Research - (2022) Volume 10, Issue 1
Controlling Multidrug-Resistant (MDR) Infections Using Leucas aspera: In Silico Identification of Phytocompounds Inhibiting DNA Gyrase and Tyrosyl-tRNA Synthetase
*Correspondence: Abubucker Peer Mohideen, Department of Basic Medical Sciences, College of Medicine, Prince Sattam bin Abdulaziz University, Al-Kharj,, Saudi Arabia, Email:
Abstract
The on-going issue of multidrug resistance (MDR) among bacterial pathogens worldwide has got further worsened during the pandemic COVID-19. The drug-resistant mechanisms evolved by pathogens have disabled the functions of most antibiotics, and the bacteria use several mechanisms in overcoming the effect of antibiotics. MDR infections are so severe that they cause longer morbidity and, left untreated, may lead to the death of the infected. This scenario brings about the necessity for the development of novel antimicrobial drugs from various sources. Since time unknown, different plants and plant materials have been used for the treatment of many of the dreaded infections. Therefore, the present study focuses on the identification of phytochemical compounds present in Leucas aspera that inhibit the antibacterial targets responsible for replication. Phytocompounds present in Leucas aspera were identified using KNApSAck database. The compounds were docked against DNA gyrase subunit B and tyrosyl-tRNA synthetase. The compounds showing higher binding energies were subjected to protein-ligand interactions and Druglikeness analysis. From the analysis, five compounds showed binding energies (>-8 Kcal/mol) higher than other compounds. All the compounds showed interaction on binding sites of the target proteins. Among them, four compounds satisfied druglikeness properties. Thus, the compounds Chrysoeriol, Apigenin, Acacetin and (-)-Chicanine were identified as possible agents that could be developed as novel antibacterial drugs to treat MDR infections.
Keywords
MDR infections, Leucas aspera, Molecular docking, Druglikeness analysis, DNA Gyrase subunit B, tyrosyltRNA synthetase
Introduction
The prevalence of microbial illnesses has risen considerably during the previous few decades. The widespread use of antimicrobial medications to treat infections has resulted the formation of resistance among many microorganism strains. MDR is characterized as a microorganism’s insensitivity or resistance to antimicrobial medications (which are structurally unrelated and target various molecular targets) [1]. According to the WHO, these resistant microorganisms (such as bacteria, fungi, viruses and parasites) can withstand antimicrobial drug attacks, resulting in poor treatment and infection persistence and transmission. Although MDR is a natural phenomenon, an increase in the number of immunocompromised patients, such as HIVinfected patients, diabetic patients, people who have had organ transplants and people who have had severe burns, makes the body an easy target for hospital-acquired infectious diseases, contributing to the spread of MDR [2].
Almost all capable infecting organisms (e.g., bacteria, fungi, virus and parasite) have used high degrees of multidrug resistance (MDR), which has resulted in increased morbidity and mortality; consequently, they are known as "superbugs." Superbugs are bacteria that have developed resistance to the medications that are supposed to kill them. Controlling and treating drug-resistant bacteria and fungi are difficult. Superbugs are bacteria that have developed drug resistance. It's also possible that they're fungus. Antibiotics are a crucial class of drugs that save a lot of lives. From minor urinary tract infections to life-threatening sepsis, they treat a wide range of infections. The recent rise in superbugs, on the other hand, is partly due to antibiotic abuse, which adds to antibiotic resistance. Antibiotic resistance is a natural aspect of the evolution of bacteria; hence there is no way to prevent it completely. Nonetheless, both doctors and patients must take precautions to avoid antibiotic resistance [3].
Certain multi-resistant opportunistic bacteria, such as Escherichia coli and Staphylococcus aureus, can colonise niches where many other species can’t (environments with severe antibiotic pressure) and even displace commensal flora. This is an example of how antimicrobial resistance can boost the virulence or fitness of some species in specific settings, allowing them to colonise new niches. Escherichia coli has recently emerged as a leading multi-drug resistant pathogen in hospitals and the community, causing urinary tract and bloodstream infections [4].
Antibiotic resistance is a major public health issue, resulting in over 23,000 deaths in the United States (the US) per year (Centre for Disease Control, Antibiotic Resistance. Threats in the United States, 2019 (2019 Antibiotic Resistance Threats Report) and over 33,000 in Europe. Although estimates are difficult to make in this subject, the number of deaths is expected to rise to more than 10 million by 2050, which is already frightening. Overall, mortality rates among patients in India with MDR infections were highest among those caused by Gram-negative bacteria (17.7%), as opposed to those caused by Gram-positive bacteria (10.8%), particularly in the ICUs, where 26.9% of patients with Gram-negative MDR infections died, Antibiotic-resistant illnesses can affect anybody, but young children, cancer patients and individuals over the age of 60 are at the greatest risk [5].
Antibiotic overuse has long been a clinical concern and antibiotic exposure has been associated with changes in gut microbiota, which have been connected to increased risks of chronic diseases like cardiovascular disease and cancer. In addition, the length of antibiotic exposure may be a risk factor for death. Efforts to treat drug-resistant illnesses are being hampered by a lack of investment and creativity in the invention of new antibiotics, which will result in a shortage of new medicines. As a result, the development of novel antibiotics based on plant extracts will aid in treating illnesses [6].
Therefore the present study evaluates the anti-MDR activity of the phytocompounds present in Leucas aspera by in silico analysis. Compounds present in Leucas aspera were identified using the KNApSAck database. The compounds were docked against DNA gyrase subunit B and tyrosyl-tRNA synthetase. The compounds showing higher binding energies were subjected to protein-ligand interactions and druglikeness analysis.
Materials and Methods
Target proteins preparation
In this present study, DNA Gyrase subunit b (PDB ID: 1KZN) and the tyrosyl-tRNA synthetase (PDB ID: 1JIJ) were used as target proteins and their 3D structures were procured from the Protein Data Bank (http:// www.rcsb.org/). The Pymol tool is utilized to visualize the target proteins and then ligands, protein-related water molecules and co-crystal ligands are eliminated (Figure 1). Auto Dock Tools were utilized to prepare the proteins and it is an open-source, free software by adding up charges and Swiss PDB viewer will help in energy minimization and next converted to pdbqt format.
Figure 1: 3D structures of the target proteins. (a) Tyrosyl tRNA synthetase (b) DNA gyrase.
Retrieval and preparation of ligands
Leucas aspera containing the bioactive compounds were recognized and retrieved by utilizing the KNApSAck database (http://www.knapsackfamily.com/ KNApSAcK/).
In this present study, a total of 18 bioactive compounds were utilized (Table 1). The ligand preparation is carried out by identifying its torsion root, assigning charges, correcting torsion angle, optimising utilizing UFF (universal force field) and finally 3D atomic coordinates of the molecules formed by converting into pdbqt format.
| S. No | Compound name |
|---|---|
| 1 | Chrysoeriol |
| 2 | Apigenin |
| 3 | Acacetin |
| 4 | Nectandrin B |
| 5 | 2alpha,3beta-Dihydroxyolean-12-en-28-oic acid |
| 6 | meso-Dihydroguaiaretic acid |
| 7 | (-)-Chicanine |
| 8 | Macelignan |
| 9 | Machilin C |
| 10 | Myristargenol B |
| 11 | Asperphenamate |
| 12 | Leucasperol A |
| 13 | Leucasperol B |
| 14 | Leucasperone A |
| 15 | Leucasperone B |
| 16 | Leucasperoside A |
| 17 | Leucasperoside B |
| 18 | Linifolioside |
Table 1: Compounds used in the study.
Determination of functional sites of targets
Precise evaluation of the active (Functional) site is required for the significant docking analysis. CASTp online server (Computed Atlas for Surface Topography) [7,8]. Is used to identify amino acid residues in the active pocket site synthesis target proteins. Protein topology and active site pockets are analysed by using CASTp, which is a simple and handy tool. Active site determination is censorious for setting the grid box previous to docking.
Molecular docking and protein-ligand interaction analysis
The PyRx tool via autodock wizard is utilized for all compounds which should be docked. It was believed that the ligands were flexible, and the protein was rigid throughout the docking process. The grid parameter configuration file is created in PyRx utilizing the grid boxes for 6 W41 (x=-12.59, y=-18.04, z=83.05) and 6 LU7 (x=17.27, y=30.68, z=48.04) [9]. After docking, the ligand with the highest binding energy (mostly negative) was identified as having the highest binding affinity. The ligands having greater binding energy (-7Kcal/mol) were identified and Biovia Drug discovery studio 2019 is utilized to analyse the interaction between ligand and the protein at the binding sites.
Screening of the of ligands for druglikeness
Compound’s druglikeness is assessed by using Swiss ADME (http://swissadme.ch/index.php). The druglikeness of a molecule is a critical criterion for validating it as a possible agonist for therapeutic targets.
Lipinski’s Rule of Five (RO5) is utilized for screening the drug likeness property of compounds showing the highest binding energy [10].
Binding site analysis and Molecular docking
CASTp was used to evaluate the functional pockets in target proteins (tyrosyl-tRNA synthetase and DNA Gyrase). CASTp is an online tool for examining the amino acid residues in a protein's binding site. Figure 2 represents the binding site images for tyrosyl-tRNA synthetase and Figure 3 represents the binding site images for DNA Gyrase. Amino acids are present in the binding site and their positions are tabulated (Table 2). Grid box for molecular docking analysis was created covering the binding sites of the target protein.
| S. No | Target Protein | Amino acid residues in binding sites |
|---|---|---|
| 1 | Tyrosyl-tRNA synthetase (PDB ID: 1JIJ) | TYR-36, CYS-37, GLY-38, ALA-39, ASP-40, PRO-41, THR-42, ALA-43, SER-45, HIS-47, ILE-48, GLY-49, HIS-50, LEU-52, PRO-53, PHE-54, LEU-70, GLY-72, THR-75, GLY-76, MET-77, ILE-78, GLY-79, ASP-80, SER-82, GLY-83, LYS-84, SER-85, GLU-86, GLU-87, ARG-88, VAL-89, LEU-90, GLN-91, VAL-96, ILE-103, ASN-124, TYR-170, GLN-174, ASP-177, GLN-190, 191-VAL, GLY-192, GLY-193, SER-194, ASP-195, GLN-196, ILE-200, ILE-221, PRO-222, LEU-223, VAL-224, 231-LYS, PHE-232, GLY-233, LYS-234, GLY-238, ALA-239, TRP-241 |
| 2 | DNA gyrase (PDB ID: 1KZN) | GLU-58, ILE-60, GLN-72, ASP-73, ASP-74, VAL-133, GLN-135, LYS-162, THR-163, GLY-164, THR-165, MET-166 |
Table 2: Amino acid residues in the active sites.
Figure 2:Binding sites of Tyrosyl tRNA synthetase analysed using castP.
Figure 3:Binding sites of DNA Gyrase analysed using CastP.
PyRx was used for docking of all 18 compounds to their target proteins (tyrosyl-tRNA synthetase and DNA Gyrase). The binding energies of the phytocompounds were examined and those with the least binding energy (<-8.0 Kcal/mol) against dual targets were identified. Around 5 demonstrated significant binding scores (<-8.0 Kcal/mol) for both the target proteins. Table 3 shows the binding energies of the compounds against target proteins.
| S. No | Compound | tyrosyl-tRNA synthetase (1JIJ) | DNA Gyrase subunit (1KZN) |
|---|---|---|---|
| 1 | Chrysoeriol* | -9.6 | -8 |
| 2 | Apigenin* | -9.5 | -8.3 |
| 3 | Acacetin* | -9.5 | -8 |
| 4 | Nectandrin B | -8.3 | -7.3 |
| 5 | 2alpha,3beta-Dihydroxyolean-12-en-28-oic acid | -7 | -5.9 |
| 6 | meso-Dihydroguaiaretic acid | -7.3 | -7 |
| 7 | (-)-Chicanine* | -8.8 | -8.8 |
| 8 | Macelignan | -7.7 | -7.4 |
| 9 | Machilin C | -8.2 | -6.8 |
| 10 | Myristargenol B | -8.1 | -7.3 |
| 11 | Asperphenamate* | -8.8 | -8.2 |
| 12 | Leucasperol A | -6.5 | -6.4 |
| 13 | Leucasperol B | -7 | -6.2 |
| 14 | Leucasperone A | -6.4 | -6.5 |
| 15 | Leucasperone B | -7.4 | -6.4 |
| 16 | Leucasperoside A | -9.5 | -7.6 |
| 17 | Leucasperoside B | -9 | -6.7 |
| 18 | Linifolioside | -8.6 | -6.7 |
| *Compound showing binding energy higher than -8Kcal/mol | |||
Table 3: Molecular docking analysis.
Analysis of protein-ligand interaction
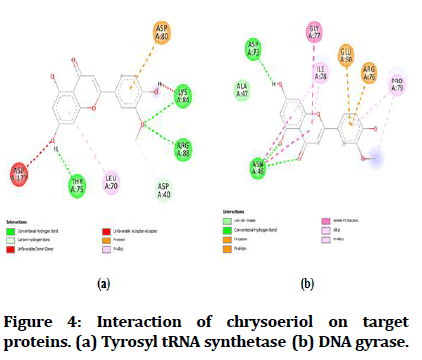
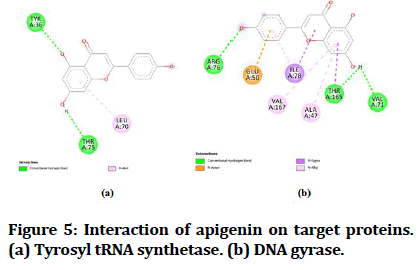
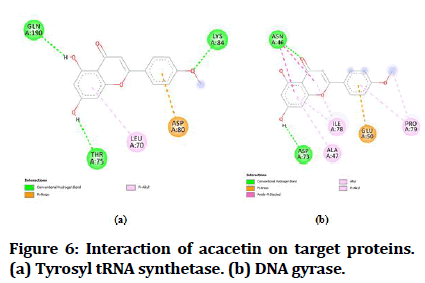
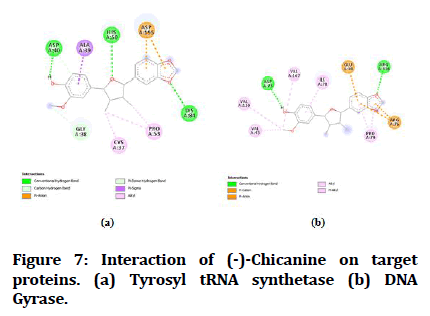
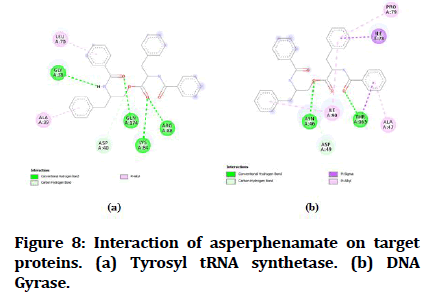
The best-docked compounds (<-8 Kcal/mol) were further subjected to interaction on active sites using Biovia Accelrys Discovery Studio Visualizer software. As crucial as binding affinity, bonding, the number of hydrogen bonds and hydrophobic interactions all play a vital role in protein-ligand interactions. Table 4 illustrates the number of hydrogen bonds produced and the amino acids involved in the interactions. Figures 4-8 demonstrate the hydrogen bonds and other hydrophobic interactions of the ligands on the binding sites of the target proteins. All the five compounds showed H-bond formation on binding sites of the target proteins.
| S. No | Compound | tyrosyl-tRNA synthetase (1JIJ) | DNA gyrase subunit (1KZN) | ||
|---|---|---|---|---|---|
| No. of H-bond | Aminoacid residue | No. of H-bond | Aminoacid residue | ||
| 1 | Chrysoeriol | 3 | THR A:75, LYS A:84, ARG A:88 | 1 | ASP A:73 |
| 2 | Apigenin | 2 | TYR A: 36, THR A:75 | 1 | THR A: 165 |
| 3 | Acacetin | 3 | THR A:75, LYS A:84, GLN A:190 | 1 | ASP A:73 |
| 4 | (-)-Chicanine | 3 | ASP A:40, HIS A:50, LYS A:84 | 1 | ASP A:73 |
| 5 | Asperphenamate | 4 | GLY A:38, LYS A:84, ARGA:88, GLY A:38 | 1 | THR A: 165 |
Table 4: Protein-ligand interactions on binding site analysis.
Figure 4:Interaction of chrysoeriol on target proteins. (a) Tyrosyl tRNA synthetase (b) DNA gyrase.
Figure 5:Interaction of apigenin on target proteins. (a) Tyrosyl tRNA synthetase. (b) DNA gyrase.
Figure 6:Interaction of acacetin on target proteins. (a) Tyrosyl tRNA synthetase. (b) DNA gyrase.
Figure 7:Interaction of (-)-Chicanine on target proteins. (a) Tyrosyl tRNA synthetase (b) DNA Gyrase.
Figure 8:Interaction of (-)-Chicanine on target proteins. (a) Tyrosyl tRNA synthetase (b) DNA Gyrase.
Druglikeness analysis of the selected compounds
The substantial interaction of inhibitors with a receptor protein or enzyme does not guarantee the acceptability of an inhibitor as a drug; hence, druglikeness of inhibitor compounds is more crucial in drug development.
Therefore, all the selected compounds are screened for their Druglikeness property. From the analysis of the five compounds showing higher binding energies, four compounds showed druglikeness.
The compounds satisfying RO5 are Chrysoeriol (MW: 300 g/mol; H-bond acceptors: 6; H-bond donors: 3; MLogP: 0.22), Apigenin (MW: 270 g/mol; H-bond acceptors: 5; H-bond donors: 3; MLogP: 0.52), Acacetin (MW: 284 g/mol; H-bond acceptors: 5; H-bond donors: 2; MLogP: 0.77) and (-)-Chicanine (MW: 342 g/ mol; H-bond acceptors: 5; H-bond donors: 1; MLogP: 2.38) satisfied Ro5 without any violations whereas Asperphenamate (MW: 506 g/mol; H-bond acceptors: 4; H-bond donors: 2; MLogP: 4.43) failed to possess druglikeness.
Discussions
Multidrug resistance (MDR) is a worldwide threat following COVID-19. The drug-resistant mechanisms evolved by pathogens have disabled the functions of antibiotics. There are several drug-resistant mechanisms involved in overcoming the antibiotics.
MDR infection left untreated leads to mortality. This creates a necessity for developing novel anti-microbial drugs for the treatment of MDR infection [5].
Therefore the present study focussed on the identification of phytochemical compounds present in Leucas aspera inhibiting the antibacterial target enzymes responsible for replication.
Bacterial gyrase is the ubiquitous enzyme essential for bacterial viability and is absent in higher eukaryotes. Bacterial gyrase-focused treatments are unique. The bacterial topoisomerase DNA gyrase regulates DNAdependent activities by producing transitory breaks in both DNA strands and reducing torsional stress in the DNA molecule by introducing negative supercoils. DNA gyrase is a heterotetrameric protein that consists of two GyrA subunits that contain the DNA cleavage site and two GyrB subunits that hydrolyze ATP to supply energy for the enzyme's catalytic action. Drugs that target bacterial topoisomerases work in one of two ways: they either stabilize the enzyme or they inhibit it [11].
Aminoacyl-tRNA synthetases (aaRSs) are important enzymes that catalyze the transfer of amino acids to their matching tRNAs during the synthesis of proteins. Because they recognize this information, which includes concurrent tRNA molecules and amino acid structures, they must transform coded information into protein structures in nucleic acids. TyrRS are members of the aaRS family and can be found in all living things. TyrRS belongs to the class I tRNA synthetase family and its active site contains two substantially symmetric sequence motifs, HIGH and KMSKS. TyrRS in bacteria differs from TyrRS in humans in several ways. Smallmolecule TyrRS inhibitors with these features could be potential therapeutic candidates for antibacterial medicines with excellent selectivity and broad-spectrum. TyrRS is required for protein production; therefore blocking these enzymes is detrimental to cells. TyrRS is also highly conserved among prokaryotes, making it a promising target for broad-spectrum antibiotic development [12].
Leucas aspera is a plant species belonging to the Leucas genus and Lamiaceae family. Although the species is called by various names depending on where it is found, Thumbai or Thumba is the most popular name. Leucas aspera is a branched annual plant with a stout and hispid sharply quadrangular stem with branches that grows to a height of 15-60 cm. Leaves are sub-sessile or shortly petiolate, linear or linearly lanceolate, obtuse, pubescent up to 8.0 cm long and 1.25 cm wide, with entire or crenate margins; petiole 2.5-6 mm long; flowers are white, sessile small, in dense terminal or axillary whorls; bracts are 6 mm long, linear, acute, bristle-tipped, ciliate with long slender hairs. Leucas aspera, is found across India from Himalayas down to Ceylon. It grows best in dry, open, sandy soil and is abundant in waste areas. The herb has been used as an antipyretic and pesticide for centuries. In terms of medicine, it has been shown to have antifungal, antioxidant, antibacterial, antinociceptive and cytotoxic properties. Insecticides such as Leucas aspera are widely utilised [14].
Total of 18 compounds were identified from KNApSAck database and these compounds were subjected to docking against the dual targets. Compounds showing higher binding energies (<-8kcal/mol) were investigated for protein-ligand interaction. Formation of H-bonds proves the stability of the docked complex. Therefore the, H-bond formation on binding sites of the target proteins was evaluated. 5 compounds showed significant interactions with H-bonds on binding sites. Druglikness analysis was carried out based on the Lipinski rule of five (RO5). RO5 is a rule of thumb to determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely active drug in humans. From the analysis, 4 compounds showed druglikeness properties.
In vitro antibacterial activity of the L. aspera leaf extracts was tested against 11 bacterial pathogens by Akter et al. [15]. Leaf extracts showed activity against all the test pathogens. Higher antibacterial activity was observed against Pseudomonas aeruginosa, Salmonella paratyphi, Salmonella typhi, Shigella dysenteriae, Staphylococcus aureus. In another study, Chew et al. [6] investigated the antibacterial activity of root, flower, leaf and stem extracts against six pathogens. Root and flower extracts showed inhibition zones against all the test pathogens. Root extracts showed higher inhibition on compared with other extracts.
Conclusion
Phytocompounds present in Leucas aspera were identified using KNApSAck database. The compounds were docked against DNA gyrase subunit B and tyrosyltRNA synthetase. The compounds showing higher binding energies were subjected to protein-ligand interactions and druglikeness analysis. From the analysis five compounds showed binding energies (>-8Kcal/mol) higher than other compounds. All the compounds showed interaction on binding sites of the target proteins. About four compounds satisfied druglikeness properties. Thus, the compounds Chrysoeriol, Apigenin, Acacetin and (-)-Chicanine can be used for the treatment of MDR bacterial infections.
Acknowledgements
The author is thankful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al- Kharj, Saudi Arabia for the support in conducting the research analysis and publishing this report.
Conflict of Interest
The author declares that there are no conflicts of interest relevant to this article.
References
- Levy SB, Marshall B. Antibacterial resistance worldwide: Causes, challenges and responses. Nature Med 2004; 10:S122-9.
- Elbossaty WF. Antibiotic drugs and multidrug resistance bacteria. Int J Pub Health Safe 2017; 2:3.
- Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: A rundown of a global crisis. Infect Drug Resist 2018; 11:1645.
- Jacopin E, Lehtinen S, Débarre F, et al. Factors favouring the evolution of multidrug resistance in bacteria. J Royal Society Inte 2020; 17:20200105.
- Kakoullis L, Papachristodoulou E, Chra P, et al. Mechanisms of antibiotic resistance in important gram-positive and gram-negative pathogens and novel antibiotic solutions. Antibiotics 2021; 10:415.
- Lehtinen S, Blanquart F, Lipsitch M, et al. On the evolutionary ecology of multidrug resistance in bacteria. PLoS Pathogens 2019; 15:e1007763.
- Sanjay prasad S, Shanthi S. Identification of angiotensin converting enzyme (ACE) Inhibiting phytochemical Compounds from Aegle marmelos , Euphorbia hirta , Senna auriculata , Ocimum tenuiflorum and Hibiscus rosasinensis by In Silic. Int J Pharma Bio Sci 2020; 11:79–85.
- Tian W, Chen C, Lei X, et al. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res 2018; 46:W363-7.
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Chem Biol 2015; 243-250.
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports 2017; 7:1-3.
- Collin F, Karkare S, Maxwell A. Exploiting bacterial DNA gyrase as a drug target: Current state and perspectives. Appl Microbiol Biotechnol 2011; 92:479-97.
- Skupińska M, Stępniak P, Łętowska I, et al. Natural compounds as inhibitors of tyrosyl-tRNA synthetase. Microb Drug Resist 2017; 23:308-20.
- Prajapati MS, Patel JB, Modi K, et al. Leucas aspera: A review. Pharmacogn Rev 2010; 4:85.
- Akter M, Khan MA, Muhsin MD, et al. In vitro studies on antibacterial, antifungal, and cytotoxic properties of Leucas aspera. Biol Med 2012; 4:183.
- Chew AL, Jessica JJ, Sasidharan S. Antioxidant and antibacterial activity of different parts of Leucas aspera. Asian Pac J Trop Biomed 2012; 2:176-80.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Department of Basic Medical Sciences, College of Medicine, Prince Sattam bin Abdulaziz University, Al-Kharj,, Saudi ArabiaCitation: Abubucker Peer Mohideen, Controlling Multidrug-Resistant (MDR) Infections Using Leucas aspera: In Silico Identification of Phytocompounds Inhibiting DNA Gyrase and Tyrosyl-tRNA Synthetase, J Res Med Dent Sci, 2022, 10(1): 186-192
Received: 17-Dec-2021, Manuscript No. JRMDS-21-47710; , Pre QC No. JRMDS-21-47710 (PQ); Editor assigned: 20-Dec-2021, Pre QC No. JRMDS-21-47710 (PQ); Reviewed: 03-Jan-2022, QC No. JRMDS-21-4771001; Revised: 06-Jan-2022, Manuscript No. JRMDS-21-47710 (R); Published: 13-Jan-2022