulm Med 2019; 19:174.
- Shujaat A, Minkin R, Eden E. Pulmonary hypertension and chronic cor pulmonale in COPD. Int J Chron Obstruct Pulmon Dis 2007; 2:273-282.
- Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr 2019; 43:181-193.
- Stolz D, Meyer A, Rakic J, et al. Mortality risk prediction in COPD by a prognostic biomarker panel. Eur Respir J 2014; 44:1557-1570.
- Qian SZ, Jin D, Chen ZB, et al. Hypoalbuminemia, a novel prognostic factor for prediction of long-term outcomes in critically ill patients with septic shock. Int J Clin Exp Med 2019; 12:7401-7409.
- Akirov A, Masri-Iraqi H, Atamna A, Shimon I. Low albumin levels are associated with mortality risk in hospitalized patients. Am J Med 2017; 130.
- Yap FH, Joynt GM, Buckley TA, et al. Association of serum albumin concentration and mortality risk in critically ill patients. Anaesth Intens Care 2002; 30:202-207.
- Pahal P, Hashmi MF, Sharma S. Chronic obstructive pulmonary disease (COPD) compensatory measure. In: Stat Pearls. Treasure Island (FL): StatPearls Publishing 2020.
- Cukic V. The changes of arterial blood gases in COPD during four-year period. Med Arch 2014; 68:14-18.
- Roberts CM, Stone RA, Buckingham RJ, et al. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax 2011; 66:43-48.
- Ilhan S, Günay R, Ozkan S, et al. Arterial blood gas analysis in chronic obstructive pulmonary disease patients undergoing coronary artery bypass surgery. Turk Thorac J 2016; 17:93-99.
- Oliver P, Buno A, Alvarez-Sala R. Arterial blood gas analyses in chronic obstructive pulmonary disease: In the clinical laboratory or as point-of-care testing? Austin J Pulm Respir Med 2015; 2:1024.
- Dev SP, Hillmer MD, Ferri M. Arterial puncture for blood gas analysis. N Engl J Med 2011; 364:e7.
- Kozacı N, Gungor F, Ay MO, et al. Can venous blood gas values be used instead of arterial blood gas values in respiratory alkalosis? Turk J Biochem 2014; 39:113–118.
Author Info
Sema Avci1 and Gokhan Perincek2
1Department of Emergency Medicine, Usak University Medical Faculty, Usak, Turkey2Department of Pulmonology, Kars Harakani State Hospital, Kars, Turkey
Citation: Didem Atabek, Nagehan Akta?, Didem Sakaryal? Uyar, Two Years Clinical Evaluation of Sonic-Resin Placement System with Self-Etch and Total-Etch Adhesive Modes, J Res Med Dent Sci, 2021, 9 (4):84-92.
Received: 15-Feb-2021 Accepted: 26-Mar-2021
