Research - (2025) Volume 13, Issue 5
Comparative Assessment of Effectiveness of Juglan Regia and Cinnamon Bark Mouthwash for the Treatment of Chronic Periodontitis-A Clinical Trial
Chanchal Katariya* and Jaiganesh Ramamurthy
*Correspondence: Chanchal Katariya, Department of Periodontics, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, India, Email:
Abstract
Background: An antiplaque agent with minimal side effects that can be used as an effective adjunct to mechanical plaque control is needed. The current study is designed to evaluate the efficacy of juglan regia and cinnamon bark mouthwash in reduction of plaque and gingivitis. Methods: Thirty individuals with chronic generalized periodontitis were randomly assigned to three groups: 1) Group A, placebo mouthwash; 2) Group B, herbal mouthwash; and 3) group C, chlorhexidine (CHX) mouthwash. All individuals were instructed to rinse with their respective mouthwash twice daily. 1) Probing depth (PD); 2) Gingival index (GI); 3) Oral hygiene indexsimplified (OHIS) was measured at baseline and 10 days. Results: All three groups showed gradual reduction in PD, GI, and OHIS levels from baseline to 10 days. A significant difference was noticed with respect to reduction in PD, GI and OHIS in group I compared with groups II and III. Mean value of 2.727 ± 0.54 at baseline and 0.732 ± 0.35 after 10 days’ post-operative for gingival index was seen. However, no significant differences were found between groups II and III for any parameters at any time intervals. Conclusions: Herbal mouthwash was found to decrease inflammatory parameters from baseline to follow-up intervals. Because improvement in periodontitis was comparable with that of CHX mouthwash, Herbal mouthwash can be considered a potential therapeutic agent in the treatment of periodontitis.
Keywords
Chlorhexidine, Juglans regia, Cinnamon, Periodontitis
Introduction
Periodontitis is defined as an infectious disease resulting in inflammation within the supporting tissues of the teeth, progressive attachment and bone loss and is characterized by pocket formation and/or recession of the gingiva. Inadequate oral hygiene is the most common cause of gingivitis, the mildest type of periodontal disease [1]. Plaque and calculus are one of the major etiological factors for progression of periodontal disease. Mechanical plaque prevention measures are used as a cornerstone to improve oral hygiene. Since mechanical plaque control techniques take time and require motivation and expertise to be effective, antimicrobial agents have been widely used as a supplement to mechanical cleaning. One of the most common aids used is mouthwash. Chlorhexidine is considered the gold standard for the same. It is the most effective and safe agent for plaque control. Chlorhexidine (CHX) is a cationic biguanide microbicide with a broad spectrum of activity against bacteria and fungi. It has broad spectrum antibacterial activity with substantivity of 8-12 hours [2]. Even though it is considered as the gold standard in plaque removal, it has some drawbacks, such as altered taste sensation and tooth and tongue staining, which restrict its use. Because of chlorhexidine's restrictions, natural oral hygiene products have been developed. It is important to bring alternative products which has similar efficiency and can even overcome the above mentioned drawbacks.
Since ancient times, walnuts (Juglans regia L., Juglandaceae) have been used in human nutrition. The walnut tree is native to Central Asia, the western Himalayan chain, and Kyrgyzstan, but it was first cultivated in Europe around 1000 BC [3]. The edible portion of the fruit (the seed or kernel) is eaten, fresh or toasted, alone or in other edible items, making walnut a high-value crop for the food industry. It is well-liked and respected around the world for its dietary, wellness, and sensory qualities. Bark of the walnut is proved to have lots of medicinal properties. The flavonoids, phenolic acids, and associated polyphenols found in Juglans regia are abundant. Walnut-supplemented diets have been shown to have a variety of health benefits as compared to control diets in several studies. Despite the fact that phenolic compounds have no known nutritional function, their antioxidant, antiatherogenic, anti-inflammatory, and antimutagenic properties may make them beneficial to human health [4,5]. Cinnamon is a spice obtained from the inner bark of several tree species from the genus Cinnamomum. Ground cinnamon is made up of approximately 11% water, 81 percent carbs (including 53 percent dietary fiber), 4 percent protein, and 1% fat. Ground cinnamon contains a lot of calcium (100 percent of the Daily Value (DV)), iron (64 percent DV), and vitamin K in a 100 gram serving (30 percent DV) [6]. Cinnamonaldehyde is one of the major phytochemicals of cinnamon. Cinnamon has antibacterial, antiinflammatory, and antifungal properties due to the presence of cinnamaldehyde, an aromatic aldehyde, which is highly electronegative and interferes in biological processes involving electron transfer and reacts with nitrogen containing components [7].
Natural products are now preferred by a large part of the population. These products have been shown to be a good substitute for synthetic chemical substances in the prevention of periodontal disease. The aim of the present study is to compare and assess the effectiveness of Juglan regia and cinnamon bark mouthwash against chlorhexidine for the treatment of chronic periodontitis.
Material and Method
This study was conducted in Saveetha Dental College and Hospital, Chennai after ethical clearance from the institutional ethical committee. Patients were selected from the Outpatient Department of Periodontics. The present study is a simple randomised controlled clinical trial. Total of 30 patients were included in the study, 10 in each group. Sample size was confirmed using Gpower with 85% power. Randomisation was done using computer generated codes and the evaluator will assign it to different groups. Study subjects and operators were blinded in the study.
Group distribution
Patients were randomly assigned to Group A, Group B and Group C and an informed consent was obtained from all the patients.
Group A: Patients were treated by scaling alone with placebo mouthwash (saline).
Group B: Patients were treated by scaling followed by use of herbal mouthwash.
Group C: Patients were treated by scaling followed by use of chlorhexidine mouthwash.
Inclusion criteria
• Patients between the age group of 20 and 45 are considered.
• There should be a minimum of 20 teeth in the dentition, with no clear signs of untreated caries.
• Patients diagnosed with Grade II and Grade III periodontitis according to AAP classification (2017).
• Patients who were able to give informed consent and participate in the study were chosen.
Exclusion criteria
• Subjects that have taken antibiotics or some other medications in the previous three months.
• Lactating mothers and pregnant women.
• Patients that is medically ill.
• Chronic Smokers.
• Patients with partial dentures, restorations, or bridges.
• Orthodontic appliance on a patient.
• Any patient who has previously been allergic to chemical or herbal products.
• Patients who have undergone any periodontal treatment, for the past six months.
Preparation of herbal mouthwash
Mouthwash preparation was done under the supervision of the pharmacology department. Barks of cinnamon and Juglans regia were crushed into fine powder. 50gms of each powder was mixed with 100ml of distilled water. It was reduced into half with boiling. The reduced mixture was filtered using filter paper. The extract was then mixed with 2 drops of peppermint oil for flavour, 2ml of glycerine as sweetener and 0.5g sodium benzoate as preservative and distilled water.
Clinical parameters
Prior to scaling, patients were subjected to assessment of the following clinical parameters.
• Simplified oral hygiene index (Greene and Vermillion, 1964)
• Gingival index (Loe and Silness, 1963)
• Probing depth
After recording the clinical parameters in selected patients, a thorough scaling was carried out using ultrasonic scalers in Group A and Group B and Group C patients. The recording of clinical parameters was carried out by the main investigator and treatment was carried out by co-investigator. The clinical parameters were assessed on day ‘0’ and 10th day.
Directions for mouthwash usage
Following the main investigator's recording of clinical criteria, patients in Group A, Group B and Group C were advised to use mouthwash 15 ml for 30 seconds twice daily after food, i.e. after breakfast and dinner, in order to minimize the bias following brushing in both groups. Mouthwashes often serve as a common and easy delivery method that removes bacteria and rinses the food debris from the mouth. Oral hygiene instructions were given to all patients.
Statistical analysis
The Kolmogorov and Smirnov tests were used to check for normality in the results. Non-parametric experiments were used where the data were not usually distributed. Data were analyzed using the student's t-test for normally distributed data and Mann-Whitney U-test for skewed data; comparison of before and after, Group A and Group B and Group C. A two tailed P value less than 0.05 was considered as significant. Data analysis was carried out using the SPSS version 23.
Result
Table 1 presents demographic data of the study population. There were no statistically significant differences in mean age and sex of individuals in the three groups. Table 2 presents mean ± SD values of GI, PD, and OHIS at different time intervals. There were no significant differences between groups A, B, and C in B/L scores of PI, GI, and OHIS. All three groups showed gradual decrease in PD, GI, and OHIS values from B/L to follow-up visits at 10 days. A significant reduction in PD, GI, and OHIS scores was observed for groups B and C at all-time intervals. There was a significant difference with respect to reduction in PD, GI, and OHIS in group A (placebo group) compared with group B (Test group) and group C (CHX group) (Figures 1 to 3).
| Parameters | Placebo | Herbal | CHX |
|---|---|---|---|
| Mean & SD | 9.04 ± 6.2 | 10.4 ± 5.9 | 9.07 ± 6.3 |
| Age | 25-40 | 25-45 | 30-45 |
| Gender (m/f) | 44657 | 44686 | 44686 |
Table 1: Basic demographic details of the subjects.
| Parameter | Interval | Placebo | Herbal | CHX | pValue |
|---|---|---|---|---|---|
| PD | Baseline | 4.756 ± 1.55 | 4.787 ± 1.34 | 4.704 ± 1.43 | <0.0001 |
| 10 days | 1.856 ± 0.56 | 1.772 ± 0.45 | 1.723 ± 0.76 | ||
| GI | Baseline | 2.796 ± 0.55 | 2.727 ± 0.54 | 2.707 ± 0.41 | <0.001 |
| 10 days | 0.876 ± 0.76 | 0.732 ± 0.35 | 0.752 ± 0.72 | ||
| OHIS | Baseline | 2.786 ± 0.12 | 2.677 ± 0.22 | 2.777 ± 0.21 | <0.001 |
| 10 days | 0.878 ± 0.98 | 0.745 ± 0.65 | 0.722 ± 0.65 | ||
Table 2: Values (mean ± SD) for PD, GI, and OHI-S at time intervals
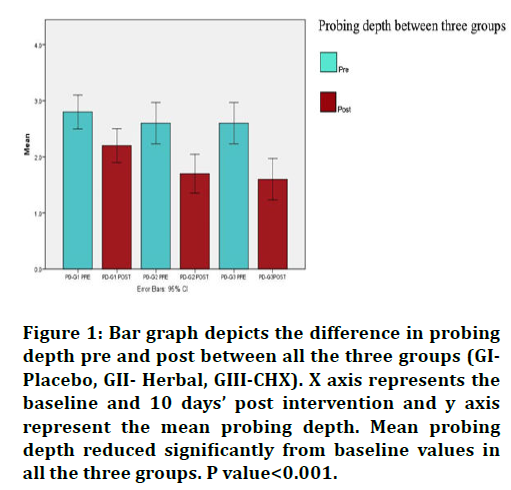
Figure 1.Bar graph depicts the difference in probing depth pre and post between all the three groups (GIPlacebo, GII- Herbal, GIII-CHX). X axis represents the baseline and 10 days’ post intervention and y axis
represent the mean probing depth. Mean probing depth reduced significantly from baseline values in all the three groups. P value<0.001.
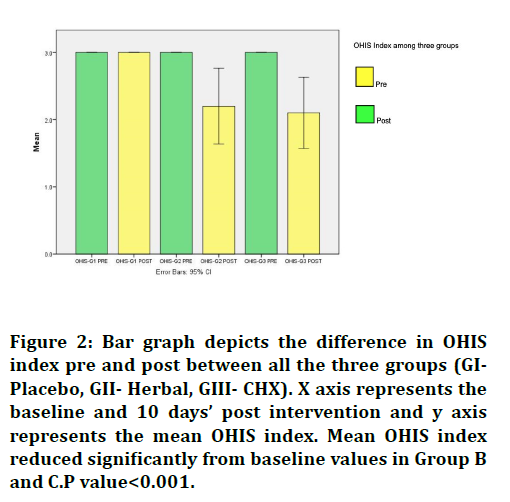
Figure 2.Bar graph depicts the difference in OHIS index pre and post between all the three groups (GIPlacebo, GII- Herbal, GIII- CHX). X axis represents the baseline and 10 days’ post intervention and y axis
represents the mean OHIS index. Mean OHIS index reduced significantly from baseline values in Group B and C.P value<0.001.
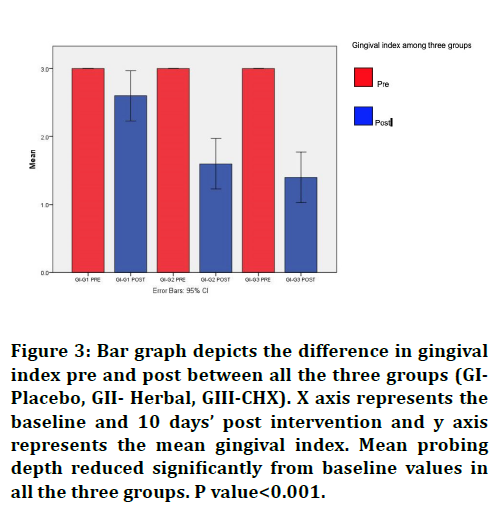
Figure 3.Bar graph depicts the difference in gingival index pre and post between all the three groups (GIPlacebo, GII- Herbal, GIII-CHX). X axis represents the baseline and 10 days’ post intervention and y axis
represents the mean gingival index. Mean probing depth reduced significantly from baseline values in all the three groups. P value<0.001.
Discussion
In the present study, the gingival index, OHIS index and probing depth by the test group was significantly lower when compared to the placebo group, and similar to CHX group. Even the placebo group also showed reductions in OHIS, PD and GI scores, which can be attributed to the Hawthorne effect i.e. individuals become more aware regarding oral hygiene maintenance and improve their oral hygiene status by awareness that they are part of any clinical trial [8].
Many studies, concluded that Juglan regia shows antimicrobial activity against Staphylococcus epidermidis MTCC-435, Bacillus subtilis MTCC-441, Staphylococcus aureus and Proteus vulgaris MTCC-321, Pseudomonas aeruginosa MTCC-1688, Salmonella typhi, Shigella dysenteriae, Klebsiella pneumonia and Escherichia coli. It is said to have antioxidant properties against scavenging effect on DPPH (2,2-diphenyl-1-picrylhydrazyl) and hydroxyl radicals [9,10]. A Study done in 2014, showed that cinnamon at the concentration of MIC=750 mg/ml had the inhibitory effects of bacteria and at the concentration of MIC=1500 mg/ml had killing effect that the course of this work compared with commonly used antibiotics (amoxicillin, metronidazole), was much weaker against porphyromonas gingivalis [11]. There was significant reduction in candidal carriage between baseline and 3 weeks in diabetic patients and in nondiabetics after using cinnamon mouthwash Results of the study proved the potential of cinnamon as an antifungal agent [12]. Cinamonaldehyde is proved to be the principle component of cinnamon bark, which has antimicrobial activity against porphyromonas gingivalis [13].
For decades, CHX has been considered gold standard mouthwash. Mainly due to its substantivity property and its antimicrobial activity against periodontopathic bacteria [14]. As everything has two sides, CHX all has some drawbacks, such as an unpleasant taste and tooth discoloration, limit its long-term usage and necessitate the use of herbal agents, which may be equally successful with less drawbacks. CHX had a positive effect on the periodontal parameters assessed, especially PD, even after the longest follow-up time (180 days) [15]. The results of a meta-analysis for CAL were favourable for CHX but statistically not significant (P > .05). For PD, we noticed an improvement after the 3 follow-up periods, but it was statistically significant at the intervals of 40 to 60 and 180 days [15] Three trials had the primary objective of evaluating the influence of CHX on PD and CAL in patients with CP who were undergoing mechanical therapy. Despite the statistically significant improvement in PD (at 40-60 and 180 days), the clinical difference was slight [16-18].
The use of herbal preparations in the treatment of periodontal disease and overall health is on the rise, and test mouthwash, with effectiveness comparable to CHX and less side effects, may be a better option for gingival inflammation and periodontal disease reduction. To validate the results of this short term clinical trial, more long-term prospective trials with larger sample sizes are required.
Conclusion
Within the study's limitations, herbal mouthwash was found to reduce inflammatory parameters and, as a result, improve gingivitis. The results were equivalent to CHX mouthwash, which has long been considered the gold standard in the treatment of gingivitis and periodontitis. Herbal mouthwash may be considered a possible therapeutic agent in the treatment of periodontal disease, according to the findings.
References
- Kumar P, Ansari SH, Ali J. Herbal remedies for the treatment of periodontal disease-a patent review. Recent Pat Drug Deliv Formul 2009; 3:221-228.
- Varoni E, Tarce M, Lodi G, et al. Chlorhexidine (CHX) in dentistry: State of the art. Minerva Stomatol 2012; 61:399-419.
- Guerrero L, Romero A, Gou P, et al. Perfil sensorial de diferentes muestras de nuez (Juglans regia L.)/Sensory profiles of different walnuts (Juglans regia L.). Food Sci Technol Int 2000; 207-216.
- Anderson KJ, Teuber SS, Gobeille A, et al. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr 2001; 131:2837-2842.
- Carvalho M, Ferreira PJ, Mendes VS, et al. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol 2010; 48:441-447.
- Olusola-Makinde OO, Makinde OS. COVID-19 incidence and mortality in Nigeria: Gender based analysis. Peer J 2021; 9:e10613.
- Kasim K, Hassan SS, Gulnaz AR. Antimicrobial efficacy of bark extracts of" Randia uliginosa" on oral pathogens: Research study. J Evol Med Dent Sci 2013; 2:4260-4264.
- McCarney R, Warner J, Iliffe S, et al. The Hawthorne Effect: A randomised, controlled trial. BMC Med Res Methodol 2007; 7:1-8.
- Rather MA, Dar BA, Dar MY, et al. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomed 2012; 19:1185-1190.
- Oliveira I, Sousa A, Ferreira IC, et al. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol 2008; 46:2326-2331.
- Abdul Azeez AR, Mahmood MS, Mahmood W. The effect of chlorhexidine mouth wash and visible blue light on Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis of patients with chronic periodontitis (an in–vitro study). IOSR J Dent Med Sci 2014; 13:1-7.
- Gujjari S. Cinnamon mouthwash as an anti-fungal agent in treatment of diabetic patients with chronic periodontitis. Adv Dent Oral Health 2018; 8:555728.
- Wang Y, Zhang Y, Shi YQ, et al. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb Pathog 2018; 116:26-32.
- Ramamurthy JA, Mg V. Comparison of effect of hiora mouthwash versus chlorhexidine mouthwash in gingivitis patients: A clinical trial. Asian J Pharm Clin Res 2018; 11:84-88.
- da Costa LF, Amaral CD, da Silva Barbirato D, et al. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J Am Dent Assoc 2017; 148:308-318.
- Faveri M, Gursky LC, Feres M, et al. Scaling and root planing and chlorhexidine mouthrinses in the treatment of chronic periodontitis: A randomized, placebo‐controlled clinical trial. J Clin Periodontol 2006; 33:819-828.
- Feres M, Gursky LC, Faveri M, et al. Clinical and microbiological benefits of strict supragingival plaque control as part of the active phase of periodontal therapy. J Clin Periodontol 2009; 36:857-867.
- Feres M, Soares GM, Mendes JA, et al. Metronidazole alone or with amoxicillin as adjuncts to non‐surgical treatment of chronic periodontitis: A 1‐year double‐blinded, placebo‐controlled, randomized clinical trial. J Clin Periodontol 2012; 39:1149-58.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Chanchal Katariya* and Jaiganesh Ramamurthy
Department of Periodontics, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, IndiaCitation: Chanchal Katariya, Jaiganesh Ramamurthy,AComparative Assessment of Effectiveness of Juglan Regia and Cinnamon Bark Mouthwash for the Treatment of Chronic Periodontitis-A Clinical Trial, J Res Med Dent Sci, 2022, 10(1): 519-523
Received: 10-Dec-2021, Manuscript No. JRMDS-21-46295; , Pre QC No. JRMDS-21-46295 (PQ); Editor assigned: 13-Dec-2021, Pre QC No. JRMDS-21-46295 (PQ); Reviewed: 27-Dec-2021, QC No. JRMDS-21-46295; Revised: 30-Dec-2021, Manuscript No. JRMDS-21-46295 (R); Published: 06-Jan-2022
