Research - (2022) Volume 10, Issue 1
Comparison of Antibacterial Efficacy of Annona Squamosa Mouthwash with Chlorhexidine for Children
Sumaia Hussein Ali and Zainab Juma Jafar*
*Correspondence: Zainab Juma Jafar, Department of Pediatric and Preventive Dentistry, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Controlling dental plaque is critical in the prevention and treatment of periodontal disease and dental caries. In addition to mechanical plaque removal techniques such as tooth brushing and flossing, the use of chemical and herbal mouthwashes is recommended because it is safe, effective and economic. Natural materials are increasingly being used in dentistry. Aim: This study will be conducted to investigate the antibacterial activity of Annona squamosa pulp mouthwash against Staphylococcus aureus and Escherichia coli in comparison with chlorhexidine. Materials and Methods: This microbiological in vitro study was conducted in the College of Dentistry/ University of Baghdad. Saliva samples were taken from children aged (7-9) years with the same socioeconomic and oral hygiene conditions, healthy child without any history of systemic medical condition. Saliva sample were obtained from the children and taken immediately to laboratory for culturing on Mannitol salt agar and Macconkey agar and incubated aerobically for 24 hours at 37 C. Following their identification and after complete extraction of annona squamosa fruits, the bacteria were plated in Mueller-Hinton agar, and the antibacterial activity of the pulp extracts was assessed using the agar-well diffusion technique. Results: A. squamosa extract have effective inhibitory concentrations against tested bacteria except in concentration 0.5 mg/ml have no effect on E. coli, and inhibition zone increase when concentration increased until 10 mg/ml have highest inhibition effect when comparable with chlorhexidine. Conclusion: A. Squamosa extract is effective as chlorhexidine mouthwash for children.
Keywords
Annona squamosa, Mouthwash, Chlorhexidine, Antibacterial, Children
Introduction
Oral health is a standard condition for the wellbeing of oral and related tissues that allow a person to do daily activities as eating, drinking, speaking and other social activities without active disease, or annoyance or confusion, which contribute to overall wellbeing [1]. In addition, the state of the teeth and periodontal status has an effect on the overall health [2]. The dental plaque is the principal cause of periodontal disease that can cause gingival tissue and periodontal attachment apparatus to be destroyed [3].
Oral hygiene begins with mechanical teeth brushing and dental flossing. However, with the extensive prevalence of periodontal disease, these mechanical procedures are insufficient for the majority of adults and children [4]. As a result, mouthwash is an auxiliary protocol to mechanical methods that prevent plaque accumulation [5].
Antiseptic and anti-plaque mouthwashes, according to some mouthwash makers, eliminate the bacteria that cause cavities, gingivitis, and bad odor. Many commercially available mouthwashes are used widely for this purpose, such as Chlorhexidine gluconate (CHX) which is the most common antiplaque and antibacterial mouthwash, and deemed the gold standard of mouthwashes because it exhibits a wide spectrum of antimicrobial activity against Gram positive and Gram negative bacteria, fungi and some viruses [6].
Now, herbal substances have been utilized for traditional medicine for thousands of years all across the world. Several of them have antibacterial and anti-inflammatory effects. They have been proven to be effective in human medicine [7]. An effort is being made to describe some of the readily available herbs and plants, as well as some fruits, that can be used as excellent mouthwashes by anyone. It may be possible to overcome several common dental diseases if people can employ and promote such cost-effective techniques of preserving oral health that are also free of any negative side effects.
Staphylococcus aureus (S. aureus) is a prominent human bacterial pathogen that causes a variety of clinical symptoms. It can be found in the environment, as normal flora on the skin and the nasopharynx of the healthiest humans as well as in the oral cavity in spite of being considered as a temporary reservoir [8]. It can be associated with multiple oral infections like angular chelitis, dry mouth, denture stomatitis, and acute dentoalveolar/ gingival/periodontal abscesses, as well as cause systemic diseases when found in the oral cavity like infective endocarditis and rheumatoid arthritis, etc. [9].
Escherichia coli (E. coli) are type of the Gram-negative bacteria that is commonly seen in humans' large intestines as normal flora living in a symbiotic relation in conversation with the host [10]. However, when their environment changes or immunity is compromised, it becomes opportunistic bacteria in respiratory tract and causes pathological diseases as well as enhanced oral mucosal infections such as angular cheilitis, dry socket and denture stomatitis [3]. E. coli and S. aureus are considered transient (non-resident) bacterium in the oral cavity rather than a part of oral flora and colonize the hospitalized patient's teeth [11]. Transient oral microorganisms are frequently linked to oral mucosal infections. Many organism resistant to synthetic drugs and the side effect of it encourage the search about antimicrobial agents from natural products of plants.
Annona (family Annonaceae), is one of the biggest tropical tree, shrub, and liana families with approximately 2300 species distributed worldwide, A. squamosa is one of these species that is a small evergreen tree with a height of 6-8 meters and its fruit is round, conical, or oval with a wonderfully scented pulp that is similarly white with blackish seeds [12]. Flavonoids, alkaloids, diterpenoids, essential oils, glycoside, tannins, sterols and acetogenins are phytochemical compounds have all been derived from the different parts in various solvents of these plants and it has traditionally been used to treat a variety of ailments and effective as antioxidant, antifungal, antidiabetic and antibacterial [12,13]. The existence of various secondary metabolites in medicinal plants may be responsible for their curative properties. Because of their elevated alkaloid content, A. squamosa plants have historically been used as an antibacterial drug for a variety of diseases, including bacterial infections like ulcers, dysentery, and boils [14]. Before starting of this study, we did not find any study that assessed the effect of A. squamosa pulp as mouthwash on oral healthy children, so this study try to fill the gap in knowledge to assess the effectiveness of A. squamosa mouthwash in children against Staphylococcus aureus and Escherichia coli in comparison with chlorhexidine.
Materials and Methods
Before beginning this in vitro study, the protocol was approved by the scientific committee in Department of Pediatric and Preventative Dentistry/College of Dentistry/ University of Baghdad, as well as approval from the Central Ethical committee in the same college.
Subjects selection
Twenty-one healthy children, aged 7-9 years were chosen to participate in the research to collect unstimulated salivary samples from which the selected bacteria were isolated. All of the patients' guardians signed a consent form. The children appeared to be in good health, had no history of systemic diseases and did not take antibiotics for the previous three weeks. They were instructed not to eat or drink anything other than water for one hour before the saliva collection procedure [15].
Calculation of the sample size
Using G power 3.1.9.7 (Program written by Franz-Faul, Universitatit Kiel, Germany) with power of study=80%, alpha error of probability=0.05, and partial eta square is 0.18 (large effect size) thus effect size of F is 0.469 (Large effect size), with 8 groups. The basic design used is completely randomized design.
Saliva collection
The children sit comfortably and 3-5 ml of unstimulated saliva was obtained in the morning between 9–11 am using a spitting procedure, by giving children basic steps in a simple way to spit the saliva by lowering the head and positioning the plane tube near to the lips to allow saliva to weep [16]. Getting the saliva collection tubes ready and transfer them to microbiological laboratory immediately.
Fruit’s sample obtaining and preparation
Fresh ripe A. squamosa fruit’s samples were purchased from a fruit shop in a local market in Baghdad, Iraq. The fruit was rinsed with distilled water to eliminate unwanted elements, and the fruit pulp was manually cleaned from the seeds and bark, afterwards packed in plastic bags and sent to a laboratory for extraction. Fresh pulp was used for the antibacterial analysis, which was then produced in an ethanolic extract.
Preparation of A. squamosa pulp extract
The organic solvent extract method was employed by the Ministry of Science and Technology, Baghdad, Iraq. The fruit pulp was extracted with ethanol and sonicated for 30 minutes at 60°C. Following that, the extract was filtered and concentrated using a rotary evaporator under vacuum equipment. The resulting extract was lyophilized in a lyophilizer and stored in airtight bags away from light until the time of analyses [17]. The extracted material was solved with dimethyl sulfoxide (DMSO) at various concentrations (0.5, 1, 2, 4, 6, 8, and 10 mg/ml).
Antibacterial analysis
The test organism; Staphylococcus aureus and Escherichia coli bacteria were isolated on their selective culture media: mannitol salt agar and Macconkey agar respectively by streaking, and identified by high power microscope and biochemical test. A 0.5 Mcfarland standard microbial suspension with about 108 colonyforming units per milliliter (CFU/mL) was utilized for this test.
On Mueller Hinton Agar MHA media, the antibacterial activity of seven concentrations of alcoholic extract of A. squamosa’ pulp (1/2, 1, 2, 4, 6, 8 and 10 mg/ml) were studied using the agar well technique 81 , compared to 0.12% chlorhexidine as a positive control.
Then 0.1 ml of activated S. aureus and E. coli bacteria inoculums were applied separately on MHA plates and kept at room temperature for 10 minutes.
In each agar plate, multiple wells of identical width and depth (6 mm) were produced, and 0.1 ml of the A. squamosal extract and chlorhexidine were tested in every well. After that, all of the produced plates were incubated for 24 hours at 37°C.
Lastly, using a ruler, the sizes of the inhibition zones of all tested concentrations were measured in millimetres and then converted to centimetres when making statistical results.
This method was carried out in the same way for two isolated bacteria and all of the trials were carried out in ten replicates for each concentration.
Statistical analysis
Data description, analysis and presentation were performed using Statistical Package for social Science (SPSS version 21) (Chicago, USA, Illinois). Shapiro Wilk test to test the normality distribution of the quantitative (continuous or discrete variable), Levene test to test the homogeneity of variance among groups.
One Way Analysis Of Variance (ANOVA) to test the difference between k independent groups with Games- Howell and Tukey HSD post hoc tests with level of significance at (0.05).
Results
Antibacterial effects against S. aureus
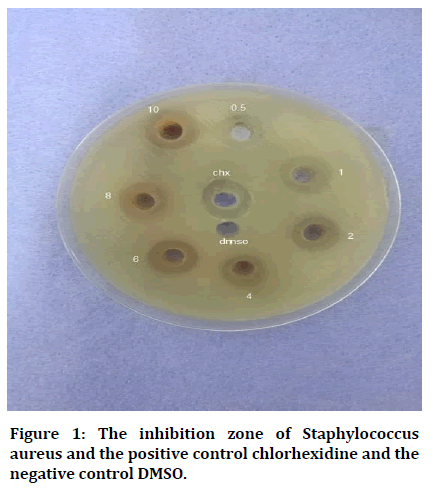
Antibacterial activity of all the seven concentrations of A. squamosa extracts was illustrated in (Table 1and Figure 1).
| Groups (mg/ml) | Mean | ± SD | ± SE | Minimum | Maximum | F | P value | ES |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 0.89 | 0.088 | 0.028 | 0.8 | 1 | 128.353 | 0 Sig. | 0.929 |
| 1 | 0.99 | 0.088 | 0.028 | 0.9 | 1.1 | |||
| 2 | 1.13 | 0.095 | 0.03 | 1 | 1.3 | |||
| 4 | 1.28 | 0.063 | 0.02 | 1.2 | 1.4 | |||
| 6 | 1.4 | 0.082 | 0.026 | 1.3 | 1.5 | |||
| 8 | 1.59 | 0.074 | 0.023 | 1.5 | 1.7 | |||
| 10 | 1.7 | 0.082 | 0.026 | 1.6 | 1.8 | |||
| CHX | 1.7 | 0.115 | 0.044 | 1.5 | 1.8 | |||
| Levene test=0.578, p value=0.771 NS | ||||||||
Table 1: Descriptive and statistical test of diameter inhibition zone (cm) of Staphylococci aureus among groups.
Figure 1: The inhibition zone of Staphylococcus aureus and the positive control chlorhexidine and the negative control DMSO.
The results show that diameter of inhibition zone of S. aureus increase with increase of concentration of A. squamosa and the highest diameter of inhibition zone was found in the CHX, with significant difference.
Following multiple comparisons by using Tukey HSD analysis, most results are found to be statistically significant when comparing each concentration with others and with CHX except the results concerning comparison of 0.5 with 1, 8 and 10 with CHX and lastly when comparing both these concentrations with each other (8 and 10), these findings are not significant, as shown in Table 2.
| (I) Groups | (J) Groups | Mean Difference (I-J) | P value |
|---|---|---|---|
| 0.5 | 1 | -0.1 | 0.168 ^ |
| 2 | -0.24 | 0.000 * | |
| 4 | -0.39 | 0.000 * | |
| 6 | -0.51 | 0.000 * | |
| 8 | -0.7 | 0.000* | |
| 10 | -0.81 | 0.000* | |
| CHX | -0.81 | 0.000* | |
| 1 | 2 | -0.14 | 0.011* |
| 4 | -0.29 | 0.000* | |
| 6 | -0.41 | 0.000* | |
| 8 | -0.6 | 0.000* | |
| 10 | -0.71 | 0.000* | |
| CHX | -0.71 | 0.000* | |
| 2 | 4 | -0.15 | 0.005* |
| 6 | -0.27 | 0.000* | |
| 8 | -0.46 | 0.000* | |
| 10 | -0.57 | 0.000* | |
| CHX | -0.57 | 0.000* | |
| 4 | 6 | -0.12 | 0.048* |
| 8 | -0.31 | 0.000* | |
| 10 | -0.42 | 0.000* | |
| CHX | -0.42 | 0.000* | |
| 6 | 8 | -0.19 | 0.000* |
| 10 | -0.3 | 0.000* | |
| CHX | -0.3 | 0.000* | |
| 8 | 10 | -0.11 | 0.093 ^ |
| CHX | -0.11 | 0.170^ | |
| 10 | CHX | 0 | 1.000 ^ |
| ^=not significant at p>0.05, *=significant at p<0.05. | |||
Table 2: Multiple Comparisons of diameter of inhibition zone (cm) of S. aureus among groups using Tukey HSD.
Antibacterial effects against E. coli
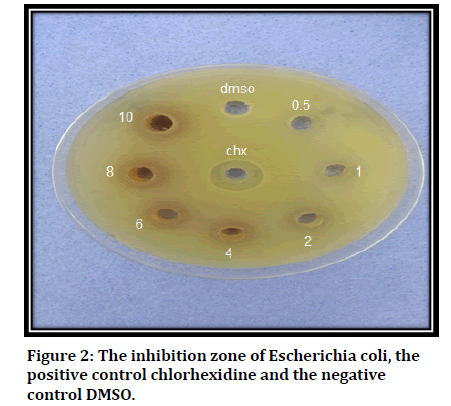
Results of antibacterial activity of all the seven concentrations of A. squamosa extracts were illustrated in (Table 3 and Figure 2). The diameter of inhibition zone (cm) increases with increase in concentration of A. squamosa until the last concentration, with the largest diameter was found in 10 mg/ml, with significant difference.
| Groups | Mean | ± SD | ± SE | Minimum | Maximum | F | P value | ES |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 0 | 0 | 0 | 0 | 0 | 476.093 | 0.000 Sig. | 0.98 |
| 1 | 1 | 0.067 | 0.021 | 0.9 | 1.1 | |||
| 2 | 1.1 | 0.082 | 0.026 | 1 | 1.2 | |||
| 4 | 1.2 | 0.082 | 0.026 | 1.1 | 1.3 | |||
| 6 | 1.3 | 0.082 | 0.026 | 1.2 | 1.4 | |||
| 8 | 1.41 | 0.074 | 0.023 | 1.3 | 1.5 | |||
| 10 | 1.5 | 0.047 | 0.015 | 1.4 | 1.6 | |||
| CHX | 1.4 | 0.082 | 0.031 | 1.3 | 1.5 | |||
| Levene test=2.325, p value=0.034 Sig. | ||||||||
Table 3: Descriptive and statistical test of inhibition zone diameter (cm) of E.Coli among groups.
Figure 2:The inhibition zone of Escherichia coli, the positive control chlorhexidine and the negative control DMSO.
Furthermore, the use of multiple comparisons demonstrates that most of the results are significant except when comparing concentration of the CHX with concentrations of 6, 8 and 10, when comparing the concentration of 1 and 2 with each other, concentration of 4 with both of 2 and 6 concentrations, and lastly the concentration of 8 with both of 6 and 10 concentrations, all these results are not significant (Table 4).
| (I) Groups | (J) Groups | Mean Difference (I-J) | P value |
|---|---|---|---|
| 0.5 | 1 | -1 | 0.000 * |
| 2 | -1.1 | 0.000* | |
| 4 | -1.2 | 0.000* | |
| 6 | -1.3 | 0.000* | |
| 8 | -1.41 | 0.000* | |
| 10 | -1.5 | 0.000* | |
| CHX | -1.4 | 0.000* | |
| 1 | 2 | -0.1 | 0.111^ |
| 4 | -0.2 | 0.000* | |
| 6 | -0.3 | 0.000* | |
| 8 | -0.41 | 0.000* | |
| 10 | -0.5 | 0.000* | |
| CHX | -0.4 | 0.000* | |
| 2 | 4 | -0.1 | 0.173^ |
| 6 | -0.2 | 0.001* | |
| 8 | -0.31 | 0.000* | |
| 10 | -0.4 | 0.000* | |
| CHX | -0.3 | 0.000* | |
| 4 | 6 | -0.1 | 0.173^ |
| 8 | -0.21 | 0.000* | |
| 10 | -0.3 | 0.000* | |
| CHX | -0.2 | 0.004* | |
| 6 | 8 | -0.11 | 0.081^ |
| 10 | -0.2 | 0.000* | |
| CHX | -0.1 | 0.280^ | |
| 8 | 10 | -0.09 | 0.076^ |
| CHX | 0.01 | 1 .00^ | |
| 10 | CHX | 0.1 | 0.179 ^ |
| ^=not significant at p>0.05, *=significant at p<0.05. | |||
Table 4: Multiple comparisons of the diameter of inhibition zone (cm) of E.Col among groups using Games-Howell post hoc test.
Discussion
The antibacterial activity of seven concentrations of A. squamosa extracts was tested using the standard well diffusion technique against gram positive bacteria (staphylococcus aureus) and gram negative bacteria (Escherichia coli) in this study. The results of this research revealed that Annona pulp extract had antibacterial activity against bacterial strains at all concentrations compared with the gold standard mouthwash chlorhexidine as a positive control except concentration 0.5 mg/ml on E. coli which had no effect. There was no zone of inhibition in the wells of dimethyl sulfoxide, which was employed as a negative control. Because it was utilized as a solvent to solubilize the extracts, so it was used as a negative control. This indicates that the antibacterial activity reported was due only to the extract and not to the solvent. According to Cousido et al. [19], in vivo investigation, which approved that the 0.12% concentrations of chlorhexidine is effective as mouthwash, so chlorhexidine mouthwash was chosen as a positive control at that concentration.
the ethanolic extract achieved a larger effect on gram positive and gram negative bacteria, that which is similar to the results of a previous study [20]. This could indicate that ethanol disbanded more active components (phytochemicals) responsible for the plant's antibacterial action such as (flavonoids, tannins, acetogenins, saponins, and alkaloids). In addition, it is in agreement to another investigation [21] that found that the highest zone of inhibition of ethanolic extract was for annona pulp against S. aureus followed by E. coli. The inhibitory effect of A. squamosa extract on S. aureus and E. coli was different depending on the concentration employed, and the inhibitory effect increased when higher concentrations were utilized. In another study, alcoholic extract of A. squamosa have larger inhibition zone and more effective on tested bacteria (E. coli and S. aureus ) more than aqueous extract [22]. This result is comparable with this study and the effect may be due to compounds of fruits such as flavonoids, carbohydrates, saponins, amino acids, tannins, glycosides and terpenoids [23].
The ethanolic extract of pulp from A. squamosa had effectively inhibited Staphylococcus aureus (0.980 cm) at concentration 125 mg/ml, but had no effect on Escherichia coli [24]. In a previous study [25], the authors reported that there was no effect of A. squamosa pulp on S. aureus and this result is different from the present results that found high inhibition zone against the same bacteria in all tested concentrations. The findings support the use of A. squamosa in folklore medicines to cure infections caused by microorganisms in the oral cavity of humans. These points to the creation of natural antimicrobial agents for the treatment of bacteria instead of chemical agents. These results may be due to the antibacterial activities of A. squamosa extracts which have been researched, but no research has been done in comparison to chlorhexidine or when used as a mouthwash in dentistry.
Conclusion
It can be concluded from this study that A. squamosa ethanolic extract has antibacterial efficacy against staphylococcus aureus and Escherichia coli especially at concentration 10 mg/ml that is nearly equivalent to that of chlorhexidine. The using the crude extracts of A. squamous fruit as a source of natural antibacterial is a viable option. These data serve as a foundation for antimicrobial researches, potentially paving the way for the discovery of new clinically active herbal mouthwash.
References
- Khamrco TY, Al-Mitras AA. The effect of residential factor on dental caries prevalence and treatment needs among the primary school children in Ninevah governorate/Iraq. Mustansiria Dent J 2004; 1:21-38.
- Yildiz Telatar G, Gürlek B, Telatar BC. Periodontal and caries status in unexplained female infertility: A case–control study. J Periodontol 2021; 92:446-454.
- Lamont RJ, Jenkinson HF. Oral microbiology at a glance. John Wiley & Sons 2010.
- Williams MI. The antibacterial and antiplaque effectiveness of mouthwashes containing cetylpyridinium chloride with and without alcohol in improving gingival health. J Clin Dent 2011; 22:179.
- Allaker RP, Douglas CI. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents 2009; 33:8-13.
- Prasanna V, Lakshmanan R. Characteristics, uses and side effects of chlorhexidine-A review. J Dent Med Sci 2016; 15:57-59.
- Ozan F, Sümer Z, Polat ZA, et al. Effect of mouthrinse containing propolis on oral microorganisms and human gingival fibroblasts. Eur J Dent 2007; 1:195-201.
- Koukos G, Sakellari D, Arsenakis M, et al. Prevalence of Staphylococcus aureus and methicillin resistant Staphylococcus aureus (MRSA) in the oral cavity. Arch Oral Biol 2015; 60:1410-1415.
- Azmi AH, Adnan SN, Ab Malik N. The Prevalence of Staphylococcus aureus in the oral cavity of healthy adults in Malaysia. Australian J Forensic Sci 2020; 49:583-591.
- Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nature Rev Microbiol 2010; 8:207-217.
- Sharma N, Bhatia S, Sodhi AS, Batra N. Oral microbiome and health. AIMS Microbiol 2018; 4:42.
- Vyas K, Manda H, Sharma RK, et al. An update review on Annona squamosa. Int J Pharm Ther 2012; 3:107-118.
- Saleem TM, Basnett H, Ravi V, et al. Phyto-pharmacological review of Annona squamosa Linn. Natural products: Indian J 2009; 5:85-88.
- Panda SK, Mohanta YK, Padhi L, et al. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016; 21:293.
- https://www.worldcat.org/title/textbook-of-clinical-cariology/oclc/30742792?referer=di&ht=edition
- Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res 1982; 61:1158-1162.
- Ribeiro PR, dos Santos RC, de Melo Filho AA, et al. Antimicrobial activity and acetilcolinesterase inhibition of oils and Amazon fruit extracts. J Med Plant Res 2020; 14:88-97.
- Valgas C, Souza SM, Smânia EF, et al. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol 2007; 38:369-380.
- Cousido MC, Carmona IT, García-Caballero L, et al. In vivo substantivity of 0.12% and 0.2% chlorhexidine mouthrinses on salivary bacteria. Clin Oral Investig 2010; 14:397-402.
- Abdulsalami MS, Aina VO, Ibrahim MB, et al. Comparative antibacterial study of aqueous and ethanolic leaf extracts of Annona muricata. J Natural Sci Res 2016; 6:141-145.
- Al-Deen FM. Evolution of antibacterial activity of various Solvents Extracts of Annona squamosa fruit. Iraqi J Sci 2017; 58:2301-2308.
- Nada KK, Iman FA, Nadhim MH, et al. Effectiveness of aqueous and alcoholic extract of” Annona Squamosa “plant against some types of Gram positive and negative bacteria. Res J Pharm Biol Chem Sci 2016; 7:294.
- Pareek S, Yahia EM, Pareek OP, et al. Postharvest physiology and technology of Annona fruits. Food Res Int 2011; 44:1741-1751.
- Trindade ML, Radünz M, Ramos AH, et al. Chemical characterization, antimicrobial and antioxidant activity of sugar-apple (Annona squamosa L.) pulp extract. Rev Chil Nutr 2020; 281-285.
- Bhardwaj A, Satpathy G, Gupta RK. Preliminary screening of nutraceutical potential of Annona squamosa, an underutilized exotic fruit of India and its use as a valuable source in functional foods. J Pharmacog Phytochem 2014; 3.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Google Scholar Cross Ref Indexed at
Google Scholar Cross Ref Indexed at
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Sumaia Hussein Ali and Zainab Juma Jafar*
Department of Pediatric and Preventive Dentistry, College of Dentistry, University of Baghdad, IraqCitation: Sumaia Hussein Ali, Zainab Juma Jafar, Comparison of Antibacterial Efficacy of Annona Squamosa Mouthwash with Chlorhexidine for Children, J Res Med Dent Sci, 2022, 10(1): 151-157
Received: 23-Dec-2021, Manuscript No. JRMDS-21-45362; , Pre QC No. JRMDS-21-45362 (PQ); Editor assigned: 27-Dec-2021, Pre QC No. JRMDS-21-45362 (PQ); Reviewed: 10-Jan-2022, QC No. JRMDS-21-45362; Revised: 13-Jan-2022, Manuscript No. JRMDS-21-45362 (R); Published: 20-Jan-2022