Research - (2021) Volume 9, Issue 4
Areca Nut (Areca catechu Linn.) Extract Induces Cell Cycle arrest and Reduces Ki-67 Activity in Oral Squamous Cell Carcinoma Cells
Liza Meutia Sari1, Gus Permana Subita2 and Elza Ibrahim Auerkari3*
*Correspondence: Elza Ibrahim Auerkari, Department of Oral Biology, Faculty of Dentistry, University of Indonesia, Indonesia, Email:
Abstract
Oral Squamous Cell Carcinoma (OSCC) is the most common form of oral cancer and the focus on finding chemotherapeutic agents have recently shifted to natural products. Areca nut is a medicinal plant with various biological activities. However, not much data is available on the anti-cancer effect of areca nut extract. Due to the current interest in the potential activity of antioxidant in areca nut extract, we investigated its ability to induce cell cycle arrest and reduce Ki-67 activity in oral cancer cell lines: HSC-2 and HSC-3. The flow cytometry was conducted for the quantification of the cells that underwent cell cycle arrest and had the expression of Ki-67 protein. All determinations were analyzed using the unpaired t-test through SPSS with p<0.01 considered as significant. The areca nut extract induced a significant increase of the cell population in subG1 phase of HSC-2 cells after 24 and 48 hours. The study also found that in HSC-3, there was significant increase in G1 phase after 24 hours treatment, but after 48 hours the areca nut extract couldn’t induce cell cycle arrest. There was significant reduction in Ki-67 expression after 24 hours treatment of areca nut extract in HSC-2 and HSC-3 cells. The increasing of subG1 population showed that apoptosis might be one of the cell death mechanisms by areca nut extract in HSC-2 cells. The cell cycle arrest and the reduction of Ki-67 activities occurred in both cells after 24 hours. These findings suggest that areca nut extract could inhibit the proliferation of OSCC cell lines.
Keywords
Areca nut extract, Oral cancer, Cell cycle arrest, Ki-67
Abbreviations
BCL-2, B-cell lymphoma 2; BD: Becton Dickinson; DMEM: Dulbecco’s Modified Eagle’s Medium; DMSO: Dimethyl sulfoxide; DNA: Deoxyribose Nucleic Acid; EDTA:EthylenediamineTetraacetic Acid; FBS: Fetal Bovine Serum; HSC: Human Squamous Carcinoma; IC50: Inhibition Concentration 50; PBS: Phosphate Buffered Saline; PI:Propidium Iodide; RNA:Ribo Nucleic Acid; WHO: World Health Organization.
Introduction
The most common malignant epithelial neoplasm in the oral cavity is the Oral Squamous Cell Carcinoma (OSCC), representing over 90% of malignancies of the oral cavity [1]. Oral squamous cell carcinoma shows a poor prognosis; the 5-year survival rate is only 35%-50% despite recent advances in radiation therapy, improvement of surgical techniques, and the advent of aggressive chemotherapy protocols [2]. Radiotherapy, alone or associated with surgery or chemotherapy, has produced a significant increase in cure rates for many malignancies of the head and neck region [3]. However, high doses of radiation in large areas, including the oral mucosa, skin, maxilla, mandible and salivary glands may result in several undesired reactions that manifest during or after the completion of therapy [4]. In this regard, the major concern has been focused on identifying new chemotherapeutic agents with fewer side effects, and increasing attention has been paid to naturally acquired compounds for new candidates of a chemotherapeutic agent.
Among naturally acquired agents explored and tested, areca nut (Areca catechu Linn., Palmaceae) has long been used empirically as folk medicine in Indonesia, Malaysia, Thailand, Taiwan, and India [5]. It is usually used in betel chewing common among the Indians and Malays as the breath freshener, digestive aid, worm expellant, aphrodisiac, and to maintain stamina [5]. The activities of areca nut effects include antioxidant and anthelmintic [5-13], antidiabetic [14], antidepressant [7], antifungal [11], antibacterial [15], antimicrobial [16], antimalarial [17], antiinflammatory [9], insecticide, psychoactive, hepatoprotective [18], and larvicidal [19], antiaging and cosmetic [20], hypolipidaemic [21] and hypoglycemic [22].Several previous studies found that areca nut extract contained high amount of carbohydrate, fats, fibres, polyphenol, alkaloids, and minerals [8, 23]. The most abundant phytochemical contents present in the areca nuts are polyphenols, such as flavonoid (catechin), phenolic, tannin (hydrolysable tannin and condense tannin), and alkaloid (arecoline, arecaine, guvacine) [6]. The polyphenol compounds determine the level of antioxidant activity in the areca nut [10].
A few studies that have been performed to evaluate the effect of areca nut on OSCC showed that areca nut has been implicated in the high occurrence of oral malignance in the south east Asia [24,25]. In contrast, we recently found that areca nut extract could inhibit growth of cancer cells, without affecting normal cells [26]. The areca nut extract also showed no oral and dermal acute toxicity in the mouse model [27,28]. Another study showed that chloroform fraction of areca nut on human breast adenocarcinoma cell line (MCF-7 cells) could decrease BCL-2 expression and induce apoptosis. In addition to affecting the apoptosis mechanism, the polyphenol compound in plants also acts as an inhibitor of cancer cell proliferation [29].
The fundamental task of the cell cycle is to ensure that DNA is faithfully replicated once during S phase and that identical chromosomal copies are distributed equally to two daughter cells during M phase [30]. The cell cycle is the series of events in which cellular components are doubled, and then accurately segregated into daughter cells. In eukaryotes, DNA replication is confined to a discrete synthesis or S-phase, and chromosome segregation occurs at Mitosis or M-phase. Two Gap phases separate S phase and mitosis, known as G1 and G2. These are not periods of inactivity, but rather periods where cells obtain mass, integrate growth signals, organize a replicated genome, and prepare for chromosome segregation [31]. Cell-cycle dysregulation is a hallmark of tumor cells. The ability of normal cells to undergo cell-cycle arrest after damage to DNA is crucial for the maintenance of genomic integrity. Checkpoints emerged as a series of cell cycle dependencies [31]. Cell cycle checkpoints are surveillance mechanisms that monitor the order, integrity, and fidelity of the major events of the cell cycle. These include growth to the appropriate cell size, the replication and integrity of the chromosomes, and their accurate segregation at mitosis [31]. Internucleosomal DNA fragmentation is one of the hallmarks of apoptosis. Because the low molecular weight DNA fragments are extracted during cell staining in aqueous solutions, apoptotic cells can be identified on DNA content frequency histograms as cells with fractional ("sub-G1") DNA content [32]. Defective checkpoint function results in genetic modifications that contribute to tumorigenesis [33].
Proliferation is a fundamental biological process in the development and continuity of tissue homeostasis. Uncontrolled cell proliferation is one of the biological mechanisms associated with carcinogenesis. One of the most important protein which acts as a proliferation marker is Ki-67. The Ki-67 protein is a non-histone nuclear protein expressed by the cell in G1, S, G2 and M phases but is not observed in cells which are not dividing (G0) [34]. This protein is also used to calculate the growth fraction of normal tissue and malignant tumors, making Ki-67 a prognostic marker of OSCC [34]. The Ki-67 cell proliferation marker is expressed by OSCC cells, particularly in well-differentiated OSCC [35]. Ki-67 plays a significant role as an indicator of proliferation in the carcinogenesis of OSCC [35]. Studies which explained the association between areca nut extract and the ability to inhibit cancer cell’s functions have not been widely conducted. However, in the present study we report the effect of areca nut extract on the proliferation of OSCC cells by assesing the cell cycle arrest and analysis of Ki-67 activity in HSC-2 and HSC-3 cells after 24 and 48 hours treatment.
Materials and Methods
Sample preparation
The study materials were obtained from areca nuts of pinang plant from Aceh Besar, Indonesia, which was determined and documented by the Botanical Division of Biological Research Center LIPI Cibinong, complete with its roots, stems, leaves, flowers, and seeds in 2018.
Extraction
The sample used was two kilograms of areca nut (gross weight). Areca nut was collected and cleansed from dirt (wet sortation), then washed with running water until clean and drained. Those seeds were dried in open air and covered from direct sunlight then continued with drying using an oven at 50°C. Dried simplicia (unprocessed natural ingredient) was crushed using a blender producing a powdered simplicia and sifted with 20 mesh sieves. The powder was macerated with 96% ethanol solvent. Around 500 grams powdered simplicia was put into a container, then 1 L of 96% ethanol was added, closed, and left for three days covered from sunlight, while repeatedly stirred. After three days the extract was strained, and the remaining extract then was dried. The dried extract was added to 500 mL of 96% ethanol and stirred, after acquiring all extract. The container was closed, left in a cool place and covered from sunlight for two days. The sediment was separated and liquid extract was obtained. Then the extract was evaporated using rotary evaporator at 30–40°C then concentrated again using water bath so a dense dried extract of areca nut would be obtained. The extract was stored in -20ºC until further use. To prepare two different concentrations (IC50 areca nut extract on HSC-2 and HSC-3 cells were 629.50 μg/mLand 164.06 μg/mL respectively), 10 mg of the powder was first dissolved in 150 μl of DMSO (276855, Sigma-Aldrich) and diluted with complete culture medium to reach the desired dilution.
Cell culture
The HSC-2 and HSC-3 cell lines were cultured in complete DMEM (D6429, Sigma-Aldrich) containing 10% FBS, nonessential amino acids, pyruvate, glutamine, and vitamins at 37°C with 5% CO2/95% air in ahumified CO2 incubator. All media were also supplemented with 100 units/ mL of penicillin and 100 mg/mL of streptomycin (15070063, Thermo Fisher Scientific). The HSC- 3 and HSC-2 cell lines used in this study were provided by the Oral Biological Laboratory, Faculty of Dentistry of the University of Indonesia. The HSC-2 and HSC-3 cell lines used in this study were given by the Section of Molecular Embriology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University. The HSC-3 cell line was derived from an OSCC of the tongue with a p53 gene mutation, namely a 4bp insertion or change in the amino acid in the form of TAAG insertion in codon 305– 306, exon 8 (JCRB0623) [36]. The HSC-2 cell line was also derived from an oral squamous cell carcinoma of the tongue with p53 intron 6 splice mutation (JCRB0622) [37]. Cell lines, placed in cryophilic liquid N2, were then moved into a 15 mL tube, then PBS (10010031, ThermoFisher Scientific) was added up to 10 mL. The thawing process started with centrifuging by using Laboratory benchtop centrifuge Liston C 2201 for 10 min at 300 × g at room temperature, the supernatant was disposed, the cell concentrate at the base of the tube (pellet) was added to 2–3 mL complete DMEM medium, and then it was pipetted to culture a plate containing 7–10 mL DMEM medium and was spread evenly. It was incubated at 37°C with a 5% CO2/95% air in a humified CO2 incubator. Media was changed by removing old medium from the culture plate by pipetting, rinsing with PBS two to three times, pouring new complete DMEM medium (around 7 – 10 mL) and then placing back into the incubator. If the cells achieved 80% confluence, then the confluence was ready to be harvested. The medium was disposed and rinsed with PBS Ca2+ and Mg2+ two to three times with the volume of 2 mL, then 1 mL Trypsin EDTA (59418C, Sigma- Aldrich) was added, then it was incubated for five to ten minutes. After the addition of complete DMEM (2 – 3 ml) and transferred into a 15 mL tube by pipetting, and centrifuging at 500 rpm for 10 minutes, the supernatant was discarded. The pellet was homogenized by pipetting, and the resuspended cells with the culture medium were ready to be used for experiment and cell counting with a hemocytometer. The flowcytometry was conducted in Clinical Pathology LaboratoriumCiptoMangunkusumo Hospital. We had performed the cell viability assay previously to evaluate the percentage cytotoxicity and IC50 of areca nut extract after treating the HSC-2 cells for 72 hours is 629.50 μg/mL while in HSC-3 cells is 164.06 μg/mL [26]. The protocol was approved by the Ethics Committee of the Faculty of Medicine, University of Indonesia no. 501/H2.F1/Etik/2014 in compliance with the International biosafety guidelines (WHO laboratory biosafety manual, 2004).
Treatment with areca nut extract
The HSC-2 and HSC-3 cells were plated at 1 × 10 5 cells/well in 60 mm dishes with DMEM. Areca nut extract (629.50 μg/mL) was added for HSC-2 cells and 164.06 μg/mL for HSC-3 cells. For combination experiments, areca nut extract was added at the same time and both cells were incubated for 24 and 48 h, before the preparation of cell extract or quantification of cell cycle and Ki-67 activity (see below). The controls are HSC-2 and HSC-3 cells that wereincubated at the same time and condition without areca nut extract treatment.
Flow cytometry analysis for determining the distribution of cells per stage of cell cycle
The principle of cell cycle profile staining procedure is to dissolve the fat in the cell membranes using a non-ionic detergent, to eliminate cytoskeletons and nuclear proteins using trypsin, as well as to digest RNA using an enzyme and to stabilize nuclear chromatin using spermine. The final result of the flow cytometry was analyzed to detect the presence of an abnormal DNA stem line (aneuploidy). This procedure also used concentrated cells of 1x105 cells/mL. The prepared cell suspension was centrifuged for five minutes at speed of 500 rpm, afterwards, the supernatant was discarded, and 200 μL of solution A, which contained trypsin in sperminetetrahydrochloride detergent buffer (340242, BD Cycletest™ Plus), was added to each tube and tapped with a finger, without a vortex. Solution A functioned to disaggregate solid tissue fragments enzymatically as well as to dissolve cell membranes and cytoskeletons. Solution A was allowed to react for ten minutes in a dark room at room temperature. Then, two hundred μL of solution B was added, which contained RNase A and trypsin inhibitor in spermine buffer (340242, BD Cycletest™ Plus), into each tube and each tube was tapped with a finger, without a vortex. Afterward, the tubes were placed in a dark room for ten minutes. Solution B functioned to inhibit trypsin activity and dissolve RNA. Two hundred μL of solution C was added, which contained PI stain and sperminetetrahydrochloride in citrate stabilizing buffer (340242, BD Cycletest™ Plus), into each tube, following by incubation for ten minutes in a refrigerator at a temperature between 2ºC and 8ºC without discarding solution A and B. Solution C functioned to bind DNA. The samples were ready to be analyzed with the flow cytometry (BD FACS Calibur Flow cytometry System type E 34297502328, San Jose, California, USA) in at least three hours after the staining procedure had been completed. The percentage of cells at each phase of the cell cycle was calculated using Modfit LT Software version 4 (Verity Software House, Topsham, USA).
Flow cytometry analysis for Ki-67 activity
After the cells were centrifuged and the supernatant had been discarded, the solution was rinsed with 100 μL of cold PBS (10010031, ThermoFisher Scientific). One mL of 70% cold ethanol was added, following by incubation in the refrigerator for thirty minutes, subsequently, the solution was rinsed again with PBS. Five μL of Alexa Fluor® 647 anti-human Ki-67 antibody solution was added (562622, BD PharmingenTM) into each tube. Incubation was performed in a dark room for twenty minutes. Following the addition of 200 μL of PI (340242, BD Cycletest™ Plus) into each tube, 200 μL of solution B was added. Incubation was performed in a dark room for ten minutes. Two hundred μL of cooled solution C was added to each tube. Following the incubation for ten minutes in the refrigerator, the tubes were ready to be analyzed with the flow cytometry (BD FACS Calibur Flow cytometry System type E 34297502328, San Jose, California, USA).
Statistical analysis
All data were presented as the mean ± standard deviation of triplicate parallel measurements. Statistical analysis used SPSS 10.0 and the data were analyzed with the unpaired t-test using a significance level of p<0.01.
Results
Cell cycle distribution by flow cytometry analysis
Twenty-four hours exposure of areca nut extract could cause a decrease in the percentage of HSC- 2 cell population in G1 phase. The decrease was also observed in S phase, in which a significant differences were found when comparing the control and after treatment with areca nut extract, 65.45%±19.39% and 21.37%±4.16% respectively (p<0.01, unpaired t-test). The G2 phase also showed a significant difference between treated and control cells, which was an 11-fold lower in the population of treated cells compared to control cells (p<0.01, unpaired t-test). We also found that there was only a significant increase of cell population in SubG1 phase, which was three times higer compared to the control cells. The low cell population in all phases of the cell cycle after 24 hours areca nut extract treatment indicated the presence of a disturbance in the synthesis and replication of RNA, DNA, cytosol organelles, as well as mitosis, causing cells unable to proliferate. Significant increase was seen only in subG1 phase, which was three times higher compared to the control cells. These results indicated that abundant apoptotic cells with DNA content had been split or cells which had undergone loss of DNA chromatin and subsequently formed apoptotic bodies. The flow cytometry results and the cell cycle profile are presented in Figure1.
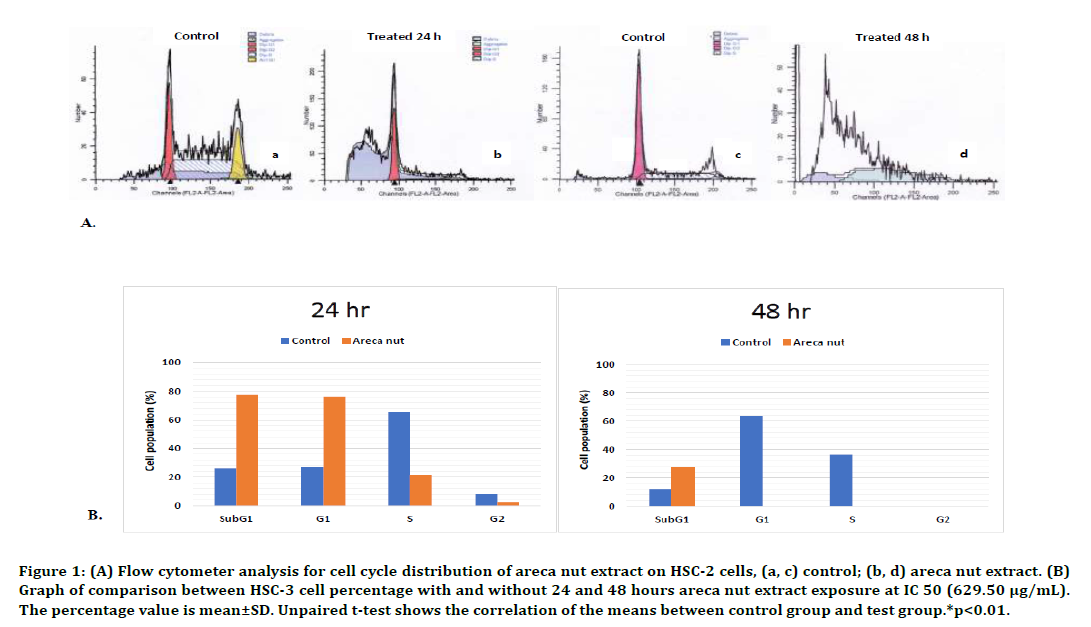
Figure 1. (A) Flow cytometer analysis for cell cycle distribution of areca nut extract on HSC-2 cells, (a, c) control; (b, d) areca nut extract. (B) Graph of comparison between HSC-3 cell percentage with and without 24 and 48 hours areca nut extract exposure at IC 50 (629.50 μg/mL). The percentage value is mean±SD. Unpaired t-test shows the correlation of the means between control group and test group.*p<0.01.
We couldn’t identified the cell cycle profile in HSC-2 cells followingarea nut extract treatment after 48 hours because there was no longer population which still underwent cell cycle. However, in subG1 phase, which is a marker for the apoptosis, statistically significant difference was observed (p<0.01, unpaired t-test) between the control cells (12.08%±36.06%) and those with the treated cells (27.06%±21.22%). The loss of cell populations in G1, S, dan G2 phases was thought to be due to apoptosis or necrosis of almost all HSC-2 cells in the absence of a preparatory phase for mitosis or proliferation after the 48-hour exposure of areca nut extract. This was observed in the results produced by the Modfit software flow cytometry analysis, in which findings of debris and aggregation due to plenty of DNA and cytosol organelle fractions, in the absence of cell cycle patterns such as that observed in the control cells histogram (Figure 1).
Furthermore, the investigation results on HSC- 3 cells demonstrated that 24-hour exposure of areca nut extract could inhibit HSC-3 cell cycle in G1 phase but no inhibition after 48-hour exposure. Statistically, a significant increase was observed in G1 phase of HSC-3 cells, which was 7.3 times higher than that of the control cells (p<0.01, unpaired t-test). The graphs and analysis results of the flow cytometry are presented in Figure 2. These results indicated that there was an increase in the number of cells which duplicated the organelle and cytosolic component as well as replicated the centrosome. However, in S and G2 phases, the number of the cell population was decreased. This decrease caused a decrease in the number of cells which conducted the DNA replication process in S phase, as well as protein synthesis and complete chromosome replication in G2 phase, and therefore the proliferation was inhibited. The flow cytometry test on the HSC-3 cells after 48-hour exposure did not show any significant changes in the cell population in G1, S, and G2 phases with or without the exposure of areca nut extract. Forty-eight hour exposure of areca nut extract at a dose of 164.06 μg/mL did not affect the cell cycle profile of HSC-3 cells.
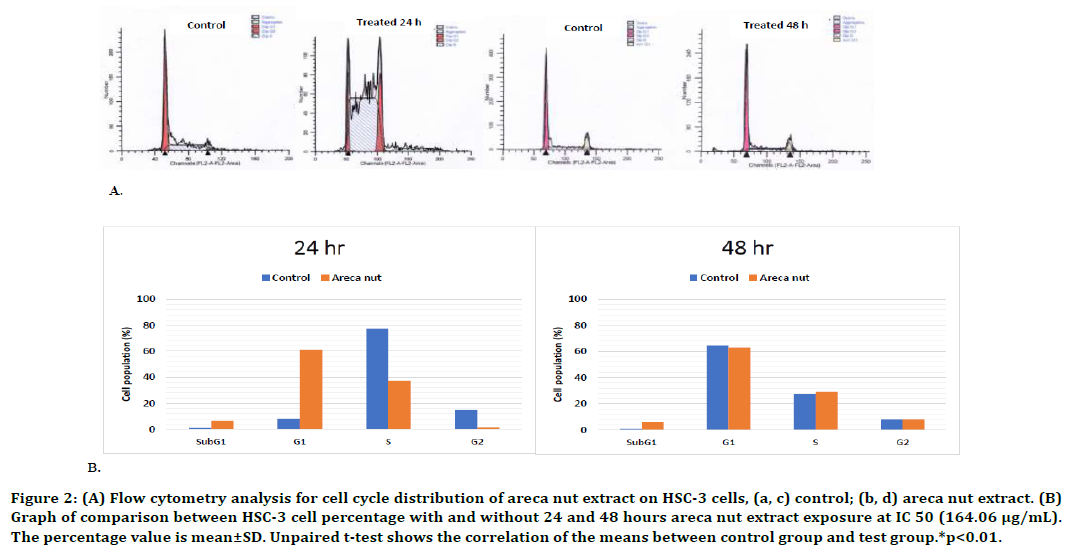
Figure 2. (A) Flow cytometry analysis for cell cycle distribution of areca nut extract on HSC-3 cells, (a, c) control; (b, d) areca nut ex tract. (B) Graph of comparison between HSC-3 cell percentage with and without 24 and 48 hours areca nut extract exposure at IC 50 (164.06 μg/mL). The percentage value is mean±SD. Unpaired t-test shows the correlation of the means between control group and test group.*p<0.01.
Ki-67 activity by flow cytometry analysis
The objective of this assay is to identify whether the areca nut extract is able to inhibit cell proliferation with the treatment at IC50 value compared to without treatment. This studyshowed a significant decrease of Ki-67 activity following the 24-hour exposure of areca nut extract in HSC-2 cells (p<0.01, unpaired t-test) (Figure 3). The 48-hour of areca nut extract exposure demonstrated a result consistent with the result of the HSC-2 cell cycle analysis, which showed that the extract was unable to induce cell cycle arrest. The M1 and M2 quadrant, which exhibited the percentage of cell population with and without Ki-67 protein, also showed a similar result in both the treatment and control groups (p<0.01, unpaired t-test). The study results showed that areca nut extract does not affect Ki- 67 activity after 48-hour exposure.
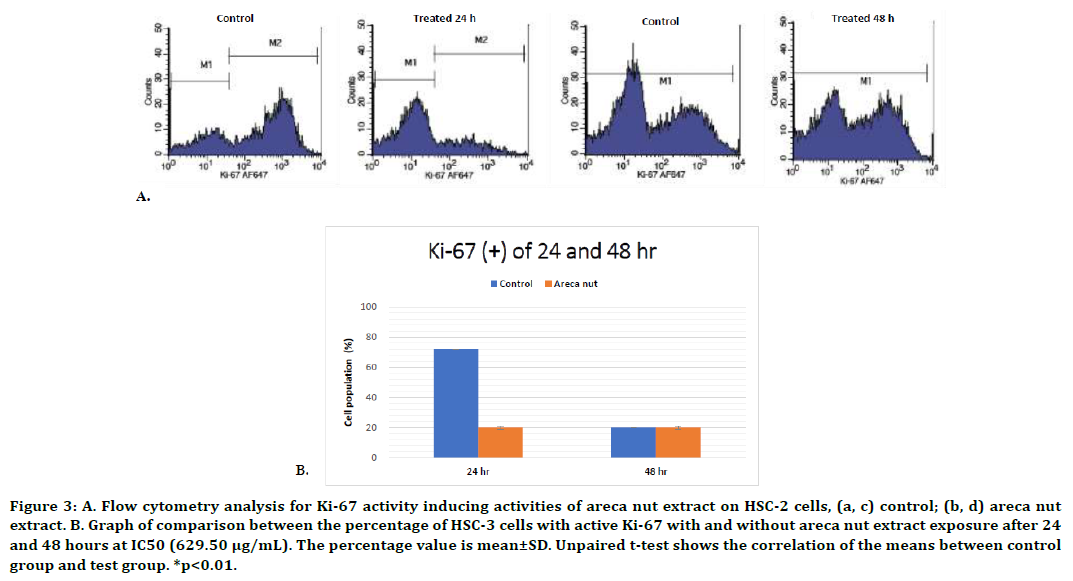
Figure 3. A. Flow cytometry analysis for Ki-67 activity inducing activities of areca nut extract on HSC-2 cells, (a, c) control; (b, d) areca nut extract. B. Graph of comparison between the percentage of HSC-3 cells with active Ki-67 with and without areca nut extract exposure after 24 and 48 hours at IC50 (629.50 μg/mL). The percentage value is mean±SD. Unpaired t-test shows the correlation of the means between control group and test group. *p<0.01.
The Ki-67 activity of HSC-3 cells was similar with the HSC-2 cells. There was a significant decrease of cell population with active Ki-67 compared to the control cells following 24-hour exposure, but not at 48-hour exposure (p<0.01, unpaired t-test). We found that the extract only inhibit HSC-3 cells proliferation after 24-hours treatment with areca nut extract, but not after 48-hour exposure. The flow cytometry analysis results and graphs are presented in Figure 4.
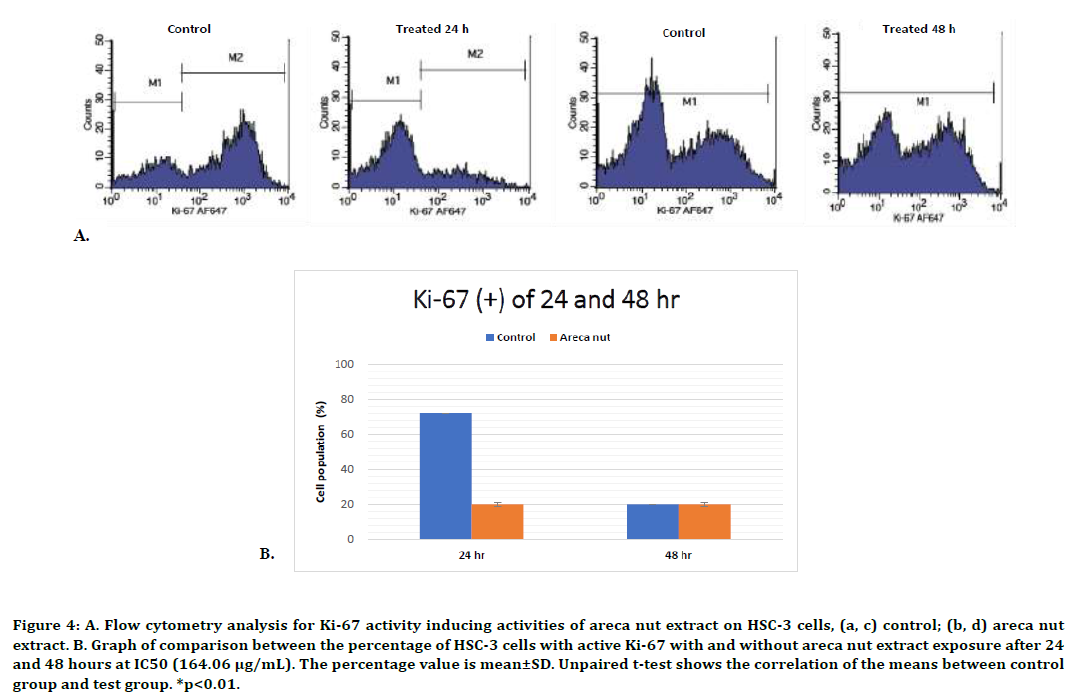
Figure 4. A. Flow cytometry analysis for Ki-67 activity inducing activities of areca nut extract on HSC-3 cells, (a, c) control; (b, d) areca nut extract. B. Graph of comparison between the percentage of HSC-3 cells with active Ki-67 with and without areca nut extract exposure after 24 and 48 hours at IC50 (164.06 μg/mL). The percentage value is mean±SD. Unpaired t-test shows the correlation of the means between control group and test group. *p<0.01.
Discussion
Herbal plants and plant-derived medicines have been widely used in traditional cultures all over the world and have gained popularity in modern society as natural alternatives to produce new potential therapeutic compounds for combating diseases [38]. Although the areca nut is reported to have multiple therapeutic properties, epidemiological studies have correlated betel quid chewing with a high incidence of oral leukoplakia, oral submucous fibrosis, and oral cancer [39]. Betel quid (a mixture of areca nut, slaked lime,with and without tobacco) chewing is a widely prevalent habbit correlated with a high incidence of oral cancer. The carcinogenic mechanism of action of betel quid chewing was there is a DNA damage induced by arecaidine from areca nut and Cu(II) under alkaline condition [40]. Areca nut is also believed to be the most widely used psychoactive substance and an important environmental risk factor for development of oral premalignant lesions and cancer. Chiang et al. found thatimportant toxic effects of arecoline could be attributed to its wide impact on various biological processes, such as repression of gene involved in xenobiotic metabolism/detoxification, chromosomal structure stability, and DNA repair system [41]. These facts raise so many controversy about the usage of areca nut as anticancer agent. However, the development of OSCC is a multistep process involving the accumulation of multiple genetic alterations modulated by genetic predisposition and environmental influences [42]. The occurrence of the OSCC’s risk in betel quid depends greatly on the composition of the compound which determines the quality of the seeds, the method of using seeds which is associated with oral hygiene, duration of use, the presence or absence of toxin caused by fungi contamination in the seeds, and the presence or absence of other carcinogenic substances such as tobacco and slaked lime [26]. The occurrence of OSCC could also be influenced by several factors such as intrinsic factors (tumor suppressor gene abnormality or mutation and oncogene) and extrinsic factors (tobacco smoking, chronic inflammation, vitamin A and iron deficiency, candidiasis, viral infections, and immunosuppression). Therefore, the present study was planned to evaluate the possible anticancer potential of areca nut extract in cell cycle distribution and the proliferation marker Ki-67 activity on oral cancer cell lines.
The results of the cell cycle evaluation show that areca nut extract arrests cell cycle progression by significantly restricting cells in G0/G1 phase in HSC-3 cells after 24-hour exposure. This implies that the areca nut extract pertubs the protein synthesis that is important to cell progression from G1 to S-phase. It is known that p53 protein and mdm2 protein are important to the progression of the cell cycle at G0/G1 [43] . It may be possible that the extract plays a role in the disturbance of these proteins, but this aspect was not investigated in this study.Although the extract has the same optimum time in both cells, there is a difference on extract effect on the cell cycle distribution, where the percentage of HSC- 2 cells undergoing apoptosis is higher than HSC- 3 cells. This result might be possible because there is a differentcharacteristic between HSC-3 cells and HSC-2 cells.[36] The mutation of HSC-3 cells was confirmed in a previous report.[44,45] We found that HSC-3 cells have the ability to evade apoptosis higher than HSC-2 cells. On p53 activation, HSC-2 cells undergo predominantly apoptosis instead of cell-cycle arrest. However, this finding may vary by the study design and so much more data must be collected to better understand these phenomena.
The cell cycle test refers to a frequency histogram of cellular DNA content in each phase of the cell cycle. A significant decrease in cell population was observed in G1, S, and G2 phases at both time points in HSC-2 cells. When the results of the HSC-2 cell cycle test were confirmed with the Ki-67 analysis results, a significant decrease in Ki-67 activity could be observed after 24-hour exposure, whereas no difference in Ki-67 activity was observed following 48-hour exposure between cells which were exposed and those which were not. The results of this analysis proved that cell cycle inhibition did not occur after 24 and 48-hour exposure of the areca nut extract in HSC-2 cells but a decrease in proliferation occured after 24-hour exposure of the extract, indicated by a decrease in the cell population in G2/M phases, while after 48-hour exposure, a decrease in proliferation occurred due to the high number of cells undergoing apoptosis (increase in subG1 phase).
Areca nut extract exerted its growth arrest on treated-HSC-3 cells by accumulating the cells at G0/G1-phase after 24 hour exposure, implying that areca nut extract may interfere with protein synthesis of HSC-3 cells thus halting their progression from G1 to S phase during their cell cycle and subsequently initiating apoptosis. This could be due to the inhibitory effects on MDM2 protein, which reduces cell proliferation and induces apoptosis by the elevation of p21, Bax and pRb levels as well as reduction of hyperphosphorylatedRb and E2F1 [46]. However, the areca nut extract was unable to inhibit proliferation after 48-hour exposure. This result was confirmed with the Ki-67 activity analysis, which indicated that a decrease in the proliferation activity due to areca nut extract only occurred after 24-hour exposure, whereas no significant difference was observed between the treated and control groups after 48-hour exposure. Twenty-four-hour exposure of the extract caused an increase of the cell count in HSC-3 cells which were retained at the G1 phase. Inhibition of cell cycle occurred due to the presence of a checkpoint at the transition of G1/S phase, causing the cells which underwent DNA damage in G1 phase to not be able to proceed to S phase [47].The p53 mutation in the HSC-3 cells caused the cell cycle to not involve the p53 pathway. This cell cycle inhibition could possibly occur from two pathways. The first pathway was activation of CycD1 by APC, leading to release of p21CIP1 which bind with Cdk4/CycD1 and inhibited Cdk2/CycE [47]. The second pathway was through an increase in the phosphorylation of Cdk2 at the site of cdc25A phosphatase, which tasked to phosphorylate this site thus inhibiting the function of Cdk2/CycE. The involvement of the Chk2 pathway which induced p53 and later activated p21 to inhibit the phosphorylation of CycE/Cdk2 did not affect the HSC-3 cell cycle inhibition.
This research using flow cytometry that has several advantages, including fast period time analysis (thousands of cells per second), single cell analysis, and multiparametric measurements (correlations with several different cell events in one unit of time), but this machine also has drawbacks; the presence of physical and enzymatic manipulations during cell preparation and staining, can trigger additional apoptosis or necrosis cell numbers. This is one of the limitations in our study.
Conclusion
The present study describe that areca nut extract can induce cell cycle arrest in HSC-3 cells after 24 hours extract exposure. The areca nut can inhibit cell’s proliferation after 24 hour extract exposure in HSC-2 and HSC-3 cells.
Competing Interests
No competing interests were disclosed.
Grant Information
The author(s) declared that no grants were involved in supporting this work.
References
- Moraes MD, Monteiro Maia CAD, Freitas RA, et al. Cell proliferation markers in oral squamous cell carcinoma. J Mol Biomark Diagn 2012; 1-5.
- JH, Hong SM, Yun JY, et al. Anti-cancer effects of cordycepin on oral squamous cell carcinoma proliferation and apoptosis in vitro. JCT 2011; 2:224-234.
- Deng H, Sambrook PJ, Logan RM. The treatment of oral cancer: an overview for dental professionals Aust Dent J 2011; 56:244-252.
- Tolentino ES, Centurion BS, Ferreira LHC, et al. Oral adverse effects of head and neck radiotherapy: literature review and suggestion of a clinical oral care guideline for irradiated patients. J Appl Oral Sci 2011; 19:448-454.
- Hamsar MN, Ismail S, Mordi M, et al. Antioxidant capacity and the effect of different parts of Areca catechu extracts on gluthatione-S-Transferase activity invitro. Free Rad Antiox 2011; 1:28-33.
- Wetwitayaklung P, Paechamud T, Limmatvapirat C, et al. The study of antioxidant capacity in various parts of Areca catechu L. Naresuan Univ J 2006; 14:1-14.
- Xing Z, Jiao W, Zhuang H, et al. Antioxidant and cytotoxic phenolic compounds of areca nut (Areca catechu L.). Chem Res Chinese Universities 2010; 26:161-164.
- Zhang WM, Wei J, Chen WX, et al. The chemical composition and phenolic antioxidants of areca (areca catechu L.) seeds. ICABE 2011; 1:16-22.
- Bhandare AM, Kshirsagar AD, Vyawahare NS, et al. Potential analgesic, anti-inflammatory and antioxidant activities of hydroalcoholic extract of Areca catechu L. nut. Food Chem Toxicol 2010; 48:3412-3417.
- Hannan A, Karan S, Chatterjee TP. A comparative study of invitro antioxidant capacity of different extract of areca seed collected from areca catechu plant grown in Assam. Int J Pharm Pharm 2012; 4:420-427.
- Jaiswal P, Kumar P, Singh VK, et al. Areca catechu L: A valuable herbal medicine againts different health problems. Res J Med Plant 2011; 5:145-152.
- Toprasri P, Chinpaisal C, Phaechamud T. Comet assay to test antioxidative effects of extracts from different parts of Areca catechu L. Thai Pharm Health Sci J 2008; 3:309-315.
- Surendiran NS, Yuvara TV. Antibacterial, antioxidant, in vitro & in vivo immuno-modulatory studies of Areca catechu in mice. J Pharm Res 2010; 3:2678-2681.
- Mondal S, Bhattacharya, Biswas M. Antidiabetic activity of Areca catechu leaf extracts against streptozotocin induced diabetic rats. J Adv Pharm Educ Res 2012; 2:10-17.
- Cyriac MB, Pai V, Varghese I, et al. Antimicrobial properties of Areca catechu (Areca nut) husk extracts against common oral pathogens. Int J Res Ayurveda Pharm 2012; 3:81-85.
- Sarpangala KB, Sarpangala M, Devasya A. Antimicrobial properties of Areca nut, Areca catechu L.: A review. Int J Res Ayurveda Pharm 2017; 8:8-12.
- Jiang JH, Jung SY, Kim YC, et al. Antimalarial effects of Areca catechu L. Korean J Physiol Pathol 2009; 23:494-498.
- Pithayanukul P, Nithitanakool S, Bavovada R. Hepatoprotective potential of extracts from seeds of areca catechu and nutgalls of quercus infectoria. Molecules 2009; 14:4987-5000.
- Amudhan MS BV, Hebbar KB. A review on phytochemical and pharmacological potential of Areca catechu L. seed. Int J Pharm Sci Res 2012; 3:4151-4157.
- Lee KK, Choi JD. The effects of Areca catechu L extract on antiaging. Int J Cosmetic Sci 1999; 21:285-295.
- Byun SJ, Kim HS, Jeon SM, et al. Supplementation of Areca catechu L. extract alters triglyceride absorption and cholesterol metabolism in rats. Ann Nutr Metab 2001; 45:279-284.
- Chempakam B. Hypoglycaemic activity of arecoline in betel nut Areca catechu L. Indian J Exp Biol 1993; 31:474-475.
- IARC. WHO-biennial report. Int Agency Res Cancer 2004; 1-192.
- Ji WT, Chuang YC, Chen HP, et al. Areca nut extracts exert different effects in oral cancer cellsdepending on serum concentration: A clue to the various oralalterations in betel quid chewers. Toxicology Reports 2014; 1:1087-1095.
- Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol 2002; 7:115-125.
- Sari LM, Subita GP, Auerkari EI. Potential antioxidant and cytotoxic activity of areca nut (Areca catechu L.) extract in human oral squamous cell carcinoma and keratinocyte cells. Asian J Pharm Clin Res 2017; 10:286-291.
- Sari LM, Suyatna FD, Utami S, et al. Acute oral toxicity study of Areca catechu Linn. aqueous extract in Sprague-Dawley rats. Asian J Pharm Clin Res 2014; 7:20-22.
- Sari LM, Suyatna FD, Subita GP, et al. Acute dermal toxicity study of Areca catechu Linn. extract in Sprague Dawley rats. Asian J Pharm Clin Res 2016; 9:1-3.
- Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr 1999; 38:133-142.
- Sherr C. Cancer cell cycle. Science 1996; 274:1672-1677.
- Barnum KJ, O'Connell MJ. Cell cycle regulation by checkpoints. Methods Mol Biol 2014; 1170:29-40.
- Kajstura M, Halicka D, Pryjma J, et al. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete ‘‘Sub-G1’’ peaks on DNA content histograms. Cytometry Part A 2007; 71A:125-131.
- Stewart ZA, Westfall MD, Pietenpol JA. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol Sci 2003; 2:139-145.
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000; 182:311-322.
- Stoll C, Baretton G, Ahrens C, et al. Prognostic significance of apoptosis and associated factors in oral squamous cell carcinoma. Virchows Arch 2000; 436.
- Kamiya Y, Ohshima T. The individual cell properties of oral squamous carcinoma and tumor suppressor gene mutation. Oral Sci Intl 2005; 2:104-117.
- Okamura M, Shimada J, Sakagami H. Comparative analysis of cell death induction by cisplatin and 5-FU in human oral squamous and hepatocellular carcinoma cell lines. Anticancer Res 2008; 28:253-260.
- Shoeb M. Anticancer agents from medicinal plants. Bangladesh J Pharmacol 2006; 1:35-41.
- Ko YC, Chiang TA, Chang SJ, et al. Prevalence of betel quid chewing habit in Taiwan and related sociodemographic factors. J Oral Pathol Med 1992; 21:261-264.
- Chen W, Liu X, Shi J, et al. Mechanism of DNA damage induced by arecaidine: The role of Cu(II) and alkaline conditions. Food Chem 2010; 119:433-436.
- Chiang SL, Jiang SS, Wang YJ, et al. Characterization of arecoline-induced effects on cytotoxicity in normal human gingival fibroblasts by global gene expression profiling. Toxicol Sci 2007; 100:66-74.
- Sayans MP, Martin JMS, Angueira FB, et al. Genetic and molecular alterations associated with oral squamous cell cancer (Review). Oncol Rep 2009; 22:1277-1282.
- Tang YQ, Jaganath IB, Sekaran SD. Phyllanthus spp. induces selective growth inhibition of PC-3 and mewo human cancer cells through modulation of cell cycle and induction of apoptosis. PLoS ONE 2010; 5:1-11.
- Sakai E, Tsuchida N. Most human squamous cell carcinoma in the oral cavity contain mutated p53 tumorsuppressor genes. Oncogen 1992; 7:927-933.
- Myoung H, Hong S, Yun P, et al. Anticancer effect of genistein in oral squamous cell cartinoma with respect to angiogenesis and in vitro invasion. Cancer Sci 2003; 94:215-220.
- Zhang Z, Li M, Agrawal S, Zhang RW. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci 2003; 100:11636–11641.
- Garrett MD. Cell cycle control and cancer. Curr Sci 2001; 81:515-522.
Author Info
Liza Meutia Sari1, Gus Permana Subita2 and Elza Ibrahim Auerkari3*
1Department of Oral Medicine, Faculty of Dentistry, University of Syiah Kuala, Banda Aceh, 23111, Indonesia2Department of Oral Medicine, Faculty of Dentistry, University of Indonesia, Jakarta, 10430, Indonesia
3Department of Oral Biology, Faculty of Dentistry, University of Indonesia, Jakarta, 10430, Indonesia
Citation: Liza Meutia Sari, Gus PermanaSubita, Elza Ibrahim Auerkari, Areca Nut (Areca catechu Linn.) Extract Induces Cell Cycle arrest and Reduces Ki-67 Activity in Oral Squamous Cell Carcinoma Cells, J Res Med Dent Sci, 2021, 9 (4): 279-306.
Received: 28-Jul-2020 Accepted: 07-Apr-2021
