Research Article - (2021) Volume 9, Issue 12
Are new bioactive materials less harmful to pulp? Real-Time Cytotoxicity Assessment in Vitro
*Correspondence: Turkay Kaykolus, Department of Dental Biomaterials, School of Dentistry/Research Center, Tehran, Iran, Email:
Abstract
Introduction: The aim of this study was to evaluate the cytotoxicity of contemporary restorative bioactive materials and glass ionomers.
Materials and methods: Fuji IX GP Capsule (GC), EQUIA Forte (GC), Biodentine (Septodont), Glass Fill (GCP Dental) and Activa BioActive Restorative (Pulpdent) material specimen’s regional toxicity determined by XTT assay and real-time cell analysis method. For statistical one-way ANOVA, post hoc Tukey's HSD, hierarchical clustering and distance correlation methods used.
Results: Activa BioActive Restorative showed the least cytotoxicity in both experiments. In XTT Glass Fill, in RTCA Fuji IX GP Capsule was found to be the most cytotoxic material. Also, undiluted concentrations of all materials were found to have cytotoxic effects on L929 cells.
Conclusion: Within the limitations of this study, we can say that restorative bioactive materials are less cytotoxic than glass ionomers.
Keywords
A Cytotoxicity, Glass ionomer cements, Bioactive materials, XTT assay, RTCAIntroduction
Dental caries, otherwise known as tooth decay, is one of the most common chronic diseases in humans worldwide and individuals are susceptible to this disease throughout their lifetime [1]. There are many materials used in the treatment of decayed teeth. However, these materials help to restore the health of the tooth, they also have the potential to produce undesirable effects on body tissues. According to primum non nocere, one of the main principles of medicine, the materials used in the treatment should not harm the body or at least the benefits should be greater than the harm. In addition, there is a growing interest in the public to whether the materials used to treat diseases of the teeth and related tissues are dangerous to the patient, the environment, the dentist or the assistant. The debates in the media, especially about dental amalgam, have contributed to a significant increase in social interest in this issue. Patients have become more likely to question whether a material is harmful to them. Biocompatibility refers to the ability of a biomaterial to perform its desired function with respect to a medical therapy, without eliciting any undesirable local or systemic effects in the recipient or beneficiary of that therapy, but generating the most appropriate beneficial cellular or tissue response in that specific situation, and optimising the clinically relevant performance of that therapy [2]. Materials used in dentistry are classified as biomaterials and the biomaterials can be scattered to the environment by corrosion and dissolution, and these components may have toxic effects on the human body. Since the components released from dental materials are very low and their LD50 values are relatively high, dental materials are not expected to produce systemic acute toxic effects but, regional interactions in developed organisms differ from systemic toxicity; substances released from dental materials may interact with pulp, gums, alveolar bone and oral mucosa locally. As a result of these interactions, cell metabolism may change and release inflammatory mediators, or apoptosis (controlled cell death) or necrosis may occur if the cell is damaged [3]. Due to all these possible negative effects, biocompatibility of restorative materials as well as all biomaterials used in the human body is very important.
Bioactivity refers to a unique property of a material that elicits a cellular response, such as the formation of hydroxyapatite. As compared to inert materials, bioactive materials are capable to produce growth factors and encourage natural mineralization. Bioactive materials can be considered as boon to dentistry; calcium hydroxide, mineral trioxide aggregate (MTA), calcium enriched mixture (CEM), MTYA1-Ca filler, tetracalcium phosphate (TTCP), sol-gel-derived bioactive glass (BAG) ceramic containing silver ions (Ag-BG), calcium phosphate, novel endodontic cement (NEC), endo sequence root repair material, Biodentine and Activa BioActive Restorative [4].
There are many studies on the possible damages of amalgam, which has been used for a long time, and the resin composite, the most popular restorative material of today [5]. Glass ionomers are considered to be more biologically acceptable than these materials. Also, they develop an interfacial ion-exchange layer with the tooth and show a degree of bioactivity when set. Therefore, glass ionomers are considered as a suitable group for cytotoxicity comparison with bioactive materials.
The aim of this study was to evaluate the cytotoxicity of contemporary restorative bioactive materials and compare them with glass ionomers. Regional toxicity of five different restoration materials [Fuji IX GP Capsule (GC), Activa BioActive Restorative (Pulpdent), EQUIA Forte (GC), Biodentine (Septodont) and Glass Fill (GCP)] were evaluated in vitro by XTT [2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium] assay and RTCA (Real Time Cell Analysis) method on L929 Mouse Fibroblasts. The null hypothesis in this study is that bioactive restorative materials do not differ favourably from glass ionomers in terms of cytotoxicity.
Materials and Methods
Preparation of cell cultures
L929 cells were cultured in DMEM (Dulbecco modified Eagle's medium) containing 10% FBS (heat inactivated, non-USA origin, sterile-filtered, Sigma Aldrich) and 1% penicillin/streptomycin (Biochrom) at 37oC with humid air containing 5% CO2. For the experiments, cells in exponential growth phase that reached 75-80% confluency were used.
Table 1: Restorative material and manufacturer, material class and indications (according to manufacturer's recommendations).
| Restorative Material and Manufacturer | Material Class | Indications (According to manufacturer's recommendations) |
|---|---|---|
| Fuji IX GP Capsule (GC) | High viscosity glass ionomer cement | Class I and II restorations in deciduous teeth. |
| Non-load bearing Class I and Class II restorations in permanent teeth. | ||
| Intermediate restorative and base material for heavy stress situation in | ||
| Class I and Class II cavities using sandwich laminate technique. | ||
| Class V and root surface restorations. | ||
| Core build-up | ||
| EQUIA Forte (GC) | Glass hybrid restorative | Class I restorations |
| Stress bearing Class II restorations | ||
| Non-stress bearing Class II restorations | ||
| Intermediate restorative | ||
| Class V and root surface restorations | ||
| Core build up | ||
| Glass Fill (GCP) | Glass carbomer | Permanent Class I and Class II restoration (non load-bearing areas) with heat |
| Class I and II restoration in deciduous teeth | ||
| Build-up material for crown and bridge | ||
| Cervical fillings | ||
| Class V | ||
| Biodentine (Septodont) | Bioceramic (also classified as tricalcium silicate cement) | In the crown: |
| Temporary enamel restoration | ||
| Permanent dentin restoration | ||
| Deep or large carious lesions | ||
| Deep cervical or radicular lesions | ||
| Pulp capping | ||
| Pulpotomy | ||
| In the root: | ||
| Root and furcation perforations | ||
| Internal/external resorptions | ||
| Apexification | ||
| Retrograde surgical filling | ||
| Activa BioActive Restorative (Pulpdent) | Bioactive composite | Bioactive filling material for pits, root surface cavities and Class I, II, Ill, IV and V restorations where there is no pulpal involvement. |
Preparation of test materials
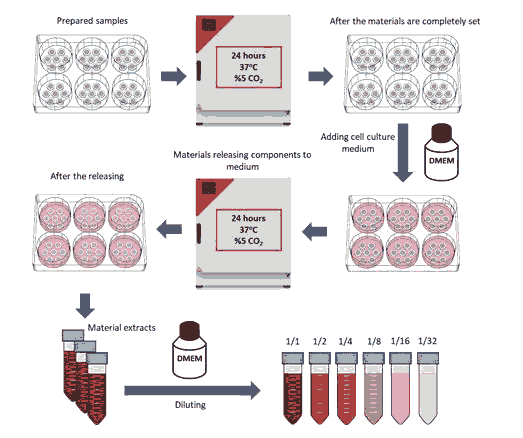
Samples of material to be tested (see table 1) were prepared in the safety cabinet (NuAire LabGard ESNU-425 Class II Type A2 Biosafety Cabinet) in the laboratory according to the instructions recommended by the manufacturers. Material samples were transferred to sterile teflon discs which on glass with a height of 2 mm and an inner diameter of 5 mm to standardize their size. The top of the teflon discs was covered with another glass to ensure a smooth surface on both sides. Samples were transferred to a 6-well plate after primary curing was achieved (see Figure 1).
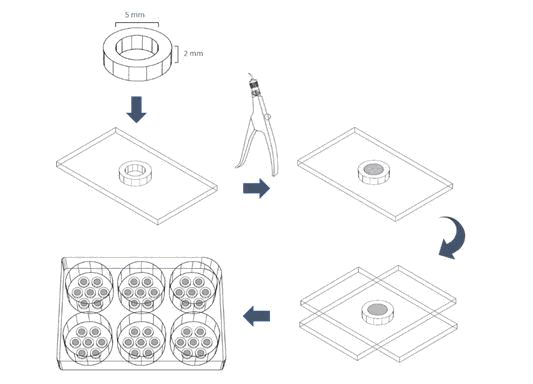
Figure 1: Schematization of the preparation of material samples.
Preparation of material extracts
To obtain material extracts, 7 samples of each group were prepared and placed in each well of a 6-well plate. The samples were incubated at 37oC for 24 hours to ensure complete curing. 3 ml of medium (DMEM) was added to the wells to ensure that the material surface area to medium volume ratio was 91.6 mm2/ml according to ISO standards. In addition to the original extract, dilutions with medium at ratio of 1/2, 1/4, 1/8, 1/16, 1/32 were prepared (see Figure 2).
Figure 2: Schematization of obtaining and diluting material extracts.
Performing of XTT test
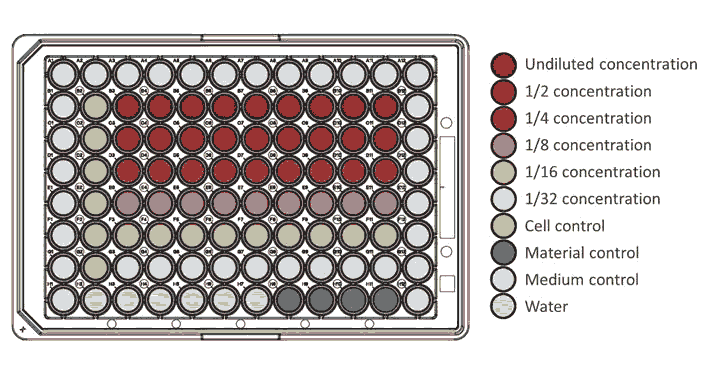
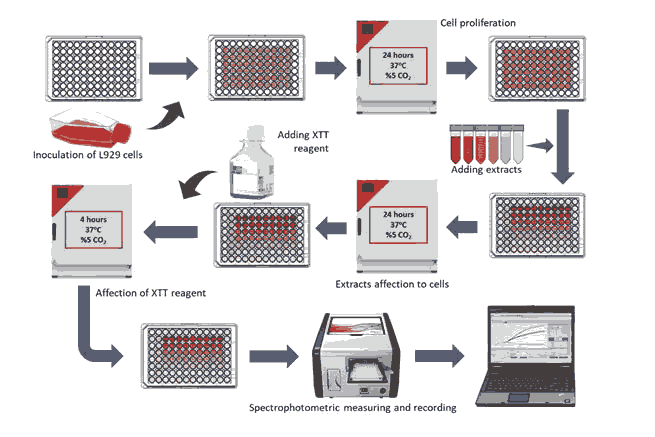
For the XTT assay, previously prepared cells were seeded at a density of 104 in a 96-well plate (Greiner Bio-One) and incubated at 37°C for 24 hours. After incubation, as shown in Figure 3, 9 wells were reserved in a 96-well plate to each concentration of each material. For the control group, 6 wells were reserved (see Figure 3).
Figure 3: Plan of 96-well plate prepared for XTT assay
100 µl of different dilutions of previously obtained material extracts were added to the separated wells. Only the medium was added to the cell control group. The 96-well plates were then incubated at 37oC for 24 hours to allow the extracts to interact with the cell cultures. After incubation, the reagent solution [Cell Proliferation Kit (XTT based), Biological Industries] was prepared according to the manufacturer's instructions, and 0.05 ml of the prepared reagent solution was added to each well. 96-well plates were incubated at 37oC in the incubator for 4 hours to allow the reagent to interact with the cell cultures. 96-well plates were measured by spectrophotometer (Epoch Microplate Spectrophotometer, BioTek Instruments) at 460 nm wavelength (see Figure 4).
Figure 4: Schematization of the XTT Assay.
The quantitative data obtained from the spectrophotometer were recorded in Microsoft Office Excel 2016 (version 16.0.4639.1000, 64 bit version). The viability percentage of the positive control group was equalled to 100%. The viability of the other groups was determined as a percentage relative to the viability of the control group. The experiment was repeated 3 times and 9x3=27 (n) observation data were obtained for each concentration of each material.
Performing real-time cell analysis
In the real-time cell analysis system, pre-warmed 50 µl of DMEM medium was added to each well of the electronic 16 well plates (E-plate 16, ACEA Biosciences) and the E-plates were kept in the safety cabinet for 30 minutes. Then, E-plates were placed in the real-time cell analysis station (xCELLigence RTCA DP, ACEA Biosciences) and background reading was done. After cell passaging and counting processes 100 µl cell suspension at 104 ml/cell density of L929 cells seeded into each wells of E-plates except medium control and material control wells. E-plates were kept in the safety cabinet for 30-60 minutes to allow the cells to adhere to the well base. The plates were then placed in the real-time cell analysis station and an impedance measurement was taken every hour. Cells adhered to the plate bases and proliferated inside the real-time cell analysis station with 5% CO2 and 95% humidification at 37°C for approximately 24 hours. Then, electronic cell culture dishes were removed from the real-time cell analysis station to add the previously prepared material extracts.
The medium in the wells was aspirated before the cells were treated with extracts. 150 µl FBS-free DMEM was added to medium control and cell control wells. 150 µl maximum dose of material concentration was added to the material control wells and a 150 µl volume of solution was added to the other wells at the determined concentrations (at ratio of 1/2, 1/4, 1/8, 1/16 and 1/32). After the process of adding material extract was completed, the E-plates were returned to the real-time cell analysis station and the device was programmed to take measurements every 15 minutes for 144 hours. The data obtained from the experiment were analysed with the RTCA 2.0 (ACEA Biosciences) software. In order to obtain more standard data between the wells, the cell index values of the wells were transformed to normalized cell index values using the RTCA 2.0 software according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (v. 25, 64-bit version). For XTT assay, homogeneity of the data was evaluated by Shapiro-Wilk test. Differences between viability percentages of experimental groups and viability percentages of control groups evaluated statistically by One-Way ANOVA and post hoc Tukey's HSD tests.
In the RTCA test, the nCI (normalized Cell Index) values of the cell control groups of all materials were averaged to compare the toxicity between the materials and the nCI values of the material extract groups were proportioned according to this average. Distance correlation method was used to sort the toxicity degree of material concentrations. For the analysis of these time series distances correlation method with Euclidean distances dissimilarity algorithm used. Hierarchical clustering method was used to evaluate the significance of the differences between toxicity levels. For hierarchical cluster analysis, between-groups linkage method used with Euclidean distance algorithm and data standardized with Z score
Results
XTT Experiment Results
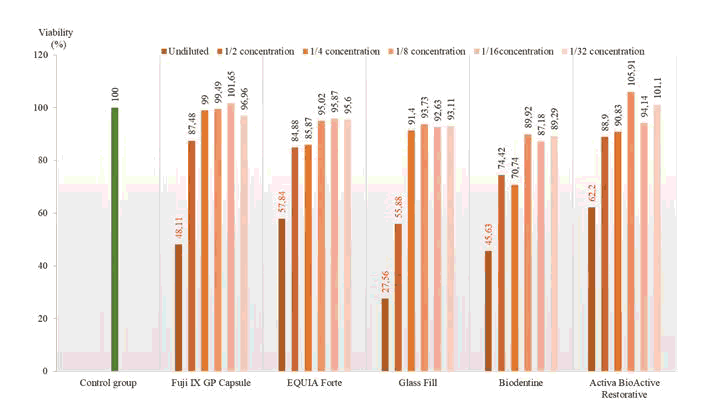
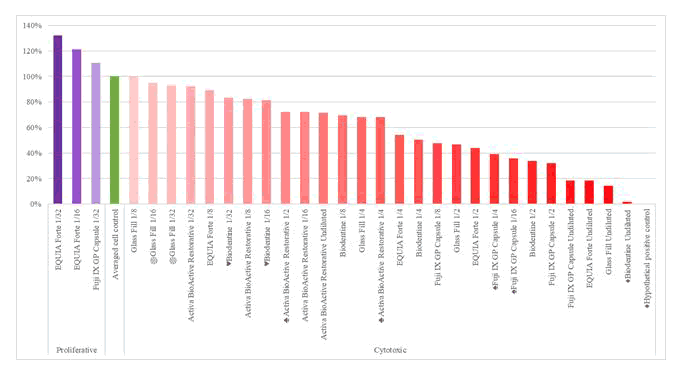
According to the results of the XTT experiment, it was observed that the material extracts affected the viability of L929 cells although they varied according to their concentration (see Figure 5). Glass Fill was found to be the most toxic alternative restoration material and reduced survival rates of L929 cells to 27,56% and extract at 1/2 concentration were statistically cytotoxic with 55.88% viability rate (p <0.05). The difference in survival rates between Glass Fill and all other materials was statistically significant (p≤0.001). Only undiluted extracts of other tested alternative restorations materials were statistically found to be cytotoxic in L929 cells (p<0.05). Fuji IX group is statistically different from Activa BioActive Restorative and EQUIA Forte groups (p≤0.001).
Figure 5: At the end of the experiment, the distribution of the mean viability percentages of L929 cells according to concentrations, red numbers indicate cytotoxic concentrations. (p <0.05).
RTCA Results
According to the results of the real-time cell analysis experiment, the material extracts were observed to affect the viability of the L929 cells although they varied according to their concentration. While most material concentrations had a toxic effect, some showed proliferative effect (see Figure 6).
distance correlation analysis. There is no statistical difference between groups with the same sign. For better understanding of the graph, distance correlation values are given as a percentage between the hypothetical positive control group (0%) and the cell control group (100%).
Figure 6: Comparison of cytotoxicity of extracts of restorative materials according to distance correlation analysis. There is no statistical difference between groups with the same sign. For better understanding of the graph, distance correlation values are given as a percentage between the hypothetical positive control group (0%) and the cell control group (100%).
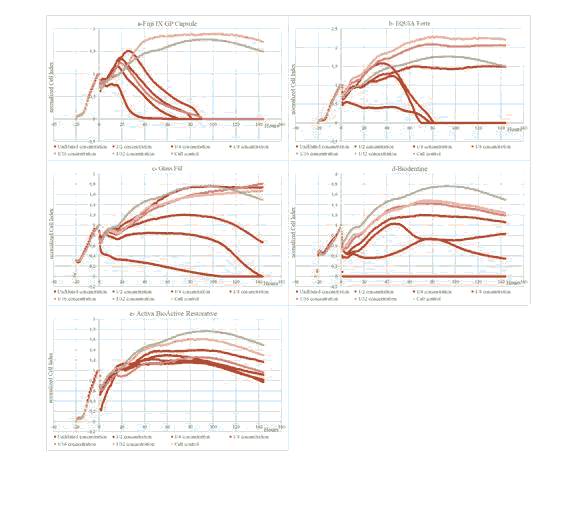
Viability completely disappeared in nearly all cell culture groups treated with Fuji IX GP Capsule’s extracts, at the end of 144th hour, only control group and cell culture group treated with extract at concentrations of 1/32 were able to survive. Also, slightly proliferative effect was also observed in cell culture group treated with extract at concentrations of 1/32 (see Figure 7a). At EQUIA Forte, Viability completely disappeared at most potent three cell culture groups treated with extracts. Close but lower nCl values were seen at 1/8 concentration compared to the control group. Also, as in Fuji IX GP Capsule, cell culture groups treated with extracts at low concentrations showed proliferative effect (see Figure 7b). At the Glass Fill’s cell culture groups treated with undiluted and diluted at concentrations of 1/2 extracts, viability completely disappeared relatively late compared to EQUIA Forte and Fuji IX GP Capsule. Although cell viability was maintained end of the 144th at 1/4 concentration, viability decreased significantly compared to the control group. At thinner concentrations cell viability compared to the control group generally remained lower but, slightly proliferative effect was observed from the 4th day of the experiment (see Figure 7c). Only cell culture group treated with Biodentine’s undiluted extract lost viability completely, at the end of 144th hour, all other cell culture groups were able to survive with lower viability. Also, unusual vitality curve patterns were observed in cell culture groups treated with Biodentine’s extracts at concentrations of 1/2 and 1/4, compared to other groups (see Figure 7d). In Activa BioActive Restorative’s, cell culture groups treated with extracts able to maintain their viability at the end of the 144th hour. Better nCl values were observed (see Figure 7e) especially at higher concentrations compared to Biodentine, which is a material used in vital therapies.
Figure 7: Change of cell viability in wells treated with contemporary alternative restorative filling material extracts according to real-time cell analysis.
Discussion
In this study, in vitro cytotoxicity of three glass ionomer based cements and two bioactive restorative materials were tested by two different methods. Our null hypothesis, bioactive restorative materials do not differ favourably from glass ionomers in terms of cytotoxicity, was rejected.
In vitro cytotoxicity tests are an essential screening step in assessing the regional toxicity of dental materials before in vivo animal or human tests. Tetrazolium reduction tests used to evaluate the viability of eukaryotic cells have been shown to be a suitable in vitro method for evaluating the cytotoxicity of dental materials[7, 8]. In XTT test, which is a different tetrazolium reduction test developed later, the process steps are reduced. Thus, faster and easier cytotoxicity tests were made possible [9]. Due to of these advantages, XTT assay was considered appropriate for examining the cytotoxic effects of the materials subject to our study. The XTT test and other cytotoxicity determination tests in general give a single measurable value for cell viability at the end of each test. In addition, relatively many processing steps are required to perform these tests, which may cause variations in the measured value. However, since cytotoxicity results can vary not only according to the test material but also the test method, many different test methods should be used and risk analyses should be carried out [10]. With the real-time cell analysis, cell viability can be read as often as desired within the specified time period, thus it is possible to monitor the viability of the cells. This provides comprehensive information during the test period. Also, in this method no labelling is required to monitor the cells, it saves resources and workload moreover allows for a more physiological measurement (see Figure 8). Due to these advantages, in our study, real-time cell analysis method was used together with XTT test to evaluate the materials in terms of cytotoxicity. By using these two test methods together, it is aimed to increase the reliability of the study. In cell culture studies, primary cells and continuous cell lines are used. Continuous cell lines are transformed primary cells with the ability to reproduce indefinitely and have a more stable phenotype. Continuous cell lines frequently used in studies are mouse fibroblasts (L929, 3T3) and human epithelial cells (HeLa) [11]. In our study, L929 mouse fibroblasts were selected for use in cell cultures, because L929 mouse fibroblast cells reacted similarly to human fibroblast cells against components released from dental materials
Figure 8: Schematic cross-sectional view of wells in the E-plates used in the real-time cell analysis system, cell presence changes the impedance between the electrodes.
In our study, only the undiluted concentrations of Fuji IX GP Capsule’s extract, which is a high viscosity glass ionomer, were found to be cytotoxic according to the XTT test results. When real-time cell analysis results are analysed, it is seen that only cell culture group treated with extract at concentrations of 1/2 able to survive at the end of the 144th hour. It was investigated how this serious difference between the XTT experiment of the Fuji IX GP Capsule and the real-time cell analysis method occurred. In RTCA, cell viability of the cell culture groups treated with Fuji IX GP Capsule extracts generally decreased rapidly after the first and second days. In XTT test, cell cultures were evaluated for cytotoxicity one day after being treated with material extracts. But due to the advantages of real-time cell analysis, the duration of the experiment has been increased to six days. Thus, the amount of data obtained for each concentration of each material was increased considerably, and how the material extracts changed cell viability in cultures over time could be observed in more detail.
We can call EQUIA Forte an improved high viscosity glass ionomer cement, also this restorative material is qualified as glass hybrid restorative by its manufacturer. It is suggested that with the addition of a very thin and highly reactive glass as a filler, a stronger matrix is formed in the cement, resulting in improved physical properties [3]. According to the results of the XTT experiment, it is seen that only the undiluted concentration of EQUIA Forte extract is cytotoxic. When the real-time cell analysis results of EQUIA Forte are examined, it is seen that the viability completely lost at the end of the 144th hour in the cell culture groups treated with undiluted, 1/2 and 1/4 concentrations of extracts. We can say that EQUIA Forte is less cytotoxic than Fuji IX GP Capsule, which can be considered its predecessor. EQUIA Forte was launched in 2015, and there is a fairly limited biocompatibility study that we can compare our findings on this material. In one of these studies, Cosgun et al. cannot find any difference in terms of cytotoxicity between Fuji IX GP Capsule and EQUIA Forte in their studies conducted with MTT assay on Vero cells and described these two materials as slightly toxic [4]. In another study, Collado-González et al. Compared EQUIA Forte and Ionostar Molar in terms of cytotoxicity with the MTT assay on hDPSCs culture and found EQUIA Forte more successful [5]. In addition, it is seen that in the groups treated with extract at the concentration of 1/16 and 1/32, the cells gave a higher normalized cell index value at the end of the 144th hour as proliferated more than the control group. Similar to what we observed in our study, Ersahan et al. Also mentioned the proliferative effect of some glass ionomer cements, including EQUIA Forte, on L929 cells.
Biodentine is a bioactive material that can be used in vital operations such as pulp lining, root perforation and internal resorption repair. Although it cannot be used as a direct restorative in general, since its resistance to abrasion is weak, it can sometimes be used as a direct restorative in cervical areas. Like other restorative materials (except Glass Fill) we tested in the XTT assay, only cell culture group treated with undiluted concentration of Biodentine extract showed cytotoxic effect. At the end of the RTCA test, vitality disappeared completely only in the "cell culture group treated with extract in undiluted concentration". As a result of these findings, we can say that Biodentine is the least cytotoxic material after Activa BioActive Restorative, which is another bioactive material. In studies related to Biodentine in the literature, MTA is generally used for comparison purposes. In one of the limited studies comparing Biodentine with the materials we use, Zhou and his colleagues examined the effects of Biodentine, MTA and Fuji IX GP on flow cytometry and human gingival fibroblasts, and observed that Biodentine and MTA produced similar responses in cells. They also found that these two materials were more biocompatible than the Fuji IX GP in the test conditions they applied. In a study, Michel et al., investigate the cytotoxicity of different dental materials on (HGF) human gingival fibroblast and hFOB (human fetal osteoblasts) cultures with the MTT assay, they found that Fuji II LC and Glass Fill are more cytotoxic than Biodentine. In addition, while Biodentine showed a similar cytotoxic effect to human gingival fibroblasts than other tested calcium silicate-based cements (ProRoot MTA, Harvard MTA, Endo Sequence putty), showed a more cytotoxic effect against hFOB 1.19 cells than other tested calcium silicate-based cements.
Glass Fill is a glass carbomer-based material. Glass carbomers are separated from glass ionomers by nano-sized powder particles, including fluorapatite and hydroxyapatite fillers. It is also recommended to use glass carbomers with a light device that can generate sufficient heat for clinical use . Glass Fill's undiluted and 1/2 concentration of extracts were found to be cytotoxic, unlike other test materials, according to the XTT assay results. In results of real-time cell analysis, at the end of 144th hour, cell viability in the cell culture groups treated with undiluted and 1/2 concentration of extracts disappeared completely. According to these findings, we can describe Glass Fill as a moderate cytotoxic restorative material compared to other materials we have tested. Since Glass Fill is a relatively new restorative material, there is very limited biocompatibility study of this material. In the study of Michel et al., they found Glass Fill more cytotoxic than Biodentine and Fuji II LC, similar to our study, with MTS assay on HGF and hFOB cells. In addition, Ülker et al. compared the self-adhesive materials in terms of cytotoxicity with MTT test on bovine pulp cells, but did not find a statistically significant difference between Glass Fill and Fuji II LC. At lower concentration slightly proliferative effect was observed from the 4th day of the RTCA experiment. We have seen similar effects in Fuji IX GP Capsule, a high-viscosity glass ionomer, and EQUIA, a glass hybrid. Therefore, we can say that glass ionomer or glass ionomer-like materials have a proliferative effect on L929 cells at low concentrations.
The most salient result we have observed is that Activa BioActive Restorative, which is qualified as a bioactive composite, gave less cytotoxic results than other restorative materials we tested. According to the XTT experiment, although only undiluted extract, like as Fuji IX GP Capsule, Glass Fill, EQUIA Forte and Biodentine, are found to be cytotoxic. Although in RTCA, this bioactive composite was the only material that could maintain the viability of the all cell culture groups at the end of the 144th hour. Activa BioActive Restorative was launched in 2013 and there are very few studies that we can compare our results about this material. Activa BioActive Restorative was found to be less cytotoxic than other materials tested in our study, even Biodentine, which is indicated for vital pulp therapies. Similarly, ElRash et al. found that Activa BioActive Restorative's biocompatibility was better than other materials in their studies where they implanted Activa BioActive Restorative, MTA-HP and iRoot BP Plus Root Repair Material into mouse subcutaneous tissues and evaluate implantation sites for up to one month [1].
Activa BioActive Restorative describes it as a “bioactive composite” and suggests that it releases calcium, phosphate and fluoride and can recharge it. In addition, although it is in the class of composites, it does not contain bisphenol A, Bis-GMA and Bis-GMA derivatives. Activa BioActive Restorative elicits a response that stimulates mineral apatite formation and remineralization, which is the defining requirement of bioactive materials. This process is claimed to knits the restoration and the tooth together, penetrates and fills micro-gaps, reduces sensitivity, guards against secondary caries, and seals margins against microleakage and failure by the manufacturer [2]. It is stated that Activa BioActive Restorative is chemically bonded to the tooth when it is first released by the manufacturer and can be used without any adhesives when retention is not required. However, in the last instruction, it is suggested to be used with a suitable adhesive agent [4].
Although the cytotoxicity of this new bioactive material is better than Biodentine and MTA, which are the materials used in vital pulp therapies, it may not be suitable to be used directly on open pulp. It should be remembered that calcium hydroxide, which has been accepted as the gold standard in pulp lining until recently, gives more cytotoxic results in cytotoxicity tests than many restorative materials. For direct pulp lining it is not enough to have good biocompatibility, the material should also induce dentin formation [2]. Activa BioActive Restorative is contraindicated in direct pulp capping according to manufacturer's instructions. More research is needed on the biocompatibility of this material
Bioactivity appears to be an increasingly popular phenomenon in restorative dentistry. Briefly, Bioactive materials are compounds that have the ability to bind to living tissues [3]. Calcium hydroxide is a bioactive material used in pulp lining for a long time in restorative dentistry. Later, with the introduction MTA and Biodentine, which are calcium silicate cements, the use of bioactive materials has expanded in restorative dentistry. With the development of bioactive resins, bioactive materials have also started to be used as direct filling materials as a new concept in restorative dentistry. It can be predicted that new bioactive materials can become more popular in restoring dental tissues.
Considering the limits of an in vitro study, it should be kept in mind that the results obtained from these studies may not be applicable to in vivo conditions. Also, considering that a biomaterial can remain in the human body for life, it can be concluded that the relevant materials are tested for a very limited time in laboratory studies. Given the highly variable conditions of the oral environment, biomaterials can corrode over time and harm the person. However, a material may not be biocompatible for all applications (e.g. a metal suitable for a full cast crown may not be suitable for the implant.) In addition to these, besides regional toxicity, biomaterials may have systemic toxicity, nontoxicity, allergy or tautological effects. It is therefore not meaningful to say whether a biomaterial is biocompatible, without extensive testing, without specifying for what purpose and where it is used
Conclusion
• At undiluted concentration all materials have cytotoxic effects on L929 fibroblast cells.
• If we need to sort the toxicity of test materials to L929 cells in the XTT experiment: Activa BioActive Restorative <Biodentine <EQUIA Forte <Fuji IX GP Capsule <Glass Fill
• If we need to sort the toxicity of test materials to L929 cells in the RTCA experiment: Activa BioActive Restorative <Biodentine<Glass Fill <EQUIA Forte <Fuji IX GP Capsule
• Real-time cell analysis method in evaluating the cytotoxicity of dental materials has higher potential to provide more useful information than XTT method.
• Glass ionomers or glass ionomer-like materials may have a proliferative effect on L929 cells at low concentrations.
References
- Selwitz, Robert H, Ismail Amid I, and Pitts Nigel B. "Dental caries." The Lancet 369, (2007): 51-59.
- Williams, David F. "On the mechanisms of biocompatibility."Biomaterials29, (2008): 2941-2953.
- Sonarkar, Snehal, and Rucheet Purba. "Bioactive materials in conservative dentistry."Int J Contemp Dent Med Rev2015, (2015): 1-4.
- Bates, Michael N, Fawcett Jackie, Garrett Nick, and Cutress Terry, et al. "Health effects of dental amalgam exposure: a retrospective cohort study."Int J Epidemiol 33, (2004): 894-902.
- Sidhu, Sharanbir K, and Nicholson John W. "A review of glass-ionomer cements for clinical dentistry."J Funct Biomater7, no. 3 (2016): 16
Author Info
Department of Dental Biomaterials, School of Dentistry/Research Center, Tehran, IranCitation: Turkay Kaykolus Are new bioactive materials less harmful to pulp? Real-Time Cytotoxicity Assessment in Vitro , J Res Med Dent Sci, 2021, 9(11): 1-8
Received: 01-Dec-2021 Accepted: 15-Dec-2021 Published: 22-Dec-2021