Research - (2021) Volume 9, Issue 3
Early Recovery of COVID-19 Patients by Using Immunoglobulins Present in Cow Colostrum Food Supplement-A Clinical Study
*Correspondence: Swati Khartode S, Consultant Dietitian, Department of Dietetics and Nutrition, Vishwaraj Superspeciality Hospital, Pune, Maharashtra, India, Email:
Abstract
Background: Immunoglobulins in cow colostrum have proven benefits against various dis-eases including recurrent infections.
Objective: To evaluate the effect of Immunoglobulins present in cow colostrum for early re-covery in COVID-19 patients.
Materials and Methods: Randomized, controlled trial of cow colostrum supplement performed at ‘vishwaraj super speciality hospital’ Pune, Maharashtra, India in September 2020, after appropriate approval by Institutional Ethics Committee. 200 COVID-19 patients were selected by randomization method, in which 100 patients in study group who received colostrum supplement and 100 patients in control group who did not receive colostrum supplement. COVID-19 patients categorized in Mild (n=55), Moderate (n=39) and Severe (n=6) cases, depending on HRCT Chest scan severity score and severity of symptoms present in patients.
Results: Comparative statistical analysis done to check any significant difference in days required for cure in the symptoms and hypoxia in between study and control group. The test used was‘t’ test for two independent samples. Level of significance is less than 0.05(p<0.05).The early recovery is significant in study group compared to control group at significance level 0.05 (p<0.05) for all symptoms in mild, moderate, severe and all combined categories (p<0.05) but not significant for Off O2 day in mild, CRP blood test in moderate, both Off O2 day and CRP blood test in Severe COVID-19 and CRP blood test in all three combined categories (p>0.05).
Conclusion: This study concluded that cow colostrum food supplement may be beneficial in COVID-19 patients for early recovery in mild, moderate and severe categories. Further study can be done in critically ill patients admitted in an ICU and those who are on nasogastric tube feed (RT feed).
Keywords
Cow colostrum, COVID-19, CRP blood test, Early recovery, Early relief from hypoxia, Immunoglobulins
Introduction
Since the time immemorial, man has sought some alternative methods to enhance and improve the immune system of human body in order to fight against diseases. Historically, Ayurveda physicians (Doctor of Alternative Indian Medicine) have used bovine colostrum for therapeutically application in Asia, particularly in India for thousands of years [1]. Colostrum is the initial milk secreted by bovine during parturition and the first few days after birth. Colostrum is a gift of nature used to protect the newborn’s immune system and provides passive immunity against pathogens. The presence of bioactive components such as insulin-like growth factor I and II (IGF-I and IGF-II), lactoferrin, lysozyme, lacto peroxidase, and immuno-globulins make the colostrum active against many pathogens. Immunoglobulins are considered an important bioactive component in colostrum, and it contains high levels of immunoglobulin G (Ig G). Immuno-supplementation with bovine milk antibodies has been shown to provide local protection to the gastrointestinal tract against disease [2]. The concentration of many nutrients and biologically active substances is many times higher in colostrum than in milk [3]. Historically, bovine colostrum use in India has occurred since the domestication of this animal species [4] and presenting therapeutic action in the fight against influenza in older adult patients [5] and to irrigate the eye during surgeries in the ocular region is documented in an ancient Indian medicine known as Ayurveda [6]. The most important part is about colostrum digestion, absorption in human body and methods used for the production of bovine colostrum supplement. IgGs are less susceptible to digestion throughout the GI tract and therefore may provide for a distinctive nutritional requirement unique to patients with intestinal disorders and diseases which other dietary proteins cannot provide. There is comprehensive review of human studies regarding the survivability of orallyadministered Ig preparations, with a focus on IgG [7]. Orally-administered Ig (particularly IgGs) from bovine colostrum survives gastric exposure and resists proteolytic digestion in the stomach and intestinal tract. Also the absorption of serum-derived bovine immunoglobulin/ protein isolate (SBI) leads to increases in plasma essential amino acids during transit through the gastrointestinal tract [8]. Greater proportion of active immunoglobulin’s present in colostrum retain by freeze drying meth-od. Chemical preservatives cannot preserve colostrum satisfactorily; chilling and freezing are the most preferred methods [9]. The Immurich capsule (cow colostrum supplement) which was used in our study is well efficient and beneficial in the management of deep wounds dressing. This Immurich colostrum powder which was procured from Immurich capsules used for deep wound dressing and due to this dressing there were decreased hospital stay, promote ulcer healing and decreased pain in cases of deep ulcers [10]. Bovine Colostrum is provided as a therapeutic option for children with recurrent URTI and diarrhea [11]. Bovine colostrum benefited on the treatment of viral upper respiratory tract (URT) infections in IgA-deficient children [12]. Coronavirus disease 2019 (COVID-19) is an acute respiratory disease caused by a newly identified β-coronavirus. This global pandemic has caused dramatic impacts on nations' healthcare systems and socio-economic stability. It transformed into a worldwide public health emergency in a short time. The common clinical features of COVID-19, include cough, fever (not in all), sore throat, headache, fatigue, lethargy, myalgia, anorexia and breath-lessens [13]. No approved treatment exists for COVID-19 currently, so the prevention principles are used as the best approach to control this infection [14]. The search for vaccines to offer immune protection against SARS-CoV-2 and for pharmacological treatments to prevent the virus from replicating is underway. In the meantime significance given to individual’s immune system and nutrition is at the forefront of this. Through experimental research and studies of people with deficiencies, a number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) have been demonstrated to have key roles in supporting the human immune system and reducing risk of infections [15]. Thus in COVID-19, patient’s nutritional care is a key component of a global care. Although a lot of effort is targeted on antiviral therapy, dedicated trials should evaluate whether early more intensive nutritional intervention could improve the clinical outcome of the Covid-19 patients [16]. Vitamin C can be effective in the treatment of SARS-CoV-2 due to its antioxidant effect, its antiviral properties, its capacity to boost the immune system, and its anti-inflammatory properties [17]. Also patients hospitalized with COVID-19 are likely to have pre-existing comorbidities, and the ensuing inflammatory response may result in increased metabolic demands, protein catabolism, and poor glycaemic control [18]. Most important, the unusual conditions in this pandemic situation may make it difficult to obtain and prepare nutritious food. Therefore, nutritional supplements might also be taken into consideration [19]. Thus we have seen the immune system plays a vital role in COVID-19 patients, Immunoglobulin rich bovine colostrum plays a vital role to enhance the immune system and it is easily available in most places, easily digested with good absorption. Cow Colostrum is rich in nutrients specially Immunoglobulins which is beneficial in HIV patients, Gastrointestinal diseases, recurrent URTI and diarrhea in pediatric patients, but there is a lack of study of this immunoglobulin rich cow colostrum use in COVID-19 positive patients hence the present study carried out to evaluate the effect of Immunoglobulins present in Cow Colostrum food supplement on COVID-19 positive patients to get early relief from its symptom and thus for early recovery rate. (Food supplement instead of actual colostrum as a food has been used just for convenient intervention).
Objective
To evaluate the effect of Immunoglobulins present in cow colostrum for early recovery in COVID-19 patients.
Materials and Methods
Study Design
Randomized, Controlled Trial of Cow Colostrum Food Supplement performed at ‘Vishwaraj Super speciality Hospital’ Pune, Maharashtra, India from 4th September to 5th October 2020.
Ethical approval
Appropriate ethical approval was obtained from the Vishwaraj Super Speciality Hospital Ethics Committee, the study protocol, Consent form, Assent form and Case record form was designed and approved by the Institutional Ethics Committee before the start of the study and all subjects of study and control groups had given written, informed consent (In English, Marathi and Hindi language).
Selection of samples
Regarding the methods of cleaning the prosthesis, 12 (33.3%) elderly people only use water to wash and remove debris and 24 (66.7%) elderly 200 COVID-19 patients were selected by Randomization method in which,
Study group-100 COVID-19 patients, received cow colostrum food supplement Cap. Immurich
Control group-100 COVID-19 patients, did not receive any colostrum food supplement.
Categories of COVID-19 patients
Depending on HRCT Chest scan severity score and severity of symptoms present in COVID-19 patients, there were three categories of COVID-19, Mild, Moderate and Severe [20]. Each group (Study and Control) consisted Mild - 55, Moderate- 39 and Severe- 6 cases of patients and all of them received same nutritional/Vitamin supplements, same age groups, similar comorbidity with same locality.
Colostrum food supplement used
Cap. Immurich 300 mg.
Dose of colostrum food supplement
1 capsule /5 kg, Immurich Cow Colostrum capsules used in our study is produced by ‘Dhanwantari Distributors Private limited, Maharashtra, India by freeze drying method which derived from first 48 hours milking post-partum after fully feeding of new born calf (Surplus). The powder is naturally high in whey proteins and rich in immunoglobulins (52 %). It contains Total Immunoglobulins 52%, Lactoferrin 2.1%, Proline Rice Polypeptides 5.3%, Insulin Growth Factor-I 320 ng/100 gm, Transforming Growth Factor beta (TGF-β)- 136 μg/100 gm, Leptin 5.2 mg/100 gm, Calcium 1120 mg/100 gm. This form of colostrum capsules is commercially available as a health promoting food supplement in India.
Inclusion criteria
To be eligible for the study, Patients with following criteria were selected:
99 Male or female patients of age above 5 years.
99 Confirmed clinical diagnosis of Mild, Moderate and severe COVID 19 patients.
99 Symptomatic patients (presence of all major symptoms of COVID-19) with and without O2 requirement.
Exclusion criteria
Patients with any of the following conditions were excluded from the study:
99 Patient who is having Lactose Intolerance (Allergic to milk).
99 Those who are ‘Pure Vegan’ (Not taking any animal product including milk and milk products).
99 Patients below 5 years of age.
99 Patients admitted in an ICU or transferred from ward to ICU due to worsen health conditions.
99 Patients who admitted just for hospital Isolation (asymptomatic or having very mild symptoms (just 1-2 symptoms).
99 Pregnant female patients.
Statistical analysis
The Variable ‘Number of Days required for Cure of the Symptoms’ is “Continuous” and the Comparison of this Continuous Variable in Two Independent Groups (Study and Control) is done by ‘t’ test for Independent Samples. The Significant Variables are those with p value less than 0.05, level of significance.
Results and Discussion
This clinical study performed on 200 hospitalized COVID-19 patients, 100 patients in study group and 100 patients in control group. All participants were selected by randomized method in which there was presence of all major symptoms of COVID-19 and excluded those who were asymptomatic or only one or two symptoms present. First, participants in study group were selected and then participants in control group were selected according to the similar demographics and symptoms (Continuous variables) which were presented in study group. Majority patients were male that is 69 (69%) and females were 31 (31%) (Table 1). Patients having similar comorbities were included in both study and control groups (Table 2). Early recovery from symptoms and signs measured by comparative statistics in the form of days required for cure in the symptoms i.e. on which day majority of patients (%) in study and control groups improved symptomatically and in oxygen level. Days are divided into 1-5 days, 6-10 days, 11-15 days and 16-20 days for better understanding. The detailed results with the frequency of distribution and statistical test whether there is any significant difference by t test and discussions are present in table 3. Study and Control group comprised a wide range of age group from 9 years to 84 years with the mean age 43.3 years in study group and 43.5 years in control group, with exclusion of below 5 years.
Demographics
Sex
Table 1: The frequency distribution of patients according to Sex across both groups (Control and Study) is as given below.
| Sex | Study Group | Total | Control Group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |||
| F | 22 | 8 | 0 | 30 | 23 | 7 | 1 | 31 |
| % | 22 | 8 | 0 | 30 | 23 | 7 | 1 | 31 |
| M | 33 | 31 | 6 | 70 | 32 | 32 | 5 | 69 |
| % | 33 | 31 | 6 | 70 | 32 | 32 | 5 | 69 |
| Total | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
| % | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
Table 2: The frequency distribution of patients according to Co-Morbidity across both groups (Control and Study) is as given below.
| Comorbidity | Study Group | Total | Control Group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |||
| None | 48 | 26 | 5 | 79 | 50 | 24 | 5 | 79 |
| % | 48 | 26 | 5 | 79 | 50 | 24 | 5 | 79 |
| Co-morbidities like bronchial asthma, Hypertension and diabetes | 7 | 13 | 1 | 21 | 5 | 15 | 1 | 21 |
| % | 7 | 13 | 1 | 21 | 0 | 0 | 0 | 21 |
| Total | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
| % | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
Age
Table 3: The frequency distribution of patients according to age across both groups (Control and Study) is as given below.
Age |
Study group | Total | Control group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |||
| 6 to 15 years | 3 | 0 | 0 | 3 | 3 | 0 | 0 | 3 |
| % | 3 | 0 | 0 | 3 | 3 | 0 | 0 | 3 |
| 16 to 25 years | 7 | 0 | 1 | 8 | 7 | 1 | 0 | 8 |
| % | 7 | 0 | 1 | 8 | 7 | 1 | 0 | 8 |
| 26 to 35 years | 12 | 10 | 0 | 22 | 17 | 5 | 0 | 22 |
| % | 12 | 10 | 0 | 22 | 17 | 5 | 0 | 22 |
| 36 to 45 years | 14 | 10 | 0 | 24 | 12 | 11 | 1 | 24 |
| % | 14 | 10 | 0 | 24 | 12 | 11 | 1 | 24 |
| 46 to 55 years | 9 | 10 | 2 | 21 | 9 | 11 | 1 | 21 |
| % | 9 | 10 | 2 | 21 | 9 | 11 | 1 | 21 |
| 56years& Above | 10 | 9 | 3 | 22 | 7 | 11 | 4 | 22 |
| % | 10 | 9 | 3 | 22 | 7 | 11 | 4 | 22 |
| Total | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
Comorbidity
Comorbidity is the presence of one or more additional conditions often associated with a primary condition. In COVID-19, patients with any comorbidity yielded poorer clinical outcomes than those without comorbidity [21]. Both study and control groups comprised 21 (21%) patients with comorbities like bronchial asthma, hypertension and diabetes.
Continuous Variables
Following is the detailed ‘comparative statistical data’ for both Study and Control groups according to which day COVID 19 patients were cured symptomatically and from hypoxia by making range of days as a 1-5 days, 6-10 days, 11-15 days and 16-20 days.
Appetite
There is a high prevalence of GI symptoms (74%) in patients with COVID-19 and the most common GI symptom is anorexia (low appetite) (53%) [22]. All 100 patients (100%) in study group had improved their appetite within first five days, while 76 patients (76%) patients in control group improved their appetites in next 6-10 days (Table 4).
Table 4: The frequency distribution of patients according to appetite across both groups (control and study) is as given below.
| Improved Appetite (in Days) | Study group | Total | Control group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |||
| 1 to 5 Days | 55 | 39 | 6 | 100 | 10 | 6 | 1 | 17 |
| % | 55 | 39 | 6 | 100 | 10 | 6 | 1 | 17 |
| 6 to 10 Days | 0 | 0 | 0 | 0 | 44 | 27 | 5 | 76 |
| % | 0 | 0 | 0 | 0 | 44 | 27 | 5 | 76 |
| 11 to 15 Days | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 7 |
| % | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 7 |
| Total | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
| % | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
General/muscle weakness
All 100 patients (100%) in study group got relief from general or muscle weakness within first five days, while 74 patients (74%) patients in control group improved their general or muscle weakness in next 6-10 days and 9 (9%) patients by 11-15th days in control group (Table 5).
Table 5: Frequency distribution of patients according to general/muscle weakness across both groups (Control and Study).
| Relief from general/muscle weakness | Study group | Total | Control group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| (in Days) | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| 1 to 5 Days | 55 | 39 | 6 | 100 | 9 | 6 | 1 | 16 |
| % | 55 | 39 | 6 | 100 | 9 | 6 | 1 | 16 |
| 6 to 10 Days | 0 | 0 | 0 | 0 | 44 | 25 | 5 | 74 |
| % | 0 | 0 | 0 | 0 | 44 | 25 | 5 | 74 |
| 11 to 15 Days | 0 | 0 | 0 | 0 | 2 | 7 | 0 | 9 |
| % | 0 | 0 | 0 | 0 | 2 | 7 | 0 | 9 |
| 16 to 20 Days | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| % | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
| % | 55 | 39 | 6 | 100 | 55 | 39 | 6 | 100 |
Bodyache/headache/backacke/pain in both lower limbs
92 (n=92) patients suffered from either headache or body ache or backache or the pain in both lower limbs from both groups, 91 patients (98.9%) in study group got relief within first 1-5 days from either any one of above mentioned pain while majority 53 (57.6%) patients in control group got relief from either any one pain in 6-10 days (Table 6).
Table 6: The frequency distribution of patients according to body ache/headache/ lower limb pain across both groups (control and study).
| Relief From Body ache/Headache/ pain in both lower limbs | Study Group | Total | Control Group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| (in Days) | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| 1 to 5 Days | 50 | 35 | 6 | 91 | 22 | 14 | 2 | 38 |
| % | 54.3 | 38 | 6.5 | 98.9 | 23.9 | 15.2 | 2.2 | 41.3 |
| 6 to 10 Days | 0 | 1 | 0 | 1 | 27 | 22 | 4 | 53 |
| % | 0 | 1.1 | 0 | 1.1 | 29.3 | 23.9 | 4.3 | 57.6 |
| 11 to 15 Days | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| % | 0 | 0 | 0 | 0 | 0 | 1.1 | 0 | 1.1 |
| 16 to 20 Days | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 50 | 36 | 6 | 92 | 49 | 37 | 6 | 92 |
| % | 54.3 | 39.1 | 6.5 | 100 | 53.3 | 40.2 | 6.5 | 100 |
Cough
The most commonly experienced symptoms of COVID‐19 patients were fever, fatigue, cough and expectoration [23]. 94 (98.9%) out of 95 patients (n=95) recovered from cough within 1-5 days in study group while majority 72 (75%) out of 96 patients (n=96) recovered from cough in 6-10 days and 13 (13.5%) patients was recovered in 11-15 days (Table 7)./p>
Table 7: The frequency distribution of patients according to cough across both groups (control and study) is as given below.
| Relief from cough | Study group | Total | Control group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| (in Days) | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| 1 to 5 Days | 51 | 37 | 6 | 94 | 9 | 1 | 1 | 11 |
| % | 53.7 | 38.9 | 6.3 | 98.9 | 9.4 | 1 | 1 | 11.5 |
| 6 to 10 Days | 0 | 1 | 0 | 1 | 41 | 27 | 4 | 72 |
| % | 0 | 1.1 | 0 | 1.1 | 42.7 | 28.1 | 4.2 | 75 |
| 11 to 15 Days | 0 | 0 | 0 | 0 | 1 | 11 | 1 | 13 |
| % | 0 | 0 | 0 | 0 | 1 | 11.5 | 1 | 13.5 |
| Total | 51 | 38 | 6 | 95 | 51 | 39 | 6 | 96 |
| % | 53.7 | 40 | 6.3 | 100 | 53.1 | 40.6 | 6.3 | 100 |
Fatigue
60 (n=60) patients in study group and 59 (n=59) patients in control group were suffered from fatigue. All 60 (100%) patients in study group cured from fatigue within first 5 days, majority 52(88.1%) patients from control group got relief from fatigue in 6-10 days, while 5 (8.3%) pa-tients from control group had fatigue after 10 days also (Table 8).
Table 8: The frequency distribution of patients according to Fatigue across both groups (Control and Study) is as given below.
| Relief from Fatigue | Study Group | Total | Control Group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| (in Days) | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| 1 to 5 Days | 30 | 25 | 5 | 60 | 2 | 0 | 0 | 2 |
| % | 50 | 41.7 | 8.3 | 100 | 3.4 | 0 | 0 | 3.4 |
| 6 to 10 Days | 0 | 0 | 0 | 0 | 27 | 21 | 4 | 52 |
| % | 0 | 0 | 0 | 0 | 45.8 | 35.6 | 6.8 | 88.1 |
| 11 to 15 Days | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 5 |
| % | 0 | 0 | 0 | 0 | 1.7 | 6.8 | 0 | 8.5 |
| 16 to 20 Days | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 30 | 25 | 5 | 60 | 30 | 25 | 4 | 59 |
| % | 50 | 41.7 | 8.3 | 100 | 50.8 | 42.4 | 6.8 | 100 |
Loss of sense of taste and smell
Loss of taste and smell is a strong predictor of having been infected by the COVID-19 virus. Also, the combination of symptoms that could be used to identify and isolate individuals includes anosmia, fever, persistent cough, diarrhea, fatigue, abdominal pain and loss of appetite [24] (Table 9). Six (n=6) patients in study and control group each had loss of sense of smell and taste, All 6 (100%) patients regained their sense of smell and taste within first 5 days while 6 (83.3%) patients in control group regained their sense of smell and taste in next 6-10 days.
Table 9: The frequency distribution of patients according to Loss of sense of taste and smell across both groups (control and study).
| Regain the Sense Of Taste and Smell | Study Group | Total | Control Group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| (in Days) | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| 1 to 5 Days | 3 | 3 | 0 | 6 | 0 | 0 | 0 | 0 |
| % | 50 | 50 | 0 | 100 | 0 | 0 | 0 | 0 |
| 6 to 10 Days | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 6 |
| % | 0 | 0 | 0 | 0 | 83.3 | 16.7 | 0 | 100 |
| Total | 3 | 3 | 0 | 6 | 5 | 1 | 0 | 6 |
| % | 50 | 50 | 0 | 100 | 83.3 | 16.7 | 0 | 100 |
ff O2 day (by O2 mask)
Oxygen therapy is recommended by the World Health Organization (WHO) [25] and Centers for Disease Control and Prevention (CDC) [26] as the first‐line therapy for treating COVID‐19‐ induced respiratory distress and hypoxia. 23 (92%) patients out of 25 (n=25) in study group had off O2 day within first 5 days while ma-jority 19 (63.3%) patients out of 30 (n=30) in control group had off O2 day in 6-10 days and 4 (13%) patients in control group had Off O2 day in between 11-15 days (Table 10).
Table 10: The frequency distribution of patients according to Off O2 day (By O2 Mask) across both groups (control and study) is as given below.
| Off O2 day (By O2 Mask) | Study group | Total | Control group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| (in Days) | Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| 1 to 5 Days | 3 | 15 | 5 | 23 | 1 | 5 | 1 | 7 |
| % | 12 | 60 | 20 | 92 | 3.3 | 16.7 | 3.3 | 23.3 |
| 6 to 10 Days | 0 | 1 | 1 | 2 | 1 | 17 | 1 | 19 |
| % | 0 | 4 | 4 | 8 | 3.3 | 56.7 | 3.3 | 63.3 |
| 11 to 15 Days | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 4 |
| % | 0 | 0 | 0 | 0 | 0 | 6.7 | 6.7 | 13.3 |
| Total | 3 | 16 | 6 | 25 | 2 | 24 | 4 | 30 |
| % | 12 | 64 | 24 | 100 | 6.7 | 80 | 13.3 | 100 |
CRP blood test
Elevated CRP level could be a valuable marker to predict the possibility of aggravation of nonsevere COVID-19 patients [27]. Within first 5 days 39 (54.2%) patients out of 72 (n=72) patients had lowered CRP value up to normal range in study group, while 31 (43.7%) patients out of 71 (n=71) in control group had lowered CRP value up to normal range in 6-10 days (Table 11).
Table 11: The frequency distribution of patients according to CRP blood test across both groups (control and study).
| Lowers the value of CRP blood test (in Days) | Study group | Total | Control group | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |||
| Did not Lower | 14 | 7 | 0 | 21 | 9 | 19 | 2 | 30 |
| % | 19.4 | 9.7 | 0 | 29.2 | 12.7 | 26.8 | 2.8 | 42.3 |
| 1 to 5 Days | 20 | 16 | 3 | 39 | 4 | 6 | 0 | 10 |
| % | 27.8 | 22.2 | 4.2 | 54.2 | 5.6 | 8.5 | 0 | 14.1 |
| 6 to 10 Days | 3 | 8 | 1 | 12 | 16 | 11 | 4 | 31 |
| % | 4.2 | 11.1 | 1.4 | 16.7 | 22.5 | 15.5 | 5.6 | 43.7 |
| Total | 37 | 31 | 4 | 72 | 29 | 36 | 6 | 71 |
| % | 51.4 | 43.1 | 5.6 | 100 | 40.8 | 50.7 | 8.5 | 100 |
Symptoms and signs
Appetite, General/Muscle weakness, Body ache/ Headache/ Pain in both lower limbs, Cough, Fatigue, Loss of sense of taste and smell, Off O2 day (By O2 Mask), CRP blood test.
Aim
To test that whether there is any significant difference in days required for cure in the symptoms and signs (hypoxia) stated above among Groups (Study and Control). The test used is ‘t’ test for two independent samples.
Mild category of COVID 19 patients
In Mild COVID-19 (n=55), the symptoms Appetite, General/Muscle weakness, Body ache / Headache/ Pain in both Lower Limbs, Cough, Fatigue, Loss of Sense Of Taste and Smell and CRP blood test were statistically significant.
(p<0.05) but not significant for Off O2 day (By O2 Mask) (p>0.05) (Tables 12 and 13).
Table 12: Descriptive
| Group Statistics* | ||||
|---|---|---|---|---|
| Symptoms and Signs | Groups | N | Mean | Std. Deviation |
| Appetite | Study | 55 | 2.6 | 0.81 |
| Control | 55 | 7 | 1.89 | |
| General/Muscle weakness | Study | 55 | 2.64 | 0.7 |
| Control | 55 | 7.27 | 2.01 | |
| Body ache/Headache/Backache/Pain in both Lower Limbs | Study | 50 | 2.36 | 0.53 |
| Control | 49 | 6.08 | 1.79 | |
| Cough | Study | 51 | 2.78 | 0.94 |
| Control | 51 | 7.31 | 1.69 | |
| Fatigue | Study | 30 | 2.63 | 0.85 |
| Control | 30 | 7.77 | 1.48 | |
| Loss of Sense Of Taste and Smell | Study | 3 | 3.33 | 0.58 |
| Control | 5 | 8.2 | 0.45 | |
| Off O2 day(By O2 Mask) | Study | 3 | 3.33 | 0.58 |
| Control | 2 | 6.5 | 2.12 | |
| CRP blood test | Study | 37 | 2.86 | 2.44 |
| Control | 29 | 5.07 | 3.88 | |
* Severity = Mild
Table 13: T test.
| Independent Samples Test* | |||
|---|---|---|---|
| Symptoms and Signs | t-test for Equality of Means | ||
| t | df | P value (2-tailed) | |
| Appetite | -15.91 | 73.156 | 0 |
| General/Muscle weakness | -16.12 | 66.992 | 0 |
| Body ache/Headache/ Pain in both Lower Limbs | -13.98 | 56.059 | 0 |
| Cough | -16.7 | 78.441 | 0 |
| Fatigue | -16.49 | 46.297 | 0 |
| Loss of Sense Of Taste and Smell | -13.48 | 6 | 0 |
| Off O2 day(By O2 Mask) | -2.06 | 1.1 | 0.269 |
| CRP blood test | -2.67 | 44.695 | 0.01 |
* Severity=Mild
Means Plot:
The means plot showing the average days for the cure of significant symptoms and signs is as given below (Figure 1).
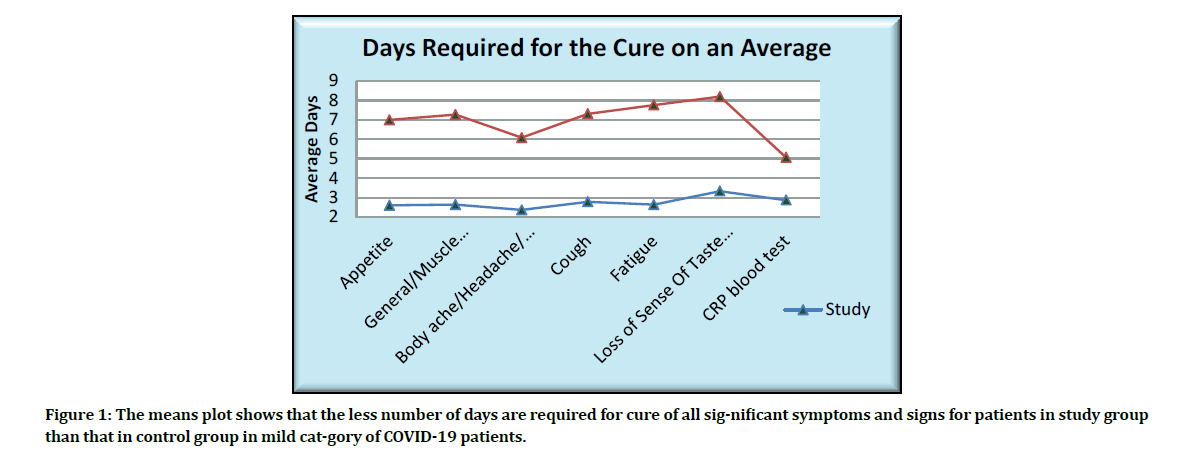
Figure 1: The means plot shows that the less number of days are required for cure of all sig-nificant symptoms and signs for patients in study group than that in control group in mild cat-gory of COVID-19 patients.
Moderate category of COVID-19 patients
In Moderate COVID-19(n=39),There were statistically significant effect on symptoms Appetite, General/Muscle weakness, Body ache/ Headache/ Pain in both Lower Limbs, Cough, Fatigue and Off O2 day (By O2 Mask) (p <0.05) but not significant to CRP blood test (p>0.05) (Tables 14 and 15).
Table 14: Descriptive.
| Group Statistics* | ||||
|---|---|---|---|---|
| Symptoms and Signs | Groups | N | Mean | Std. Deviation |
| Appetite | Study | 39 | 2.79 | 0.73 |
| Control | 39 | 8.56 | 2.93 | |
| General/Muscle weakness | Study | 39 | 2.79 | 0.7 |
| Control | 39 | 8.77 | 3.34 | |
| Body ache/Headache/ Pain in both Lower Limbs | Study | 36 | 2.58 | 0.91 |
| Control | 37 | 6.38 | 2.34 | |
| Cough | Study | 38 | 3.29 | 1.11 |
| Control | 39 | 9.69 | 2.35 | |
| Fatigue | Study | 25 | 3.08 | 0.76 |
| Control | 25 | 8.8 | 1.85 | |
| Loss of Sense Of Taste and Smell | Study | 3 | 3 | 0 |
| Control | 1 | 9 | N.A. | |
| Off O2 day(By O2 Mask) | Study | 16 | 3.5 | 1.32 |
| Control | 24 | 6.58 | 2.32 | |
| CRP blood test | Study | 31 | 3.52 | 2.29 |
| Control | 36 | 3.17 | 3.72 | |
*Severity = Moderate; N.A. = Not Applicable
Table 15: T test.
| Independent Samples Test* | |||
|---|---|---|---|
| Symptoms and Signs | t-test for Equality of Means | ||
| t | df | P value (2-tailed) | |
| Appetite | -11.94 | 42.734 | 0 |
| General/Muscle weakness | -10.95 | 41.293 | 0 |
| Body ache/Headache/ Pain in both Lower Limbs | -9.19 | 46.834 | 0 |
| Cough | -15.33 | 54.504 | 0 |
| Fatigue | -14.31 | 31.877 | 0 |
| Loss of Sense Of Taste and Smell | Not Applicable | ||
| Off O2 day(By O2 Mask) | -4.81 | 38 | 0 |
| CRP blood test | 0.47 | 59.225 | 0.641 |
* Severity = Moderate
Means Plot
The means plot showing the average days for the cure of significant symptoms is as given below (Figure 2).
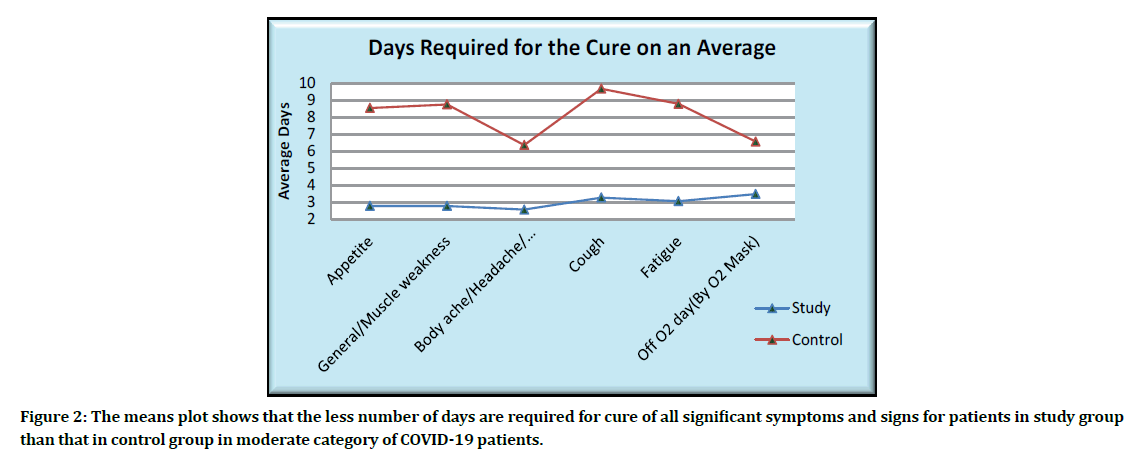
Figure 2: The means plot shows that the less number of days are required for cure of all significant symptoms and signs for patients in study group than that in control group in moderate category of COVID-19 patients.
Severe category of COVID-19 patients
In Severe COVID-19 (n=6), the symptoms Appetite, General/Muscle weakness, Body ache /Headache/ Pain in both Lower Limbs, Cough, Fatigue, Loss of Sense Of Taste and Smell are statistically significant at 0.05 level of significance.(p <0.05) but not significant for Off O2 day (By O2 Mask) and CRP blood test (Tables 16 and 17).
Table 16: Descriptive
| Group Statistics* | |||
|---|---|---|---|
| Symptoms and Signs | Groups | N | Mean |
| Appetite | Study | 6 | 3 |
| Control | 6 | 8.17 | |
| General/Muscle weakness | Study | 6 | 3 |
| Control | 6 | 8.33 | |
| Body ache/Headache/ Pain in both Lower Limbs | Study | 6 | 2.17 |
| Control | 6 | 6.5 | |
| Cough | Study | 6 | 3.17 |
| Control | 6 | 8.67 | |
| Fatigue | Study | 5 | 3 |
| Control | 4 | 9.25 | |
| Loss of Sense Of Taste and Smell | Study | 0† | Not Applicable |
| Control | 0† | ||
| Off O2 day(By O2 Mask) | Study | 6 | 4.17 |
| Control | 4 | 8.5 | |
| CRP blood test | Study | 4 | 4.25 |
| Control | 6 | 4.83 | |
* Severity = Severe
Table 17: T test.
| Independent Samples Test* | |||
|---|---|---|---|
| Symptoms and Signs | t-test for Equality of Means | ||
| t | df | P value (2-tailed) | |
| Appetite | -5.1 | 10 | 0 |
| General/Muscle weakness | -5.22 | 10 | 0 |
| Body ache/Headache/ Pain in both Lower Limbs | -5.87 | 5.536 | 0.001 |
| Cough | -4.43 | 10 | 0.001 |
| Fatigue | -8.33 | 7 | 0 |
| Off O2 day(By O2 Mask) | -1.86 | 3.612 | 0.144 |
| CRP blood test | -0.34 | 6.442 | 0.742 |
* Severity = Severe
Means Plot:
The means plot showing the average days for the cure of significant symptoms is as given below (Figure 3).
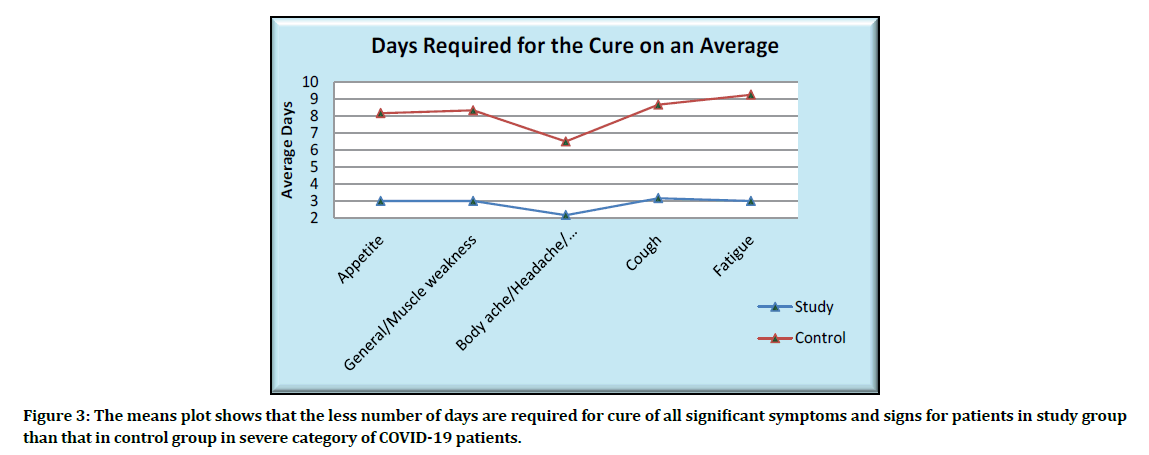
Figure 3: The means plot shows that the less number of days are required for cure of all significant symptoms and signs for patients in study group than that in control group in severe category of COVID-19 patients.
Mild, Moderate and Severe all Combined categories of COVID-19 patients
All combined three (Mild, Moderate, Severe) categories (n=100), were the significance for symptoms Appetite, General/Muscle weakness, Body ache/Headache/ Pain in both Lower Limbs, Cough, Fatigue, Loss of Sense Of Taste and Smell and Off O2 day (By O2 Mask)(p<0.05) and non- Significance For Crp Blood Test (Tables 18 and 19).
Table 18: Descriptive
| Symptoms and signs | Groups | N | Std. Deviation | |
|---|---|---|---|---|
| Appetite | Study | 100 | 0.8 | |
| Control | 100 | 2.46 | ||
| General/Muscle weakness | Study | 100 | 0.73 | |
| Control | 100 | 2.7 | ||
| Body ache/Headache/ Pain in both Lower Limbs | Study | 92 | 0.7 | |
| Control | 92 | 2.01 | ||
| Cough | Study | 95 | 1.05 | |
| Control | 96 | 2.34 | ||
| Fatigue | Study | 60 | 0.86 | |
| Control | 59 | 1.69 | ||
| Loss of Sense Of Taste and Smell | Study | 6 | 0.41 | |
| Control | 6 | 0.52 | ||
| Off O2 day (By O2 Mask) | Study | 25 | 1.35 | |
| Control | 30 | 2.63 | ||
| CRP blood test | Study | 72 | 2.34 | |
| Control | 71 | 3.86 | ||
Table 19: T test.
| Independent Samples Test | |||
|---|---|---|---|
| Symptoms and Signs | t-test for Equality of Means | ||
| t | df | P value (2-tailed) | |
| Appetite | -19.25 | 119.571 | 0 |
| General/Muscle weakness | -18.63 | 113.282 | 0 |
| Body ache/Headache/ Pain in both Lower Limbs | -17.08 | 112.731 | 0 |
| Cough | -20.48 | 131.981 | 0 |
| Fatigue | -22.09 | 85.709 | 0 |
| Loss of Sense Of Taste and Smell | -19.23 | 10 | 0 |
| Off O2 day(By O2 Mask) | -5.5 | 53 | 0 |
| CRP blood test | -1.61 | 114.988 | 0.11 |
Means Plot:
The means plot showing the average days for the cure of significant symptoms is as given below (Figure 4).
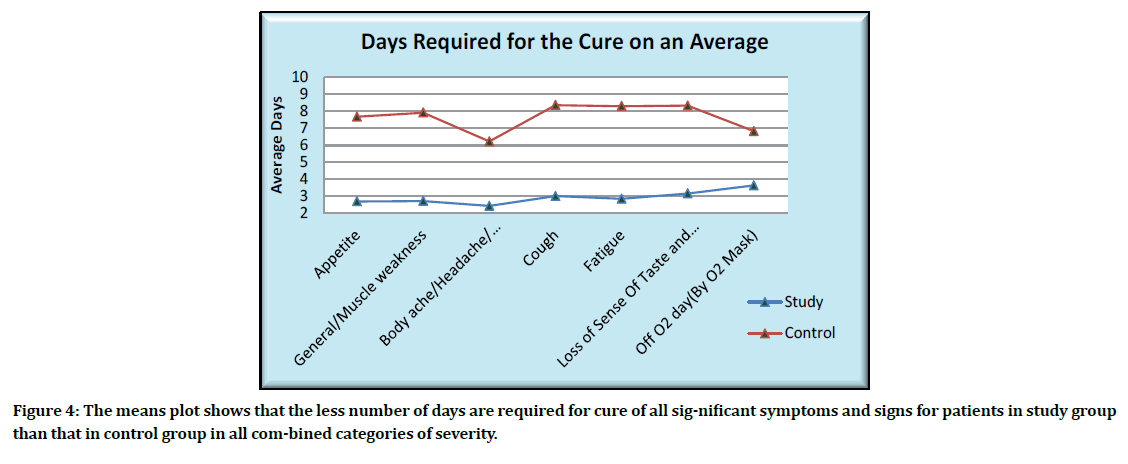
Figure 4: The means plot shows that the less number of days are required for cure of all sig-nificant symptoms and signs for patients in study group than that in control group in all com-bined categories of severity.
Comparative statistical analysis done to check whether any significant difference in ‘days required for cure in the symptoms in between study and control group’ has been done separately for all these three categories of COVID-19 and one for all combined categories. The test used was ‘t’ test for two independent samples. Level of significance is less than 0.05 (p <0.05). Above statistics shows that the early recovery was significant in study group compared to control group at significance level 0.05 (p <0.05) for all symptoms in mild, moderate, severe and all combined categories but not significant for Off O2 day in mild, CRP blood test in moderate, both Off O2 day and CRP blood test in Severe COVID-19 and CRP blood test in all three combined categories (n=100) (p>0.05). Thus the study suggested that majority patients in study group recovered within first 5 days with mean days 2-3 while majority patients in control group recovered in 6-10 days with mean days 6-8 days.Above findings from this study suggest that there was a significant effect of cow colostrum sup-plement on earlier recovery in study group compared to control group in the form of symptoms and oxygen level and CRP blood test. In this study not a single patient from study group reported adverse reaction due to colostrum. One of the major symptom fever was not included in this study as all patients were on antipyretic medicines so it would not be the significant symptom.
Limitations
Study has been done in only one Center.Required blood test or HRCT chest have not been repeated in many participants just to avoid economic burden, so this study missed the comparative study in between HRCT chest and other inflammatory blood markers.
Conclusion
This study concluded that cow colostrum food supplement may be beneficial in COVID-19 patients for early recovery in mild, moderate and severe categories. Further study can be done in critically ill patients admitted in an ICU and those who are on nasogastric tube (RT feed).
Abbreviations
ARDS-Acute respiratory distress syndrome.
BC-Bovine colostrum.
CRP-C-reactive protein.
COVID-19-Coronavirus disease of 2019.
Cap–Capsule.
DOA-Date of admission.
ESR-Erythrocyte sedimentation rate.
GI-Gastrointestinal.
HRCT-High resolution computed tomography
HIV-Human Immunodeficiency virus.
ICU-Intensive care unit
Ig-Immunoglobulin.
IgG-Immunoglobulin G.
IgA- Immunoglubulin A.
NCD-Non communicable disease.
O2-Oxygen.
RT-Ryle’s tube.
SARS-CoV-2–Severe acute respiratory syndrome coronavirus-2.
SPO2–Saturation of peripheral oxygen.
URTI-Upper respiratory tract infection.
URT-Upper respiratory tract.
References
- Seth R, Das A. Colostrum powder and its health benefits. Compendium of lectures, winter school on chemical analysis of value added dairy products and their quality assurance. Dairy Chemistry Division National, Dairy Research Institute, Deemed University, Karnal 2011; 11:59-67.
- Tripathi V, Vashishtha B. Bioactive compounds of colostrum and its application. Food Reviews Int 2006; 22:225-244.
- Georgiev IP. Differences in chemical composition between cow colostrum and milk. Bulgarian J Veterinary Med 2008; 11:3-12.
- Godhia ML, Patel N. Colostrum–its composition, benefits as a nutraceutical–A review. Current Res Nutrition Food Sci J 2013; 1:37-47.
- Conte F, Scarantino S. A study on the quality of bovine colostrum: physical, chemical and safety assessment. Int Food Res J 2013; 20.
- Buttar HS, Bagwe SM, Bhullar SK, et al. Health benefits of bovine colostrum in children and adults. In Dairy in human health and disease across the lifespan. 2017; 1 3-20.
- Jasion VS, Burnett BP. Survival and digestibility of orally-administered immunoglobulin preparations containing IgG through the gastrointestinal tract in humans. Nutrition J 2015; 14:1-8.
- Shaw AL, Mathews DW, Hinkle JE, et al. Absorption and safety of serum-derived bovine immunoglobulin/protein isolate in healthy adults. Clin Exp Gastroenterol 2016; 9:365.
- Borad SG, Singh AK. Colostrum immunoglobulins: Processing, preservation and application aspects. Int Dairy J 2018; 85:201-210.
- Kshirsagar AY, Vekariya MA, Gupta V, et al. A comparative study of colostrum dressing versus conventional dressing in deep wounds. J Clin Diagnostic Res 2015; 9:PC01.
- Saad K, Abo-Elela MG, Abd El-Baseer KA, et al. Effects of bovine colostrum on recurrent respiratory tract infections and diarrhea in children. Med 2016; 95.
- Patıroglu T, Kondolot M. The effect of bovine colostrum on viral upper respiratory tract infections in children with immunoglobulin a deficiency. Clin Respiratory J 2013; 7:21-26.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New England J Med 2020; 382:1708-1720.
- Khayyatzadeh SS. Nutrition and Infection with COVID-19. J Nutrition Food Security 2020; 5:93-96.
- Calder PC. Nutrition, immunity and COVID-19. BMJ Nutrition Prevention Health 2020; 3:74.
- Thibault R, Coëffier M, Joly F, et al. How the Covid-19 epidemic is challenging our practice in clinical nutrition—feedback from the field. Eur J Clin Nutrition 2020; 16:1-0.
- Hernández A, Papadakos PJ, Torres A, et al. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Revista Española Anestesiología Reanimación 2020; 67:245-252.
- Chapple LaS, Fetterplace K, Asrani V, et al. Nutrition management for critically and acutely unwell hospitalised patients with coronavirus disease 2019 (COVID-19) in Australia and New Zealand. Nutrition Dietetics 2020; 77:426-436.
- Khoramipour K, Basereh A, Hekmatikar AA, et al. Physical activity and nutrition guidelines to help with the fight against COVID-19. J Sports Sci 2021; 39:101-107.
- Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls 2021.
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respiratory J 2020; 55.
- Chen A, Agarwal A, Ravindran N, et al. Are gastrointestinal symptoms specific for coronavirus 2019 infection? A prospective case-control study from the United States. Gastroenterol 2020; 159:1161-1163.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol 2020; 92:1902-1914.
- Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nature Med 2020; 26:1037-1040.
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance, 13 March 2020. World Health Organization 2020.
- HCP HP. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in Healthcare Settings.
- Wang G, Wu C, Zhang Q, et al. C-reactive protein level may predict the risk of COVID-19 aggravation. In Open Forum Infectious Diseases 2020; 7:153.
Author Info
Consultant Dietitian, Department of Dietetics and Nutrition, Vishwaraj Superspeciality Hospital, Pune, Maharashtra, IndiaCitation: Swati Khartode S, Early Recovery of COVID-19 Patients by Using Immunoglobulins Present in Cow Colostrum Food Supplement - A Clinical Study, J Res Med Dent Sci, 2021, 9 (3):186-198.
Received: 02-Jan-2021 Accepted: 23-Mar-2021 Published: 30-Mar-2021
