Research Article - (2021) Volume 9, Issue 12
Attempts to Develop a Recombinant Protein with Antioxidant and Thrombol Ytic Activity - In-Vitro Study
C.Revathy and A.J. Manjula Devi*
*Correspondence: A.J. Manjula Devi, Department of Biochemistry, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, India, Email:
Abstract
The objectives were framed bearing in mind to generate a synergetic protein of higher efficacy so as to handle both the thrombotic status and ischemia mediated free radical injury to fight Myocardial infarction. Amplification of human Thioredoxin gene from lung cancer cell line A549.Cloning of human Streptokinase in pRSET A at Barn HI and Eco RI site and fusion of human thioredoxin in the recombinant clone pRSET A/Sk at Ndel and Barn HI site In this study, the human thioredoxin isolated from lung cancer cell lines is fused with streptokinase to generate a fusion construct so as to develop a synergetic protein of higher efficacy in the thrombolytic and the antioxidant activity. Expression studies and solubility of the recombinant protein was studied., Maximum soluble fraction achieved by optimizing various parameters like host strain, optical density, post induction temperature, inducer concentration etc... was subjected to ion exchange chromatography obtaining a sing1e band corresponding to the molecular weight in SDS-PAGE and confirmed by western blot as well. Activity of the purified recombinant TRX-Sk protien was confirmed using in-vitro biological assay such as: Insulin reduction assay - Retention of the biological activity of Thioredoxin in the recombinant protein in comparison with the control.Keywords
Phospholipids, Cloning, Myocardial infarction, Cellular redoxIntroduction
Sickle Streptokinase is an extracellular protein produced by various strains of hemolytic Streptococci. It is a potent thrombolytic agent comprising of 414 amino acid and mass of about 47 kDa which is used to treat acute myocardial infarction. Streptokinase is an indirect plasminogen activator which binds with free circulating plasminogen to form a complex (1:1) that can convert the plasminogen to plasmin, which intum dissolves the blood clot. SL. tokinase continues to be the cost effective fibrinolytic agent of choice in the treatment of myocardial infarction.
Thioredoxin, a REDOX protein forms the free radical scavenging system in the human body. It has proven to be so effective in part because of its ability to reduce disulfide bonds that do not respond to other cellular reductants. Furthermore, it has been demonstrated that thioredoxin reductively inactivates phospholipase A2 which is one of the contributing factors in inflammatory disorders. It may be used in all areas of inflammation. This could implicate that thioredoxin could be useful in treatment of free radical mediated injuries especially in inflammation. In research, thioredoxin can be used in the over express10n of genes encoding economically valuable proteins and peptides. Above all there have been a multitude of studies, the mechanisms of angiogenesis though incompletely understood; increasing evidence suggests that cellular redox homeostasis is an important regulator of angiogenesis.
The thioredoxin (TRX) system functions as an endogenous antioxidant that can exert influence over endothelial cell function via modulation of cellular redox status. It has become apparent that TRX participates in both canonical and novel angiogenic signaling pathways and may represent an avenue for therapeutic exploitation. Recent identified a role for TRX in ischemia-induced angiogenesis. Thus TRX system plays a major role in endothelial cell homeostasis and angiogenesis 1 So the thioredoxin_streptokinase fusion construct was designed so as to generate a potent free radical scavenger with a thrombolytic .so as to develop a synergistic protein of higher efficacy to combat the ischemia mediated free radical injury in myocardial infarction. A blood clot in a vessel is called a thrombus, and it is called an embolus when part of it detaches and travels through the bloodstream, where it can lodge in a blood vessel and block the blood flow. Blood flow can become blocked when a blood vessel gets obstructed by blood clots or other foreign matter in the bloodstream. As blood flow beyond the clot of blood or foreign matter is reduced or cut off, the part of the body thus deprived of oxygen may become damaged. If the brain becomes damaged, a stroke may result. If the heart muscle becomes damaged, a heart attack may result.A healthy haemostatic system suppresses the development of blood clots in normal circulation, but r acts extensively in the event of vascular injury to prevent blood loss. Outcomes of a failed hemostasis include stroke, pulmonary embolism, deep vein thrombosis and acute myocardial infarction. Strategies have been developed to prevent these clotting and one such approach is to use anticoagulants. This includes the use of heparin, aspirin and warfarin, which reduce the stickiness of blood at first place 2.
Pathologies involving a failure of hemostasis and the development of clot require clinical intervention consisting of intravenous administration of thrombolytic agents. Streptokinase is one such agent. Other thrombolytic or fibrinolytic agents include urokinase and the tissue type plasminogen activator \-t-PA). All these drugs digest clots by increasing the amount of plasmin (plasmin dissolves clots) in the blood. To produce plasmin, the substance plasminogen must first be activated. Plasminogen is converted into plasmin by certain enzymes known as plasminogen activators 3.
Material and Method
One of the major criteria in the production of recombinant therapeutic protein is the development of rational combinations that bear synergistic effect supplementing each other functions thereby providing a therapeutic option of higher efficacy in handling diseases. Streptokinase (SK) is a potent plasminogen activator with the widespread clinical use as a thrombolytic agent and Thioredoxin is a potent free radical scavenging protein that could protect the tissues from the hypoxic damage. This led to the ncept of cloning the most effective redox protein, human thioredoxin with streptokinase. The overall outline of the present study is given in the flowchart,
Chemicals
Fine chemicals were procured from Sigma Chemicals Company, St.Louis, USA and chemicals for media preparation were purchased from Hi-Media, Bombay , India. Restriction enzymes and T4 DNA ligase obtained from New England Biolabs Beverley , USA. Taq DNA polymerase and Hybond nitrocellulose membrane were purchased from Amersham pharmacia biotech, Birmingham, UK. Primers used in this study were synthesized in Microsynth, Switzerland and Bangalore Genei, Bangalore, India.
pRSETB vector, E.coli strain BL21 (DE3) and DH5a were purchased from invitrogen Corporation, Sandiego, USA. pRSET-A expression vector was purchased from Novegen, Merck Darmstadt, Germany. Large scale purification of plasmids and midi preparation kits were purchased from QIAGEN, Germany. Most of the immunological reagents used in the study were purchased from Pierce, USA or from Sigma, USA.
Culture media
In all the experiments the E.coli culture was grown in the Luria-Bertani (LB) Broth. To prepare LB Broth 10g/L of Tryptone, 10g/L of sodium chloride and 5g/L of yeast extract were dissolved in distilled water and the pH was adjusted to 7.3. And 1.5% agar was added to the liquid broth to prepare solid medium. LBON (LB lacking NaCl was prepared similarly without including NaCl). Media was supplemented with l00µg/ml of Ampicillin or 50µg/ml of chloramphenicol wherever required.
Gel electrophoresis
Agarose Gel Electrophoresis of DNA (Sambrook et al 1989) Horizontal submerged gels were employed in this study. The buffer for electrophoresis was TBE (89mM Tris, 89 mM Boric acid and 2mM EDTA, pH 8.0). The gel loading buffer was a solution of 20% sucrose and 0.01% bromophenol blue in TE buffer. Samples containing an appropriate quantity of DNA in TE buffer were mixed with a 1/4 volume of gel loading buffer and loaded onto the gel. Depending on the size of the fragments to be separated, 0.7- 1.2% agarose gels were run. The gels were 10cm long and 3mm thick. Electrophoresis was performed at 10 V/cm until the dye reached the bottom of the gel. Gels were stained in a 1 µg/ml solution of ethidium bromide in water for 5 min and viewed under illumination of 300nm. Photographs were taken for a permanent documentation.
SDS-polyacrylamide gel electrophoresis
Proteins present in cell extracts were analysed by SDS-PAGE according to the method of Laemmli (1970) with some modifications. The composition of the systems is desq_ribed below and also in appendix.
a) Monomer solution: 30% acrylamide and 0.8% bisacrylamide.
b) Separating gel buffer: 1.5M Tris, pH 8.8.
c) Stacking gel buffer: 1.5M Tris, pH 6.8.
d) Ammonium per sulphate (APS) - 140 mg/ml.
e) SDS 10% solution.
Temed western blotting
In order to find out the location or presence of specific proteins in the SDS-PAGE, the protein pattern were electro transferred to a nitrocellulose membrane as described 50• After the electrophoresis was complete, the gel was incubated for 10-15 min in the transfer buffer to eliminate swelling. In the meantime, the NCP/Hybond cut to the desired size was incubated for 5-10 min in transfer buffer (Tris 25mM, glycine 192mM, methanol 20%, SDS 1%). The membrane was over laid on the gel (by avoiding air bubble) and sandwiched between the filter paper and scotch brite pads. The gel was placed 'cathodic' to the membrane. The transfer was carried out at 120 mA, 20V for one and half hours by using LKB transphor 2005 electroblotting apparatus. After the transfer was complete, the molecular weight marker lane was stained with amido black (100 mg amido black in 45ml methanol with distilled water) for 2- 3 min and then destained until the background stain was eliminated or prestained protein marker was used. The rest of the membrane was blocked for 1hr at room temperature with 3% skimmed milk in PBS[1].
The membrane was washed in wash buffer for three times of 5 min duration. The membrane was incubated for 1 hr with the mouse monoclonal anti-Streptokinase antibody (primary) diluted in PBS (1:1000). After extensive washing in the wash buffer, the membrane was incubated for the anti-mouse secondary antibody (1:1000 dilutions) conjugated with alkaline phosphatase. For alkaline phosphatase staining, the blots were incubated in the buffer (l00mM Tris HCI (pH 9.5), l00mM NaCl, 5mM MgC12) for 10 min and the colour development was carried out by using 30µ1 of nitroblue tetrazolium (NBT) (50mg/ml in 70% dimethyl formamide) and 16.5µ1 of bromo-chloro-indolyl phosphate (BCIP) (50mg/ml in 100% diethyl formamide).
Results
RT PCR was carried out and the thioredoxin gene was isolated from lung cancer cell lines (A549) and the cDNA synthesized was further used for PCR amplification having Ndel and BamHl restriction sites using gene specific primers and the PCR products were analyzed in 1.8% agarose gel[2].
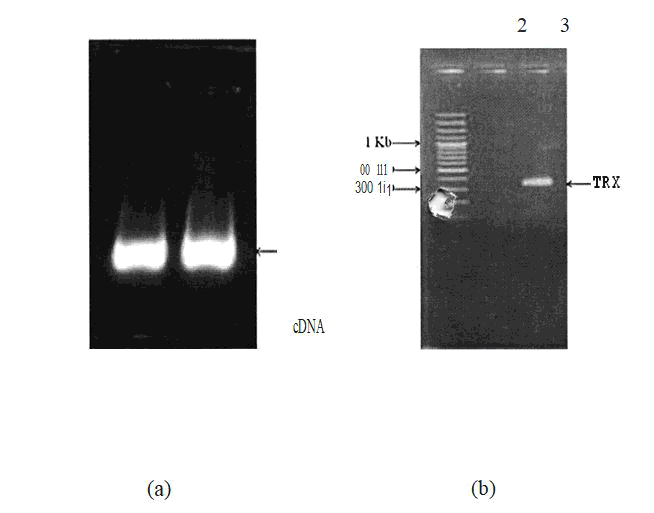
Figure 1: Constrution of the thioredoxin gene.
The Sk gene was amplified usmg gene specific pnmers containing BamHl and EcoRl restriction sites in the forward and reverse primers respectively. The amplicons were purified and then subjected to restriction digestion by BamHl and EcoRl restriction enzymes. Meanwhile the expression vectors, pRSETA were prepared for ligation by digestion with BamHI and EcoRI enzymes and subsequently purified with gel elution. The purified amplicons were ligated with the linearized vectors (pRSETA) using T4 DNA ligase. The ligation products were then transformed into DH5a and the recombinants were screened by PCR (figure 5.3) and the clones were confirmed by restriction digestion. The constructs were then transformed and expressed in various E.coli strains such as BL21 (DE3) and GJI 158 and their expression profile was analyzed in 10% SDS pAGE
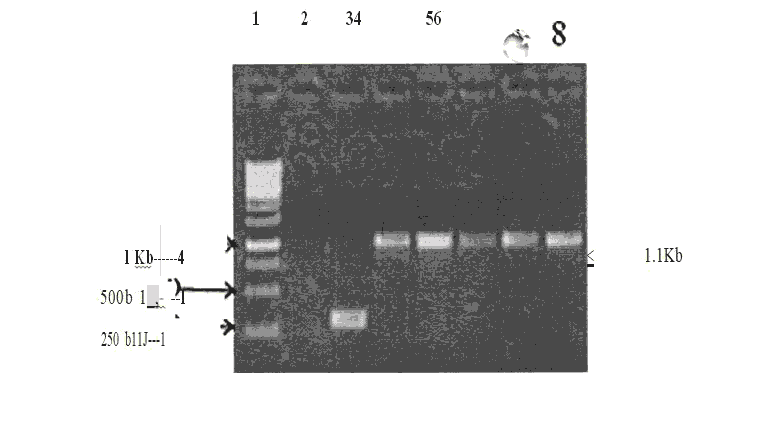
Figure 2: Screening of Positive recombinant clones pRSETA/ TRX-SK (Lysate PCR).
The recombinant plasmids pRSETA/TRX-SK isolated from the positive clones and digested with Ndel , BamHI and EcoRI for the release of inserts - Restriction Digestion of Clone pRSETA/TRX - SK and popout.
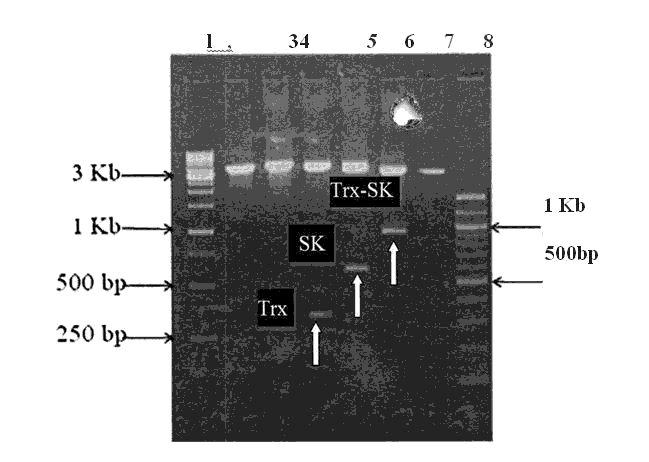
Figure 3: Agarose gel electrophoresis of PCR product for Trx, Sk and Trx-Sk ( Annealing Temp. 55°C)
This figure shows the PCR product for Trx, SK and Trx-SK respectively. PCR recations were carried out using primers (annealing temperature 55°C ). The PCR products were analyzed in 0.8% agarose gel. Lane 1: lKb Marker, Lane 2: pRSETA/ TRX - SK Ndel singlecut, Lane 3: pRSETA/ TRX - SK EcoRI singlecut, Lane 4: pRSETA/ TRX- SK Ndel + BamHI Doublecut, Lane5: pRSETA/ TRX - SK BamHI + EcoRI Doublecut, Lane 6: pRSETA/ TRX- SK Ndel + EcoRI Doublecut, Lane 7: pRSETA Ndel + SK DoubleCut and Lane 8:100bp Marker. The clone transformed in BL21 (DE3) and GJl 158 were confirmed by pop out check (Figure 5.4) an DNA sequencing[3].
The Sk gene was amplified using gene specific primers, ontaining BamHl and EcoRl restriction sites in the forward and reverse primers respectively. The amplicons were purified and then subjected to restriction digestion by BamHl and EcoRl restriction enzymes. Meanwhile the expression vectors, pRSETA were prepared for ligation by digestion with BamHl and EcoRl enzymes and subsequently purified with gel elution. The purified amplicons were ligated with the linearized vectors (pRSETA) using T4 DNA ligase.
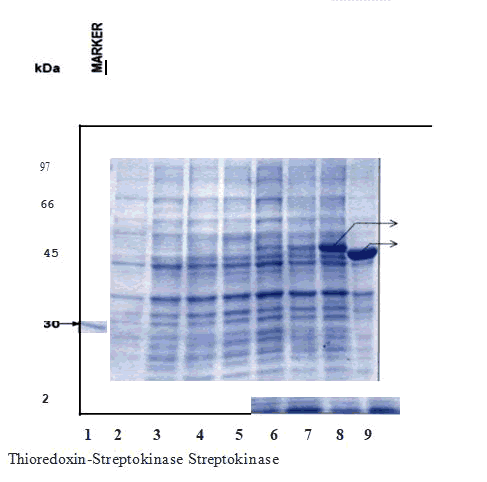
Figure 4: Expression of pRSETA -Trx-Sk in BL21 (DE3).
The soluble form of recombinant Thioredoxin- Streptokinase fused protein was purified using anion exchange chromatography. The soluble form of protein was loaded on DEAE-Sepharose column at Ph 8.0 and eluted with a linear gradient of NaCl. DEAE-Sepharose column of bed volume (10cm x 1.5cm ) was equilibrated with 50 mM Tris HCl buffer, pH 8.0, the soluble form of protein solution (pH adjusted to 8.0) was loaded on to this column at a flow rate of 1 mL/min. The column was washed with four column volumes of equilibration buffer and then the bound protein was eluted with a linear gradient of NaCl (0 - 1.0 M NaCl) at a flow rate of 2 mL/min. The absorbance was measured online with AKTA FPLC UV monitor. The peak fractions were analyzed by SDS PAGE and coomassie staining[4].
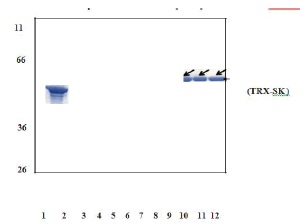
Figure 5: SDS-PAGE analysis of purified recombinant TRX-SK by Anion exchange chromatography.
The peak fractions containing activity were pooled and lyophilized. The purified samples were confirmed with western blot analysis (Figure 5.7) and bioassay.
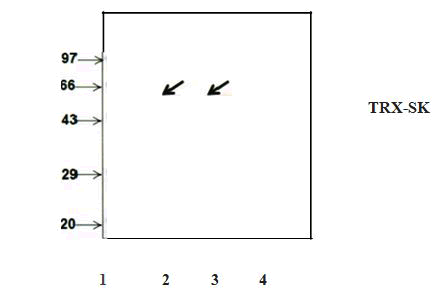
Figure 6: Western blot analysis of purified recombinant TRX-SK.
Appearance of single band corresponding to the molecular weight of 59 kDa after challenging the blot containing purified sample with antibody against streptokinase confirms the homogenous nature of purified protein.
Bioassay for thioredoxin-insulin reduction assay
Thioredoxin was shown to catalyze the reduction of insulin disulfides by dithiothreitol. rate of insulin reduction spectrophotometrically at 650 nm at 25°C as turbidity formation from the precipitation of the free insulin chain. The assay mixture contained 100 mM potassium phosphate, 2 mM EDTA (pH 7.0), 0.1? mM insulin (0.75 mg/ml), and recombinant thioredoxin. The reaction was initiated by the addition of 0.33 mM dithiothreitol (DTT). The implication of the dithiol-disulfide oxidoreductase activity of thioredoxin for the regulation of enzyme activities by thiol oxidation-reduction control is used in this assay (Holmgren 1979).
Table 1: Insulin reduction assay of recombinant TRX-SK.
| Time | Bl | B2 | Positive | Purified |
|---|---|---|---|---|
| ( £e.,Or'1l> ) | ||||
| 0 | 0.043 | 0.04 | 0.076 | 0.045 |
| 5 | 0.043 | 0.04 | 0.078 | 0-049 |
| 10 | 0.043 | 0-040 | 0.085 | 0.051 |
| 15 | 0.043 | 0.04 | 0.093 | 0.055 |
| 20 | 0.043 | 0.04 | 0.101 | 0.059 |
| 25 | 0.044 | 0.042 | 0.105 | 0.066 |
| 30 | 0.043 | 0.041 | 0.11 | 0.075 |
| 35 | 0.044 | 0.042 | 0.12 | 0.106 |
| 40 | 0.043 | 0.041 | 0.127 | 0.114 |
| 45 | 0.043 | 0.041 | 0.139 | 0.122 |
| 50 | 0.043 | 0.041 | 0.151 | 0.127 |
| 55 | 0.043 | 0.041 | 0.155 | 0.141 |
| 60 | 0.043 | 0.041 | 0.158 | 0.145 |
| 65 | 0.043 | 0.041. | 0.168 | 0.152 |
| 70 | 0.043 | 0.041 | 0.17 | 0.161 |
| 75 | 0.043 | 0.042 | 0.171 | 0.17 |
| 80 | 0.044 | 0.042 | 0.173 | 0.178 |
| 85 | 0.044 | 0.042 | 0.175 | 0.18 |
| 90 | 0.044 | 0.042 | 0.178 | 0.182 |
| 95 | 0.044 | 0.042 | 0.179 | 0.184 |
| 100 | 0.044 | 0.042 | 0.184 | 0.187 |
| 105 | 0.044 | 0.042 | 0.186 | 0.191 |
| 110 | 0.044 | 0.042 | 0.187 | 0.194 |
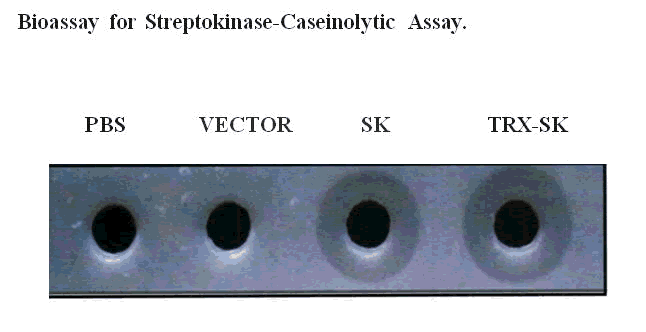
Figure 7: Caseinolytic assay of the recombinant TRXSK.
The proteins were estimated using Bradford's assay and about 5µg of the samples were loaded into the wells and kept at 37°C. Clear zones were observed around the wells aft ......... few hours of incubation showing the retention of lytic activity by the recombinant protein.
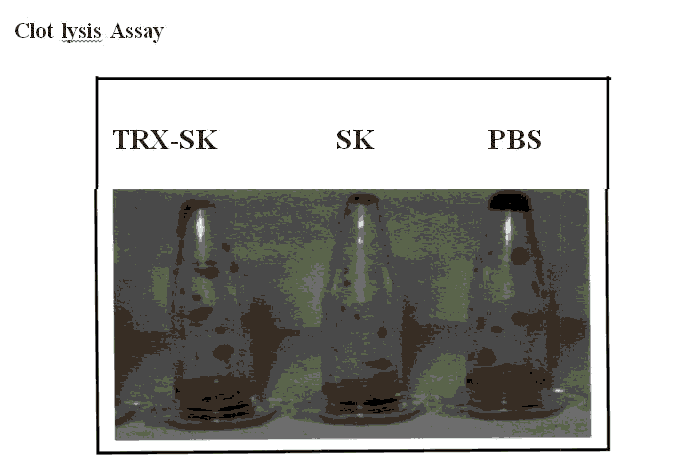
Figure 8: Clot lysis assay of the recombinant TRX-SK
Table 2: Data of Clot lysis assay for streptokinase and hybrid Thioredoxin Streptokinase.
| Patient name | Age | Sex | Time of clot; lysis (hours) | |
|---|---|---|---|---|
| SK | TRX-SK | |||
| Annadurai | 41 | M | 1.3 | 1.15 |
| Adhilakshmi | 40 | F | 1.3 | 1.14 |
| Sumathi | 38 | F | 1.3 | 1.15 |
| Radha | 37 | F | 1.31 | 1.16 |
| Ramalingam | 42 | M | 1.3 | 1.15 |
| Ganasundari | 41 | F | 1.31 | 1.15 |
| Murugammal | 40 | F | 1.31 | 1.15 |
| Murugammal | 39 | F | 1.3 | 1.15 |
| Vijaya | 42 | F | 1.3 | 1.15 |
| Rajalakshmi | 40 | F | 1.3 | 1.14 |
| Bharathi | 38 | M | 1.3 | 1.15 |
| Subramani | 41 | M | 1.31 | 1.15 |
| Fathima | 40 | F | 1.31 | 1.14 |
| Manoharan | 60 | M | 1.32 | 1.15 |
| Dhanam | 56 | F | 1.3 | 1.15 |
| Saradha | 42 | F | 1.3 | 1.14 |
| Kandhasamy | 40 | M | 1.3 | 1.14 |
| Srinivasan | 38 | M | 1.3 | 1.15 |
| Krishnan | 63 | M | 1.31 | 1.15 |
| Prema | 40 | F | 1.32 | 1.15 |
Statistical analysis
The age of the subjects was ranging from 37-63 years with mea ± SE as 42.90 ± 1.6 years. 85% were less than 50 years and 15% greater than 50 years.
The Clot lysis activity of Streptokinase for age less than 50 years was observed to be 1.3± 0.001 (mean± SE) and for age above 50 years 1.31± 0.005(mean± SE) yielding a statistically insignificant t value of 1.39(p=0. l 79).
The Clot lysis activity of Thioredoxin-Streptokinase for age less than 50 years was observed to be 1.14± 0.00l(mean± SE) and for age above 50 years 1.15± 0.000 (mean± SE) yielding a statistically insignificant t value of 0.709(p=0.488).
Table 3: Statistical analysis of clot lysis time with age.
| PROTEIN | AGE(years) | CLOTLYSISTIME (Hours) | T value | P value |
|---|---|---|---|---|
| STREPTOKINASE | <50 | 1.30 ± 0.001 I | 1.39 | 0.179 |
| >50 | 1.31 ± 0.005 | |||
| THlOREDOXIN- STREPTOKINASE | <50 | 1.14 ± 0.001 | 0.709 | 0.488 |
| >50 | 1.15 ± 0.000 |
Table 4: Statistical analysis of the clot lysis time with sex.
| PROTEIN | Sex | T value | P value | |
|---|---|---|---|---|
| Male Female | ||||
| STREPTOKJNASE | 1.30 ± 0.002 | 1.30 ± 0.001 | 0 | 1 |
| THIOREDOX.IN- STREPTOKINASE | 1.14 ± 0.0012 | 1.14 ± 0.0017 | 0.513 | 0.614 |
Discussion
Streptokinase is a monomeric multi domain protein of 47 kDa produced by several hemolytic strains of streptococci. Streptokinase (SK) is a potent plasminogen activator with the widespread clinical use as a thrombolytic agent. Handling this status with a thrombolytic alone cannot provide a complete solution as the part of ischemia mediated free radical injury is left unattended. Thioredoxin, a REDOX protein forms the free radical scavenging system in the human body. It has the ability to reduce disulfide bonds that do not respond to other cellular reductants52• Furthermore, it has been demonstrated that thioredoxin reductively inactivates phospholipaseA2 which is one of the contributing factor in inflammation and is also known to promote angiogenesis. In research, thioredoxin can be used in the over expression of genes encoding economically valuable proteins and peptides. The thioredoxin_streptokinase fusion construct thus designed so as to develop a synergetic protein of higher potency in the management of myocardial infarction.
In order to achieve maximum expression, a highly efficient system like T7 was employed in pRSETA expression vector. The T7 expression vectors are designed for high level expression, simple cloning, specific detection and efficient purification of expressed proteins. These vectors contain strong T7 promoter which provides high level expression of the target protein53 and also the presence of polyhistidine allows the target protein to be expressed as fusion proteins which facilitates the single step purification using chromatography techniques[5].
High level expression is achieve- d in T7 expression systems because the T7 RNAP is more processive than E.coli RNAP and is dedicated to the transcription of gene of interest. The T7 RNAP gene is under the control of the lacUV5 promoter which can be induced by IPTG. Therefore the expression vectors contain one of the T7 promoters to which the recombinant gene will be fused. In this case TRX-SK gene was fused to an inducibile promoter allowing its transcription and translation during expression phase. However, disadvantages still exist, such as formation of inclusion bodies in the cell cytoplasm. The pRSETA vector replicate from PUC1 origin of replication which maintains a high copy number per cell. So the host that harbours pRSETA construct has high gene dosage of the target protein. The literature reports that using a high copy number plasmid for the expression of the target protein has no advantage over the plasmids that replicate from another origin of replication(such as pBR322). However, the plasmid copy number preferably replicates in a relaxed fashion. These multi-copy numbers are stably replicated and maintained under selective conditions and plasmid fre-e daughter cells are rare.
In this study, we have integrated thioredoxin with streptokinase and the expression of TRX-SK in E.coli was carried out at different temperatures (25°C, 30°C, 35°C and 37°C), different post induction time(3 hrs and 6 hrs) and optica: .. ensities (0.6 O.D and 1.0 O.D) in BL21 (DE3) and GJl 158 expression hosts. Hyper expression was achieved in GJl 158 at around 0.6 O.D. at a post induction temperature of around 30°C.
Inclusion body formation remains a significant barrier to gene expression in the cytosol, however inclusion bodies do offer several advantages. However, these are small consolation considering the arduous task of refolding the aggregated protein (Rudolph and Lilie 1996), the uncertainty of whether refolded protein retain its biological activity and the reduction in the yield of the refolded and purified protein to date, the precise physico-chemical parameters that contribute to the formation of inclusion bodies remain unclear 55 56 57 Inclusion bodies formation can be minimized by several methods, such as co expression of molecular chaperons, choosing appropriate host, decreased cultivation temperature, increasing aeration, medium composition, low inducer concentration, and induction at low cell densities58• In E. coli GJl158, the pro U promoter governs the expression of T7 RNA polymerase which is induced by addition of NaCl. Medium used for the growth of the strain is devoid of NaCl hence hypoosmotic, on induction by the addition of NaCl b mes hyperosmotic. Under such conditions of hyperosmolarity, osmo responsive proteins that are involved in praline transport are induced and the intracellular levels of osmoprotectants such as praline, glycinebetaine have been known to increase in E. coli 59. Such osmoprotectants have been known to improve protein folding. Improperly folded proteins are toxic to the host and hence there is degradation of the proteins (Kagawa and Cao 2001, Savvas Makrides 1993). Improper folding may be related to improper formation of disulphide bonds. The E. coli GJl 158 has been shown to improve folding and activity of recombinant proteins60 case of E. coli GJl 158, the environment of osmoprotectants like praline, glycinebetaine may help in expression by conferring better folding and stability.
Expression of the soluble protein optimized and large amount of the soluble protein around more than 50% of the soluble protein was obtained at around 30°C (3 hrs induced samples) at 0.6 O.D. in GJl 158. The soluble fraction was subjected to anion exchange chromatography to yield a single band in SDS PAGE analysis and was further confirmed by western blot. The dithiol-disulfide oxidoreductase activity of thioredoxin for the regulation of enzyme activities (Holmgren 1989) can be assayed by the catalysis of the reduction of insulin disulfides with dithiot teitol. In this reaction, the rate of insulin reduction observed spectrophotometrically at 650 nm at 25°C as turbidity formation from the precipitation of the free insulin chain.[26] The caseinolytic and the clot lysing activity of the recombinant protein is compared with that . of the control Streptokinase. The positive clot lysis assay and caseinolytic assay confirms the retention and enhanced biological activity of the constituent Thioredoxin and Streptokinase of the recombinant fusion protein. Thus it was attempted to generate a recombinant protein with both antioxidant and thrombolytic property taking care of both the thrombotic status and the ischemia mediated free radical injury and promoting angiogeogenesis in the thrombo-embolic disorders like Myocardial Infarction, Cerebrovascular accidents etc.
Conclusion
Cloning the thrombolytic streptokinase along with a redox protein like thioredoxin gives the benefit of free radical scavenging effect on the ischemia mediated free radical injury of the vascular endothelium and surrounding cells. This can find potential usage in thrombo-embolic disorders like Myocardial Infarction, Cerebro Vascular Accidents etc... where the ongoing oxidative damage and reperfusion injuries can be handled along with thrombolysis.. The following were achieved by c rying out this work: Amplification of human Thioredoxin gene from lung cancer cell line A549. Cloning of human Streptokinase in pRSET A at Barn HI and Eco RI site and Fusion of human thioredoxin in the recombinant clone pRSET Al SK at Ndel and Barn HI site.
Expression of the hybrid streptokinase in BL21 (DE3) and GJ1158 and Optimization of expression at various inducer concentrations, different temperature, medium and hosts. Solubility studies & Purification studies. Bioassay for the recombinant protein in the form of Insulin reduction assay, Caseinolytic assay and Clot lysis assay were performed. The results showed that the recombinant protein is more effective than plain Streptokinase.
Acknowledgements
The encouragement and support from Bharath University, Chennai, is gratefully acknowledged. For provided the laboratory facilities to carry out the research work.
Declaration of Conflict of Interest
The authors declare no conflict of interest.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
References
- Rios-Steiner JL, Schenone M, Mochalkin I, et al. Structure and binding determinants of the recombinant kringle-2 domain of human plasminogen to an internal peptide from a group A Streptococcal surface protein. J mol bio 2001;308:705-719.
- Torréns I, Reyes O, Ojalvo AG, et al. Mapping of the antigenic regions of streptokinase in humans after streptokinase therapy. BBRC 1999;259:162-168.
- Koide A, Suzuki S, Kobayashi S. Preparation of polyethylene glycol-modified streptokinase with disappearance of binding ability towards anti-serum and retention of activity. FEBS letters 1982;143:73-76.
- Avilan L, Yarzabal A, Jürgensen C, et al. Cloning, expression and purification of recombinant streptokinase: partial characterization of the protein expressed in Escherichia coli. BJMBR 1997;30:1427-30.
- Azuaga AI, Dobson CM, Mateo PL, et al. Unfolding and aggregation during the thermal denaturation of streptokinase. Euro J biochem 2002;269:4121-4133.
Author Info
C.Revathy and A.J. Manjula Devi*
Department of Biochemistry, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaCitation: C.Revathy , A.J. Manjula Devi Attempts to Develop a Recombinant Protein with Antioxidant and Thrombol Ytic Activity-In-Vitro Study, J Res Med Dent Sci, 2021, 9(9): 1-8
Received: 01-Dec-2021 Accepted: 15-Dec-2021 Published: 22-Dec-2021
