Research - (2022) Volume 10, Issue 11
ANTIBACTERIAL, ANTI-ADHERENCE AND ANTIBIOFILM EFFECTS OF PETROSELINUM CRISPUM AND AZADIRACHTA INDICA AGAINST STREPTOCOCCUS SANGUINIS AND STREPTOCOCCUS MUTANS
Zaleha Shafiei1, Nur Insyirah Mohamad Nor2, Khairunnisa Hanifah2, Kristina Soosay Selvam2 and Alida Mahyuddin3*
*Correspondence: Alida Mahyuddin, Department of Family Oral Health, Faculty of Dentistry, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300, Kuala Lumpur, Malaysia, Email:
Abstract
Petroselinum crispum (Parsley) and Azadirachta indica (Neem) are known for their antibacterial activities. The potential of these two extracts as an organic mouthwash has not been widely studied. Objective: This study aimed to determine the antibacterial, anti-adherence and antibiofilm activities of Parsley and Neem extracts against commensal Streptococcus sanguinis (early colonizer) and cariogenic Streptococcus mutans (late colonizer). Fresh leaves of Petroselinum crispum and Azadirachta indica were cleansed, boiled and extracted using the aqueous extraction method. The decoction was centrifuged, filtered, and lyophilized to obtain sterile dried crude extract. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined using the broth microdilution method. The anti-adherence and antibiofilm activities at MIC and sub-MIC values were quantified using crystal violet dye on a 96-well microtiter plate. The MIC values of Petroselinum crispum against Streptococcus sanguinis and Streptococcus mutans were 256 mg/ml and 128 mg/ml, respectively. Meanwhile, the MIC values of Azadirachta indica against Streptococcus sanguinis and Streptococcus mutans were 128 mg/ml and 64 mg/ml, respectively. The MBC values of both extracts were 512 mg/ml against both bacteria. The anti-adherence and antibiofilm activities of Azadirachta indica and Petroselinum crispum were observed in both bacteria and their effects were higher against Streptococcus mutans than Streptococcus sanguinis. Azadirachta indica and Petroselinum crispum possess antibacterial, anti-adherence and antibiofilm properties against Streptococcus sanguinis and Streptococcus mutans, and could be used as alternatives to chemical mouthwash in preventing dental caries.
Keywords
Antibacterial, Anti-adherence Antibiofilm, Petroselinum crispum, Azadirachta indica
Introduction
Dental caries is a common chronic infectious disease resulting from specific oral bacteria, primarily the Streptococcus mutans. These bacteria metabolize sugars to produce acid which would demineralize the tooth structure, over some time. It affects an estimated 60-90% of schoolchildren and the vast majority of adults globally [1].
S. mutans is present in the oral biofilm (or dental plaque) along with Streptococcus sanguinis which make up the normal microbiota in the oral cavity. S. sanguinis is an early colonizer that binds to the tooth surface for the formation of biofilm utilizing the salivary pellicle. It serves as a chain for the adherence of other bacteria [2]. While S. mutans as a late colonizer has a receptor to adhere to salivary components of the salivary pellicle and also on the cell surface of S. sanguinis to form multispecies biofilm. Several studies have reported the inverse relationship between S. mutans and S. sanguinis. The increase in S. sanguinis level has been associated with caries-free or post-treatment oral environment[3,4].
As treatment of dental caries is costly, caries prevention is of utmost importance. One of the methods of prevention is the use of antimicrobials to eliminate cariogenic oral bacteria. However, some of these antimicrobials available commercially can have undesirable side effects such as altered taste, stained teeth and mucosal desquamation [5]. Complications arising from increased bacterial resistance to antibiotics as well as adverse effects of some antibacterial agents currently used in dentistry have indicated the need to seek alternative prevention and treatment options that are deemed safe, effective and economical. Natural phytochemicals isolated from plants that have been used as traditional medicines are considered as potential alternatives.
Azadirachta indica which is commonly known as Neem is a member of the Meliaceae family. It is native to India but can be found in most tropical and subtropical countries. It is widely used in Chinese and Indian ayurvedic medicines. The chemical constituents have been found to contain many biologically active compounds such as azadirachtin, nimbolinin, nimbin, nimbidin, nimbidol, salannin and quercetin which have the property to inhibit bacterial growth and modulate genetic pathways [6]. Neem bark is used as the active ingredient in many kinds of toothpaste and tooth powders. Dried chewing sticks of Neem showed maximum antibacterial activities against S. mutans compared to other dental cariescausing organisms, S. salivarius, S. mitis, and S. sanguinis [7]. A study on Neem leaf extract showed a significant difference in the zone of inhibition when compared with chlorhexidine against S. mutans [8]. Another study reported that organic extracts of Neem leaves showed strong antimicrobial activities against Streptococcus mutans, Streptococcus salivarius and Fusobacterium nucleatum isolated from dental caries [9].
Petroselinum crispum (P. crispum) (Parsley) is a species of flowering plant in the family Apiaceae that is native to the central Mediterranean region. Parsley is commonly used to flavor cuisines [10]. Tang, et al. [11] has shown that Parsley has antioxidant properties to destroy free radicals, protect against DNA damage that can lead to cancer, and inhibit both the proliferation and migration of cancer cells. Parsley has been employed in the food, pharmaceutical, perfume, and cosmetics industries [12]. In traditional medicine, it is a diuretic, uterine stimulant, sedative, emollient and anti-parasitic agent. It is also commonly employed for the treatment of chronic bronchitis, bronchial asthma and dyspepsia [13,14]. Various chemical composition and pharmacological properties in P. crispum such as flavonol glycosides of quercetin, apiol, myristicin, terpenes, luteolin, phthalides, furanocoumains, apiin, carotenoids, ascorbic acid and tocopherol [15,16]. Previous studies reported that the supplementation of diets with fresh P. crispum leaves increases the antioxidant capacity of plasma in rats [17] and decreases oxidative stress in humans [18]. Zheng, et al. [19] reported the inhibition of benzo[a] pyrene-induced tumorigenesis in the lungs of mice by myristicin, a major volatile aromatic constituent of parsley leaf oil. In terms of dental caries, Parsley (P. crispum) was reported to have a lower anti-S. mutans activity in vitro and its bacteriostatic activity on S. mutans strains is significantly less than that of chlorhexidine mouthwash.
Oral microbial studies of both plants are still lacking, particularly on S. sanguinis and S. mutans. Therefore, this study was conducted to investigate the antibacterial, antiadherence and antibiofilm properties of A. indica (Neem) and P. crispum (Parsley) leaves against S. sanguinis and S. mutans for the prevention of dental caries.
Materials and Methods
Ethical approval
The study protocol was reviewed and approved by the Research Ethics committee of The National University of Malaysia (The Ethics committee/IRB reference number: UKM PPI/111/8/JEP-2019-640). Written informed consent was obtained from a volunteer for saliva collection.
Preparation of plant extracts
Fresh leaves of A. indica and P. crispum were obtained from the local market in the Chow Kit area, Federal Kuala Lumpur in September 2020. The fresh leaves were washed with tap water followed by distilled water. The leaves were dried using tissue paper, weighed, and cut into small pieces before aqueous extraction using a modified method described by Shafiei, et al. [20]. A total of 100 g of fresh leaves was blended for 2 minutes and repeated several times with a total of 1000 ml of boiling distilled water. The decoction was left for 30 minutes in warm water for thorough extraction. The decoction was then filtered with muslin cloth and the clear filtrate was heated at 80°C until the volume reached 200 ml. The cold filtrate was then centrifuged at 3660 rpm at 4°C for 20 minutes and the supernatants were then filtered using Whatman No.1 filter paper. The extract was frozen overnight at -80°C followed by freeze-drying (i.e., lyophilization) using a freeze dryer (Lyotrap, Great Britain) in a sterile environment. The sterile powdered crude aqueous extracts were stored in airtight bottles at -20°C for further use.
Preparation of bacterial stock
S. sanguinis ATCC BAA-1455 (strain SK36) and S. mutans ATCC 25175 were from American Type Culture Collection (ATCC, USA) and obtained from the Balai Ungku Aziz Research Laboratory, Faculty of Dentistry, University of Malaya. Before the experiment, the respective 20% glycerol stocks of each bacterium were prepared. Briefly, 1% of the respective bacterial strains were transferred in 20 ml of sterile fresh Brain Heart Infusion (BHI) broth (Oxoid, UK), incubated overnight at 37°C, 5% CO2. The bacterial suspension was centrifuged at 7200 rpm, 4°C for 30 minutes. The supernatant was decanted, and the pellet was rinsed once with cold phosphatebuffered saline (PBS, pH 7.4) (Oxoid, UK) to remove excess supernatant. To prepare bacterial stocks in 20% glycerol, the respective pellet was suspended with 8 ml of fresh BHI broth media and 2 ml of 70% glycerol. The respective bacterial suspension was then aliquoted into Eppendorf tubes and stored at -80oC for subsequent use.
Standardization of bacterial suspension
The bacterial stock was thawed at room temperature and 1% of the strains were transferred in 20 ml of sterile fresh Brain Heart Infusion (BHI) broth (Oxoid, UK) and incubated at 37°C, 5% CO2 for 24 hours. The bacterial suspensions were then centrifuged at 7200 rpm, 4°C for 30 minutes. The supernatant was decanted, and the pellet was rinsed once with cold PBS to remove excess supernatant and suspended in fresh BHI broth (10 ml). The turbidity of each strain was standardized by adjusting the absorbance to 0.144 at OD 550 nm using a spectrophotometer [20]. The purity of the bacterial cultures was checked each time before every experiment by streaking the bacterial suspension on BHI agar and incubating at 37°C for 24 to 48 hours (until the appearance of the colonies). Gram staining was also carried out to confirm the identity of working strains.
Determination of Colony-Forming Units (CFUs) for MIC assay
The turbidity of the test bacterial suspension that matched with that of the 0.5 McFarland Standard was measured using a spectrophotometer at an optical density (OD) of 550 nm. The value was taken and adjusted to a reading of 0.144 at OD 550 nm to standardize the bacterial suspension for subsequent studies. A series of 10-fold serial dilutions until the 106 dilutions (101-106 dilutions) of the test bacterial suspension was prepared and vortexed vigorously for 10 seconds. A total of 20 μl of each serial dilution was plated on a BHI agar plate in triplicate. The plates were incubated at 37°C for 24 to 28 hours. The dilution containing from 30 to 300 colonies was taken as a valid measurement. The colony-forming unit was calculated as follows:
Total CFU/ml=Number of colonies/Volume plated (ml) × Dilution factor
The standardized bacteria at 550 nm of 0.144 which was equivalent to 106 CFU/ml was used to yield the final cell count of 105 CFU/ml in the MIC system.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
A standard broth microdilution method following the standard protocol of Clinical and Laboratory Standards Institute (CLSI, 2012) was carried out to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of A. indica and P. crispum against S. sanguinis and S. mutans [21]. A total of 4.096 g of sterile extract (A. indica) was reconstituted in 4 ml sterile distilled water giving a final concentration of 1024 mg/ml for the stock solution of extract. The reconstituted extract was serially diluted 2-fold in BHI broth to obtain a range of concentration (512 – 0.25 mg/ml) in respective test tubes labeled T1-T12. The diluted extract was transferred into the corresponding wells of a 96-well microtiter plate in triplicate to ensure reproducibility of the result. A total of 90 μl of the respective serially diluted plant extract in BHI broth (T1-T12) was added to the first three rows of the 96- well microtiter plate labeled as A1-A12, B1-B12, C1- C12 and 10 μl of standardized bacterial suspension (S. sanguinis) was pipetted into the respective wells, resulting a recommended a final cell count of 105 CFU/ ml. The respective 2-fold serially diluted plant extract without a bacterial presence in triplicate was added to the wells D1-D12, E1-E12 and F1-F12 as the blank control. A 0.12% chlorhexidine (well G1-G3 and H1- H3), and sterile distilled water (well G5-G7 and H5- H7), replaced by plant extract, served as positive and negative controls, respectively. The respective BHI broth without bacteria (well G9-G11) and with the bacterial suspension (well H9-H11) in a total of 100 μl were also prepared to ensure the fresh BHI broth used was sterile and the bacteria grew well in the medium throughout the experiment. Subsequently, the microtiter plate was incubated at 37°C, 5% CO2 for 24 hours. The MIC was determined using an ELISA microplate reader at 620 nm. The wells indicated no turbidity changes of the bacterial growth in suspension compared to that of blank control were taken as MIC values.
A similar procedure was repeated using P. crispum against S. sanguinis. The experiment was repeated for the two tested plant extracts using S. mutans.
For the MBC determination, 20 μl of the bacterial suspension from selected wells which showed almost no turbidity was inoculated on BHI agar as well as the positive control (chlorhexidine), negative control (BHI broth-distilled water mixture) and sterile BHI broth. The latter was to detect any possibility of contamination in the tested fresh broth. The agar plates were then incubated for 24 hours at 37°C. The lowest concentration of the extracts that showed no growth corresponded to the MBC.
Preparation of sterilized saliva
Saliva was prepared according to the modified method described by Nordini, et al. [22]. A single volunteer was used to collect 100 ml of stimulated whole saliva (SWS) to minimize any variation in the experiment. The SWS collection was done by expectorating in a test tube after chewing a parafilm. The donor was healthy, caries-free, had no known chronic disease and had no antibiotic taken in the previous 3 months. The SWS was centrifuged at 8000 rpm, 4°C for 15 minutes. The collected supernatant was filter-sterilised using a 0.22 μm low-protein binding membrane filter (Cellulose acetate syringe filters Sartorius, USA) and stored at -20°C for further use. Before the experiment, the sterile SWS was thawed at room temperature and centrifuged to remove any precipitate.
Anti-adherence effects of plant extracts
The anti-adherence effect of A. indica and P. crispum against S. sanguinis and S. mutans was carried out by a modified method of Kwasny, et al. [23] by preparing the plant extracts at MIC and sub-MIC concentrations in Eppendorf tubes. The experiment was carried out using one plant extract at a time. The 96-wells microtiter plate was pre-coated with 150 μl of sterile saliva in respective wells followed by incubation at 37°C for 15 minutes. The saliva was then aspirated and rinsed twice with 200 μl cold phosphate-buffered saline (PBS, pH 7.4) (Oxoid, UK) to remove the excess saliva. The wells were then treated with 150 μl of the plant extract followed by incubation at 37°C for 15 minutes. The plant extract was aspirated and rinsed twice with cold PBS (200 μl) to remove the excess extract. A total of 150 μl of S. sanguinis was added to the wells and incubated for 24 hours to form a 24- hour biofilm.
The bacterial suspension was aspirated following incubation and rinsed twice with 200 μl of cold PBS to remove the unattached biofilm. The wells were then dyed with 200 μl of 0.2% (v/v) crystal violet dye for 20 minutes followed by aspiration and cold PBS washing (200 μl) for 2 times. Each well was added with 95% (v/v) ethanol (200 μl) and the solution was measured with an ELISA microplate reader at 620 nm wavelength.
For positive and negative controls, the wells were treated with 0.12% (v/v) Chlorhexidine and sterile distilled water, respectively, instead of the plant extract. The procedure was then repeated with S. mutans. All the procedures were done in triplicate.
Antibiofilm effects of plant extracts
Biofilm reduction (antibiofilm) assay was prepared according to the modified method of Kwasny, et al. [23] by preparing the plant extracts (P. crispum and A. indica) at MIC and sub-MIC values (32 – 0.5 mg/ml) in Eppendorf tubes. The experiment was carried out using one plant extract at a time. The 96-wells were precoated with 150 μl of sterile saliva in respective wells followed by incubation at 37°C for 15 minutes. The excess saliva was rinsed twice using 200 μl of cold PBS (pH7.4). Then, the wells were added with 150 μl of S. sanguinis and incubated at 37°C, 5% CO2 for 24 hours to form a 24- hour biofilm. The bacterial suspension was aspirated and rinsed twice with 200 μl PBS to remove unattached cells in the biofilm. Then, the biofilm was treated with 150 μl of the plant extract and incubated at 37oC for 15 minutes. The wells were then aspirated and washed twice with cold PBS (200 μl; pH7.4) to remove excess plant extract. The wells were then dyed with 0.2% (v/v) crystal violet dye (200 μl) for 20 minutes followed by aspiration and rinsed twice with cold PBS (200 μl). The wells were then added with 95% (v/v) ethanol (200 μl) and read with an ELISA microplate reader at 620 nm wavelength.
For positive and negative controls, the wells were treated with 0.12% (v/v) Chlorhexidine and sterile distilled water, respectively, instead of the plant extract. All the procedures were done in triplicate and repeated with S. mutans.
Statistical analysis
Data were analysed using IBM SPSS statistical software, version 25. The Kolmogorov-Smirnova test was used to test assumptions of normality. The results were analysed using two-way ANOVA (Analysis of Variance) which is a parametric statistical technique used to test the hypothesis of more than two groups with different outcomes in the normal distribution. Meanwhile, the Kruskal-Wallis test was used as a non-parametric test to assess for significant differences on a continuous dependent variable by a categorical independent variable. Meanwhile, the Mann-Whitney U test was also used to compare two different independent groups when the dependent variable was continuous and not normally distributed. An independent t-test was used to compare two independent variables on the same continuous dependent variable. Results were significant at p<0.05. The experiments were performed in triplicate. Mean and standard deviation (SD) as OD measurements were stated in the results and were expressed in a bar chart graph.
Results and Discussion
The aqueous extraction method was chosen in this study due to water being a convenient and environmentally friendly solution with higher polarity compared to chemical extraction. This allowed more active compounds to be diluted in the water. Figure 1 showed a photograph of A. indica (Neem) and P. crispum (Parsley) and its respective dried powdered aqueous extracts used in this study. No data on the yield of the two dried powdered plant extracts obtained from aqueous extraction method.
Figure 1: Photograph of (a) Azadirachta indica (Neem) and (b) Petroselinum crispum (Parsley) and its respective dried powdered aqueous extracts.
The crude aqueous extracts of A. indica (Neem) and P. crispum (Parsley) were used to determine the antibacterial, anti-adherence and antibiofilm activities against commensal S. sanguinis and cariogenic S. mutans population. The two bacteria were selected in this study due to their presence as normal microbiota and their interactions in the oral cavity to cause dental caries. In this study, the respective S. sanguinis and S. mutans suspension were standardized at optical density 550 nm of 0.144 and were found to be equivalent to (4.33 ± 6.35) x106 and (4.63 ± 1.53) x 107 CFU/ml, respectively (Table 1). The colonies of S. sanguinis ATCC BAA-1455 (SK36) on the BHI agar plate showed small sizes and translucent colonies while S. mutans ATCC 25175 colonies were also small with white colour colonies after 24 hours incubation (Figure 2). It is also expected that a higher CFU/ml count of S. mutans than S. sanguinis at similar OD reading might be due to the distinctness in sizes and colours of the two colonies.
| Bacteria | Mean ± SD of bacterial suspension (CFU/ml) |
|---|---|
| Streptococcus sanguinis ATCC BAA-1455 | (4.33 ± 6.35) x106 |
| Streptococcus mutans ATCC 25175 | (4.63 ± 1.53) x 107 |
Table 1: The CFU/ml of S. sanguinis and S. mutans suspensions at OD 550 nm of 0.144.
Figure 2: Pure colonies of (a) Streptococcus sanguinis and (b) Streptococcus mutans on agar BHI agar plate after 24 hours incubation.
| Plant extract | Bacteria | MIC (mg/ml) | MBC (mg/ml) |
|---|---|---|---|
| Neem | Streptococcus sanguinis ATCC BAA-1455 | 128 | 512 |
| Streptococcus mutans ATCC 25175 | 64 | 512 | |
| Parsley | Streptococcus sanguinis ATCC BAA-1455 | 256 | 512 |
| Streptococcus mutans ATCC 25175 | 128 | 512 |
Table 2: Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of Parsley and Neem against S. sanguinis and S. mutans.
The standardisation of the two bacteria was needed to ensure the final concentration of 105 CFU/ml bacterium has being used for the MIC system based on CLSI protocol. In this study, the MIC values of Neem against S. sanguinis (128 mg/ml) and S. mutans (64 mg/ml) were lower than Parsley for both bacteria [S. sanguinis (256 mg/ml) and S. mutans (128 mg/ml)] (Table 2). The lower MIC value of Neem against both the bacteria indicates it has higher inhibitory activities compared to Parsley. Ghapanchi, et al. [24] confirmed that the Parsley (P. crispum) has a lower anti-S. mutans activity and it is significantly less than that of chlorhexidine mouthwash.
This is the first report on the antibacterial effects of Neem and Parsley against S. sanguinis which indicated that the leaves extract of Parsley is ineffective in inhibiting the growth of S. sanguinis compared to S. mutans. However, the efficiency of Neem as anti-S. sanguinis exhibited similar efficacy with Parsley against S. mutans (Table 2). Alzohairy et al. [6] supported the finding that Neem (A. indica) has shown antibacterial activity against isolated oral bacteria from an adult mouth.
In this study, the MIC (64 mg/ml) and MBC (512 mg/ ml) values of A. indica against S. mutans were higher compared with what has been reported by Mistry, et al. [25] which is 125 μg/ml and 250 μg/ml, respectively. This could be due to the difference in the bacterial strain used which was from clinical isolate whereby this study used bacterial strain purchased from ATCC. Besides that, the difference in extraction method can influence the MIC and MBC values as the extraction method used were aqueous extraction while the previous study used methanol extraction [25]. Subramaniam, et al. [26] stated that fewer active compounds are extracted using an aqueous extraction method compared to the ethanol extraction method where organic dissolvent such as ethanol is more effective due to the active compounds belonging to phenol groups will dissolve better in organic dissolvent. However, in this study distilled water is used as it is a more economical, safer and environmentally friendly procedure while the ethanol extraction method is more expensive and possibly causes side effects to the human lung following inhalation. However, total phenolics content of Neem leaves extract by using Folin– Ciocalteu reagent and gallic acid as a standard showed the highest amount of total phenolics was obtained in butanol crude extract (107.29 mg/g dry crude extract) compared to water extraction (51.02 mg/g dry crude extract) [27]. Azizah, et al. [28] also reported that the boiling method for 2-6 min resulted in significant losses (18.3%) of total phenolic compounds of pumpkin but increased its carotenoid content (fat-soluble compound). Phenolic compounds are water-soluble (flavonoid) and water-insoluble (tannin) compounds. The heating process used in this study was less than the boiling point (70 - 80°C) to ensure that the active compounds were not degraded by heat. Thus, all the potential effects shown by the selected plant extracts throughout this study were derived from the retained phenolic compounds.
Azadirachtin belongs to the class of limonoids and is present as a secondary metabolite in both Neem seeds and leaves but is most extracted from its seeds [29]. According to Hayes' Handbook of Pesticide Toxicology (2010), azadirachtin is stable in crystalline form and must be stored in the dark, have a higher decomposition rate when exposed to high temperature, acidic condition and especially in light. Neem formulations can retain at least 59% of the azadirachtin content for about a year when it is stored at 10-15°C in the dark [30].
The MBC value was higher than the MIC value for all the extracts. The MBC value was the same for both extracts against the two bacteria which was 512 mg/ ml, indicating that it exerts similar bactericidal effects against the two bacteria at higher concentrations. The active compounds that contributed to the antibacterial activities of aqueous extract of P. crispum leaves are flavonoids apigenin, cosmosiin, oxypeucedanin hydrate, apiin [7] and kaempferol [31].
According to Alzohairy, et al. [6] acute oral toxicity effects of A. indica against mice are approximately 13 g/ kg of the body weight. There is no proven oral toxicity of P. crispum. Awe, et al. [32] stated P. crispum is nontoxic orally at doses of 10-100 mg/kg for 8 weeks in rats. The MBC obtained throughout this experiment is 512 mg/ml which is far less than the proven toxicity.
Chlorhexidine (0.12% v/v) is a gold standard in the treatment of periodontal disease and was chosen as a positive control for comparison to the plant extracts. Some studies reported that chlorhexidine is effective as an antibacterial agent, but it may cause staining; alter taste perception and tartar formation. Due to the several side-effects of chlorhexidine (a chemical mouth rinse), thus a safer new alternative, economically and effective from natural plant extracts was extensively studied. Thus, the potential of the leaves aqueous extracts of A. indica (Neem) and P. crispum (Parsley) at MIC and sub- MICs against S. sanguinis and S. mutans was further determined as anti-plaque and anti-caries agents using anti-adherence and antibiofilm assays. The theory behind the pre-coated plant extract on surface saliva in a 96-well microtiter plate was to inhibit the bacteria from adhering to the salivary pellicle to form biofilm. This process is known as anti-adherence or anti-plaque assay. While the ability to disperse a formed 24-hour biofilm by the plant extract is represented as antibiofilm or anti-caries assay. In this study, the anti-adherence and antibiofilm activities were determined using the modified methods of Kwasny, et al. [23]. In which the amount of bacterial suspension in the microtiter plate, frequency of washing and the agents used to wash (sterile distilled water), OD (600) and usage of acetic acid for final rinse were modified. Generally, results showed that all the plant extracts at MIC and sub-MIC values were able to inhibit the adherence of S. sanguinis and S. mutans at lower and higher concentrations, respectively when compared to the negative control (Figures 3 and Figure 4).
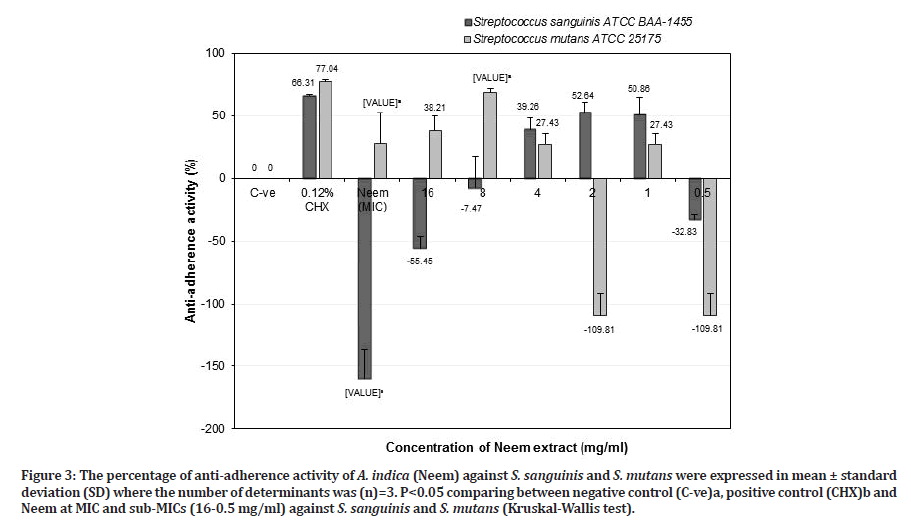
Figure 3:The percentage of anti-adherence activity of A. indica (Neem) against S. sanguinis and S. mutans were expressed in mean ± standard deviation (SD) where the number of determinants was (n)=3. P<0.05 comparing between negative control (C-ve)a, positive control (CHX)b and Neem at MIC and sub-MICs (16-0.5 mg/ml) against S. sanguinis and S. mutans (Kruskal-Wallis test).
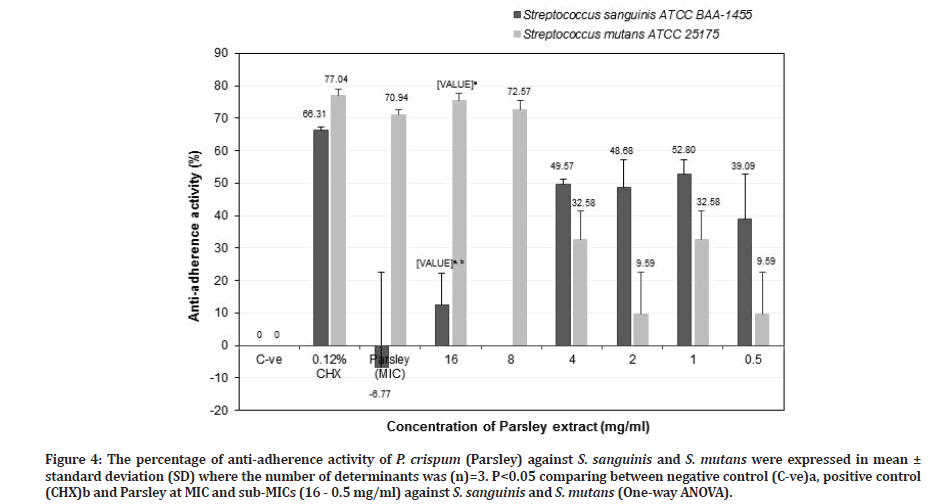
Figure 4:The percentage of anti-adherence activity of P. crispum (Parsley) against S. sanguinis and S. mutans were expressed in mean ± standard deviation (SD) where the number of determinants was (n)=3. P<0.05 comparing between negative control (C-ve)a, positive control (CHX)b and Parsley at MIC and sub-MICs (16 - 0.5 mg/ml) against S. sanguinis and S. mutans (One-way ANOVA).
Specifically, Neem at MIC value (64 mg/ml) showed a significant negative result of anti-adherence activity against S. sanguinis (-159.87 ± 23.29%) (p=0.037) and effective anti-adherence activity against S. mutans (27.83 ± 24.80%) (p=0.037) compared to the negative control. However, it was not found to be as effective as 0.12% chlorhexidine for both bacteria. Neem (A. indica) at a potent sub-MIC of 8 mg/ml indicated that it was significantly more effective against S. mutans (68.39 ± 3.17%) (p=0.037) than S. sanguinis (-7.47 ± 25.44%) (p=0.487) compared to the negative control. When compared with chlorhexidine against S. sanguinis, Neem at sub-MIC of 8 mg/ml (-7.47 ± 25.44%) was less potent than chlorhexidine (66.31 ± 1.03%). However, it showed greater anti-adherence activity against S. mutans (68.39 ± 3.17%) as compared to chlorhexidine (77.04 ± 1.82%) (p=0.513) (Figure 3).
While Neem at the value of 2 mg/ml, showed an effect against S. sanguinis (52.64 ± 8.15) than S. mutans (-109.81 ± 17.75%) compared to the negative control. Meanwhile, for S. mutans, it showed less anti-adherence activity (compared to chlorhexidine (77.04 ± 1.82%) but for S. sanguinis, Neem (52.64 ± 8.15%) showed better activity with a result close to chlorhexidine (66.31 ± 1.03%) (p > 0.05) (Figure 3). Neem at higher concentration (MIC and sub-MIC of 8 mg/ml) against S. sanguinis showed negative results rather than lower concentration of 2 mg/ml, suggesting that the aggregation process occur between Neem extract and S. sanguinis that tightly adhered to the salivary pellicle, allows this bacterium retained the colour of extract and interfere with crystal violet dye that adsorbed in the cell wall of the bacterium, resulting in higher OD reading. However, this result contradicted with had been reported by Gorniak, et al. [33] that flavonoids from plant extracts inhibit the virulence factors, biofilm formation and reduce the bacterial population through aggregation rather than bactericidal activity. Further study needs to be done to confirm the results. Based on the result in Figure 3, Neem at 8 mg/ml is a good candidate as an alternative antiplaque agent due to the ability to reduce cariogenic S. mutans population and retained commensal S. sanguinis population.
The percentage of anti-adherence activities of P. crispum (Parsley) against S. sanguinis and S. mutans was shown in Figure 4. Generally, Parsley had proficiency to reduce S. mutans population at higher concentration and lower concentration for S. sanguinis. Results indicated that Parsley at MIC value (128 mg/ml) showed higher anti-adherence activity against S. mutans (70.94 ± 1.57%), which was close to chlorhexidine (77.04 ± 1.82) (p=0.330). However, for S. sanguinis, the anti-adherence activity of Parsley (-6.77 ± 29.49) was much lower when compared to chlorhexidine (66.31 ± 1.82%) (p=0.105). Parsley at sub-MIC of 16 mg/ml showed a greater antiadherence activity towards S. mutans (75.46 ± 2.13%) (p=0.07) than S. sanguinis (12.38 ± 10.01%) (p=0.021) when compared to the negative control. The antiadherence activity against S. sanguinis was much lower compared to chlorhexidine but for S. mutans, the antiadherence activity was similar to the latter (p > 0.05).
Parsley at sub-MIC of 1 mg/ml showed better antiadherence activity against S. sanguinis (52.80 ± 4.31%) than S. mutans (32.58 ± 8.79) compared to the negative control (Figure 4). However, Parsley at that concentration was less effective as an anti-adherence or anti-plaque agent against both S. sanguinis and S. mutans compared to chlorhexidine (p > 0.05). Therefore, Parsley at 16 mg/ml is suitable as an anti-plaque agent in reducing cariogenic S. mutans and retained commensal S. sanguinis populations. Zhu, et al. [34] reported that S. sanguinis adhered tightly to salivary pellicle and compete with cariogenic S. mutans in reducing dental caries and retaining commensal S. sanguinis population will create healthy oral cavity as well as associated with periodontal health. Referring from the results in Figures 3 and 4 indicated that Neem (8 mg/ml) is two times more effective as an anti-plaque or anti-S. mutans agent compared to Parsley (16 mg/ml).
Figures 5 and 6 show the percentage of antibiofilm activities of A. indica (Neem) and P. crispum (Parsley) against S. sanguinis and S. mutans. Both plant extracts were found to effectively reduce S. sanguinis and S. mutans biofilms when compared to the negative control. Neem at MIC value (64 mg/ml) showed a significant negative result against S. sanguinis (-1.97± 19.59%) (p=0.023) but effective antibiofilm activity against S. mutans (69.21 ± 2.91%) (p=0.115). However, it was not as effective in comparison to chlorhexidine for both S. sanguinis (45.50 ± 10.38%) (p=0.208) and S. mutans (79.16 ± 1.92%) (p=0.624), respectively. Based on Figure 5, Neem at sub- MIC of 8 mg/ml was potent as an antibiofilm agent, but it was greater effective against S. mutans (78.46 ± 3.19%) (p=0.114) than S. sanguinis (34.51± 6.33%) (p=0.052) compared to the negative control. When compared with chlorhexidine, Neem at sub-MIC of 8 mg/ml against S. sanguinis (34.51 ± 6.33%) was less than chlorhexidine (45.50 ± 10.38%) (p=0.275) but with S. mutans (78.46 ± 3.19%) showed almost as effective as chlorhexidine (79.16 ± 1.92%) but it is not significant (p =0.397). Pai, et al. [35] reported the effectiveness of Neem leaves extract against S. sanguinis biofilms in the oral cavity. Neem extract reduced the plaque index and bacterial count significantly as compared with a control group [35]. In another study by Harjai, et al. [36] showed that the adhesion of Pseudomonas aeruginosa (P. aeruginosa) to uroepithelial cells was reduced significantly in the presence of Neem extract, indicating that Neem can inhibit the adhering ability of P. aeruginosa. It is also indicated that Neem extract was also effective in altering the structure of biofilm, which may result in the penetration of antibiotics easily and hence may be effective even at low doses. But their study used an ethanolic extraction method [36].
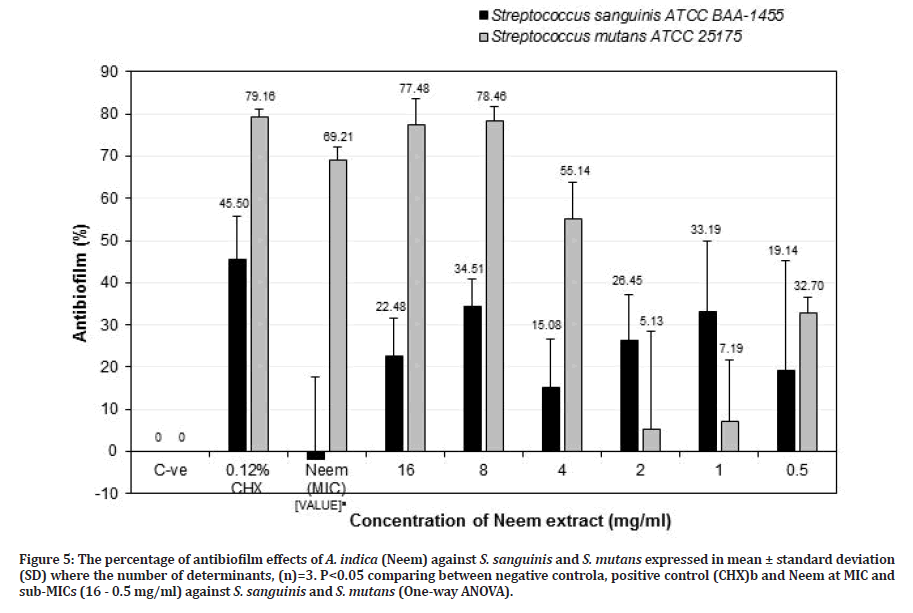
Figure 5: The percentage of antibiofilm effects of A. indica (Neem) against S. sanguinis and S. mutans expressed in mean ± standard deviation (SD) where the number of determinants, (n)=3. P<0.05 comparing between negative controla, positive control (CHX)b and Neem at MIC and sub-MICs (16 - 0.5 mg/ml) against S. sanguinis and S. mutans (One-way ANOVA).
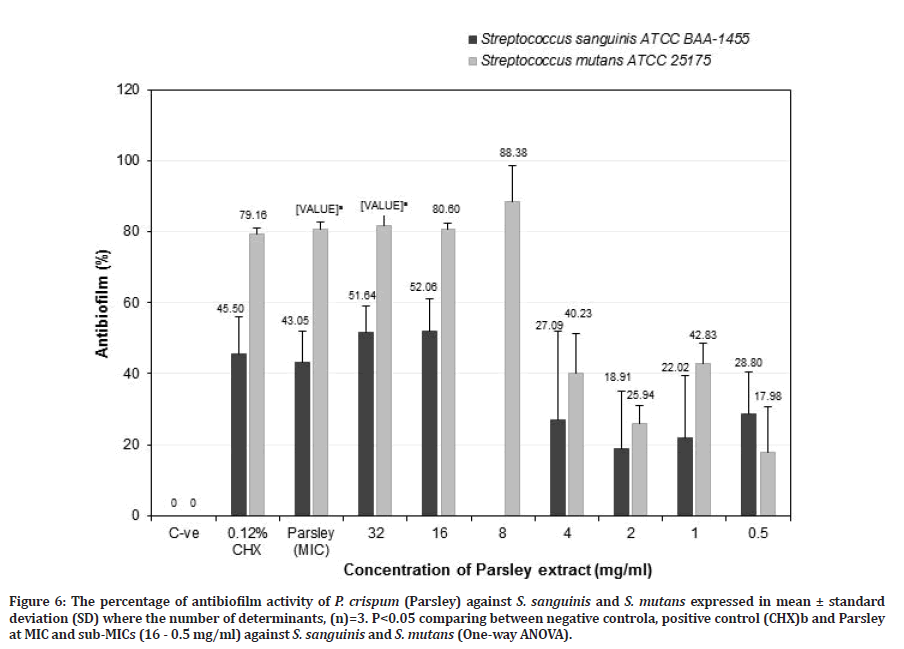
Figure 6: The percentage of antibiofilm activity of P. crispum (Parsley) against S. sanguinis and S. mutans expressed in mean ± standard deviation (SD) where the number of determinants, (n)=3. P<0.05 comparing between negative controla, positive control (CHX)b and Parsley at MIC and sub-MICs (16 - 0.5 mg/ml) against S. sanguinis and S. mutans (One-way ANOVA).
Parsley at MIC value showed better antibiofilm activity towards S. mutans (80.66 ± 1.93%) (p=0.017) compared to S. sanguinis (43.05 ± 8.90%) (p=0.054), but it was not as effective as chlorhexidine (45.50 ± 10.38%) (p=0.656) for S. sanguinis. However, for S. mutans, it showed higher antibiofilm activity than chlorhexidine (79.16 ± 1.92%) but was not significant (Figure 6). Parsley at a potent sub-MIC of 32 mg/ml showed a greater antibiofilm activity towards S. mutans (81.65 ± 2.96%) (p=0.024) than S. sanguinis (51.64 ± 7.47%) (p=0.085) compared to the negative control. While for Parsley at sub-MIC of 16 mg/ml showed higher antibiofilm activity against S. mutans (80.60 ± 1.82%) than S. sanguinis (52.06 ± 9.09%) compared to the negative control. However, Parsley at sub-MIC of 32 and 16 mg/ml exhibited a slightly higher antibiofilm activity compared to chlorhexidine against S. mutans (79.16 ± 1.92%) and S. sanguinis (45.50 ± 10.38%) but it is not significantly different for both bacteria (Figure 6). Parsley at 8 mg/ml showed the greater anti-S. mutans biofilm but no data for S. sanguinis. The results indicated that Parsley at 8 mg/ ml is effective in reducing S. mutans biofilm and it is more effective compared to chlorhexidine. Parsley at 16 mg/ml is also suitable as an anti-caries agent due to the ability to retain almost halves of S. sanguinis biofilm (48%) and a greater reduction of S. mutans biofilm (80.6%). This study confirmed the ability of Parsley at 16 mg/ml or 8 mg/ml was effective as anti-S. mutans biofilm. This is the first study reporting the antibacterial, anti-adherence and antibiofilm effects of Neem and Parsley against S. sanguinis biofilm. The above results indicate that Parsley at 16 mg/ml is as effective as Neem and chlorhexidine in reducing S. mutans biofilm and retaining S. sanguinis biofilm. Further study was needed to be carried out on the anti-adherence and antibiofilm activities of Parsley at 8 mg/ml against S. sanguinis.
Conclusion
A. indica and P. crispum demonstrated antibacterial, antiadherence and antibiofilm effects against S. sanguinis and S. mutans, thus effective as anti-plaque and anticaries agents. Neem at 8 mg/ml and Parsley at 16 or 8 mg/ml are suitable candidates as anti-plaque and anticaries agents and show potential as oral healthcare products in the form of mouthrinses in the prevention of dental plaque and dental caries. However, further studies need to be conducted to confirm the effect of P. crispum against S. sanguinis.
References
- http://www.mah.se/CAPP/ Country-Oral-Health-Profiles
- Pramesti HT. Streptococcus sanguinis as an opportunistic bacteria in human oral cavity: Adherence, colonization, and invasion. Padjadjaran J Dent 2016; 28.
- Patidar D, Sogi S, Singh V, et al. Salivary levels of Streptococcus mutans and Streptococcus sanguinis in early childhood caries: An in vivo study. J Indian Soc Pedod Prev Dent 2018; 36:386.
- Ge Y, Caufield PW, Fisch GS, et al. Streptococcus mutans and Streptococcus sanguinis colonization correlated with caries experience in children. Caries Res 2008; 42:444-448.
- Tartaglia GM, Kumar S, Fornari CD, et al. Mouthwashes in the 21st century: A narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin Drug Deliv 2017; 14:973-982.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med 2016; 2016.
- Chaves DS, Frattani FS, Assafim M, et al. Phenolic chemical composition of Petroselinum crispum extract and its effect on haemostasis. Natural Product Communications 2011; 6:1934578X1100600709.
- Christy S, Nivedhitha MS. Antimicrobial efficacy of Azadirachta indica against Streptococcus mutans-An in vitro study. Asian J Pharm Technol 2019; 9:149-153.
- Lekshmi NC, Sowmia N, Viveka S, et al. The inhibiting effect of Azadirachta indica against dental pathogens. Asian J Plant Sci Res 2012; 2:6-10.
- Wong PY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem 2006; 97:505-515.
- Tang EL, Rajarajeswaran J, Fung S, et al. Petroselinum crispum has antioxidant properties, protects against DNA damage and inhibits proliferation and migration of cancer cells. J Sci Food Agric 2015; 95:2763-2771.
- Azab AE, Abushofa FA, Abdul Rahman HM. Nephroprotective effect of aqueous extract of parsley against nephrotoxicity induced by carbon tetrachloride in the male rats. J Biotechnol Bioeng 2019; 3:16-26.
- World Health Organization. Continuous improvement of oral health in the 21st century-the approach of the WHO Global Oral Health Program. The world oral health report. 2003.
- Petrolini FV, Lucarini R, Souza MG, et al. Evaluation of the antibacterial potential of Petroselinum crispum and Rosmarinus officinalis against bacteria that cause urinary tract infections. Brazilian J Microbiol 2013; 44:829-834.
- Fejes S, Kery A, Blazovics A, et al. Investigation of the in vitro antioxidant effect of Petroselinum crispum (Mill.) Nym. ex AW Hill. Acta Pharm Hungarica 1998; 68:150-156.
- Vora SR, Patil RB, Pillai MM. Protective effects of Petroselinum crispum (Mill) Nyman ex AW Hill leaf extract on D-galactose-induced oxidative stress in mouse brain. Indian J Exp Biol 2009; 47:338-342.
- Hempel J, Pforte H, Raab B, et al. Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Food 1999; 43:201-204.
- Nielsen SE, Young JF, Daneshvar B, et al. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr 1999; 81:447-455.
- Zheng GQ, Kenney PM, Zhang J, et al. Inhibition of benzo [a] pyrene-induced tumorigenesis by myristicin, a volatile aroma constituent of parsley leaf oil. Carcinogenesis 1992; 13:1921-1923.
- Shafiei Z, Rahim ZH, Philip K, et al. Antibacterial and anti-adherence effects of a plant extract mixture (PEM) and its individual constituent extracts (Psidium sp., Mangifera sp., and Mentha sp.) on single-and dual-species biofilms. Peer J 2016; 4:e2519.
- Wikler MA. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. Clsi (Nccls) 2006; 26:M7-A7.
- WI WN, Fathilah AR, Rahim ZH. Plant extracts of Psidium guajava, Mangifera and Mentha sp. inhibit the growth of the population of single-species oral biofilm. Altern Integr Med 2013; 2:1000102.
- Kwasny SM, Opperman TJ. Static biofilm cultures of Gram‐positive pathogens grown in a microtiter format used for anti‐biofilm drug discovery. Curr Protoc Pharmacol 2010; 50:13A-8.
- Ghapanchi J, Bazargani A, Zariean A, et al. Evaluation of the anti-Streptococcus mutans potential of Petroselinum crispum, an in vitro study. Eur J Med Plants 2016; 15:1-8.
- Mistry KS, Sanghvi Z, Parmar G, et al. Antibacterial efficacy of Azadirachta indica, Mimusops elengi and 2% CHX on multispecies dentinal biofilm. J Conserv Dent 2015; 18:461.
- Subramaniam SK, Siswomihardjo W, Sunarintyas S. The effect of different concentrations of Neem (Azadiractha indica) leaves extract on the inhibition of Streptococcus mutans (In vitro). Dent J 2005; 38:176-179.
- Al-Jadidi HS, Hossain MA. Studies on total phenolics, total flavonoids and antimicrobial activity from the leaves crude extracts of neem traditionally used for the treatment of cough and nausea. Beni-Suef University J Basic Applied Sci 2015; 4:93-98.
- Azizah AH, Wee KC, Azizah O, et al. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschato). Int Food Res J 2009; 16:45-51.
- Sharma V, Walia S, Kumar J, et al. An efficient method for the purification and characterization of nematicidal azadirachtins A, B, and H, using MPLC and ESIMS. J Agric Food Chem 2003; 51:3966-3972.
- Ujváry I. Chapter 3: Pest control agents from natural products. In: Krieger R. Hayes' Handbook of Pesticide Toxicology 3rd Ed.New York: Academic Press.
- Gadi D, Bnouham M, Aziz M, et al. Flavonoids purified from parsley inhibit human blood platelet aggregation and adhesion to collagen under flow. J Complement Integr Med 2012; 9.
- Awe EO, Banjoko SO. Biochemical and haematological assessment of toxic effects of the leaf ethanol extract of Petroselinum crispum (Mill) Nyman ex AW Hill (Parsley) in rats. BMC Complement Altern Med 2013; 13:1-6.
- Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev 2019; 18:241-272.
- Zhu B, Macleod LC, Kitten T, et al. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol 2018; 13:915-932.
- Pai MR, Acharya LD, Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel: A 6-week clinical study. J Ethnopharmacol 2004; 90:99-103.
- Harjai K, Bala A, Gupta RK, et al. Leaf extract of Azadirachta indica (neem): A potential antibiofilm agent for Pseudomonas aeruginosa. Pathog Dis 2013; 69:62-65.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Zaleha Shafiei1, Nur Insyirah Mohamad Nor2, Khairunnisa Hanifah2, Kristina Soosay Selvam2 and Alida Mahyuddin3*
1Department of Craniofacial Diagnostics and Biosciences, Faculty of Dentistry, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300, Kuala Lumpur, Malaysia2Oral Health Division, Ministry of Health, Malaysia
3Department of Family Oral Health, Faculty of Dentistry, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300, Kuala Lumpur, Malaysia
Citation: Zaleha Shafiei, Nur Insyirah Mohamad Nor, Khairunnisa Hanifah, Kristina Soosay Selvam, Alida Mahyuddin, Antibacterial, Anti-Adherence and Antibiofilm Effects of Petroselinum crispum and Azadirachta indica against Streptococcus sanguinis and Streptococcus mutans, J Res Med Dent Sci, 2022, 10 (11): 72-82.
Received: 11-Oct-2022, Manuscript No. JRMDS-22-76883; , Pre QC No. JRMDS-22-76883(PQ); Editor assigned: 13-Oct-2022, Pre QC No. JRMDS-22-76883(PQ); Reviewed: 27-Oct-2022, QC No. JRMDS-22-76883(Q); Revised: 31-Oct-2022, Manuscript No. JRMDS-22-76883(R); Published: 07-Nov-2022
