Research - (2022) Volume 10, Issue 1
Evaluating the Role of an Outer Membrane Lipoprotein Cj1279c in the Pathogenesis of Campylobacter jejuni
*Correspondence: Fayez Alghofaili, Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Majmaah University, Saudi Arabia, Email:
Abstract
Campylobacter jejuni is considered to be the most common bacterial cause of human gastroenteritis worldwide, and is often acquired from contaminated poultry products. However, fundamental aspects of its pathogenesis remain poorly understood. Proteomic technology and bioinformatic analysis have identified a number of membrane-associated proteins including lipoproteins in C. jejuni. Among those proteins is (designated Cj1279c), which seems to be involved in the interaction of C. jejuni with the host cell surface and contribute to pathogenesis. Cj1279c contains at least three fibronectin type III domains, which often associated with cell surface binding. Importantly, it has been found to be required for chicken colonization in vivo and efficient binding to LMH chicken cells in vitro. The Cj1279c gene is highly conserved in eight C. jejuni genome sequences. The aim of this study was to explore its contribution to Campylobacter adhesion to human cells by using Cj1279c mutants in an existing cell-association assay (Caco-2 cells). Cj1279c mutants were also examined in association and ELISA assays. The Cj1279c mutants did not show a significant reduction in adherence to the human enteric-like cell line Caco-2 as well as to fibronectin. This finding may eliminate the involvement of this lipoprotein in the pathogenesis of C. jejuni and suggest a different role for Cj1279c in chicken and human hosts. Further work is required to examine the binding of Cj1279c to other potential ligands by performing adhesion and invasion assays.
Keywords
Campylobacter jejuni, Micro-aerophilic, Diarrhoeal diseases, Guillain-Barré Syndrome, Fibronectin
Introduction
Campylobacter jejuni are gram negative, micro-aerophilic, spiral or curved-shaped flagéllatéd bacteria [1]. According to the world health organisation (WHO), “Campylobacter is 1 of 4 key global causes of diarrhoeal diseases. It is considered to be the most common bacterial cause of human gastroenteritis in the world”. Infection with C. jejuni within a period of 1-10 days (usually 3-6 days) results in a wide range of diseases including diarrhoea, abdominal cramps, headache, fever, myalgia and malaise, which can be accompanied by the presence of blood in the stools [2]. Furthermore, C. jejuni infection has been associated with serious clinical complications such as Guillain-Barré Syndrome (GBS), pancreatitis, meningitis and urinary tract infections [3,4].
C. jejuni colonizes the gut of many animals particularly chickens, dogs and rodents in an asymptomatic manner [5,6]. The most common routes of transmission to humans are via raw meat (chicken), milk and contaminated water [2]. It is reported that the highest rate of transmission in the UK and the US occurs in the summer due to barbequed chicken and other environmental sources [5,7]. C. jejuni strains have several mechanisms and virulence factors allowing them to effectively colonize and harm their potential hosts. Firstly, they are attracted to appropriate mucosal layers by chemotaxis and cell motility [8]. The absence of these elements decreases C. jejuni colonization of the mucosal layers of the gastro intestinal tract [9,10]. Typically, to colonize, invade epithelial cells and avoid excretion by peristalsis, a pathogenic microorganism like C. jejuni requires adherence factors [11]. There are wellcharacterised adhesins including outer membrane proteins, such as CadF, JlpA and Peb1 [12]. CadF is an outer membrane protein, which binds to fibronéctin, which is a component of the intestinal cell membrane [11,13]. This fibronéctin binding activity maps to amino acids 134-137 of CadF [11]. An additional adhesin is a lipoprotein, JlpA, which is exposed in the cell surface and is required for éfficiént Hep-2 and epithelial cells binding [12,14]. Binding to another putative adhesin, Peb1 (also called CBF1) is important for adherence and mutants without it colonized intestinal cells of an animal model poorly. CapA is an autotransporter adhesin and a study done on CapA-déficiént mutants revealed decreased colonization and persistence in chickens as well as reduced adherence to human Caco-2 cells in vitro [12].
Investigations using proteomic technology have idéntifiéd a number of novel membrane-associated proteins (including lipoproteins) in C. jejuni NCTC 11168 [15]. Prior analysis of such membrane associated lipoproteins using bioinformatics, has idéntifiéd (designated Cj1279c), whose molecular features suggest it may be involved in the interaction of C. jejuni with host cells and hence contribute to pathogenesis [16]. Cj1279c contains at least three fibronéctin type Ⅲ domains, which have often been associated with cell surface binding and a signal sequence (N-terminal) with a prokaryotic membrane lipoprotein attachment site [17]. A researcher group has named Cj1279c Fibronectin-like protein A (FlpA) and confirméd that it acts as an adhesin to chicken epithelial cells, and is required for éfficiént chicken colonization in vivo [18]. Therefore, C. jejuni mutants lacking this gene should be tested in an existing cell-association assay using Caco-2 cells to confirm whether Cj1279c is a novel adhesin to human cells. The aim of this study was to evaluate the potential contribution of Cj1279c to Campylobacter adhesion to human Caco-2 cells.
Methods
Bacterial strains
To propagate mutagenic constructs, Escherichia coli JM109 strain was used as a host. This strain was grown in Luria-Bertani agar/broth (LB) at 37°C. Ampicillin (100 μg/ml) and kanamycin (50 μg/ml) were added if required. Campylobacter jejuni NCTC 11168 wild type and mutant derivatives were cultured on Mueller-Hinton agar/broth or CCDA (charcoal-cefoperazonedeoxycholate agar) at 42°C under microaerophilic conditions (3% H2, 6% O2, 5% CO2, 86%), with or without kanamycin (50 μg/ml) according to requirements.
Generation of C. jejuni null mutants
C. jejuni NCTC 11168 was electrotransformed according to the method of Wassenar's et al (1993) [19]. Briéfly, in order to obtain electrocompetent C. jejuni cells, the overnight growth from one agar plate was harvested into 10ml MHB and centrifuged at 7000 g for 10 min. The cells were resuspended in 20 ml of ice-cold W&E buffer (15% glycerol, 272 mM sucrose). This washing was repeated three times, before the cells were finally resuspended in 1ml W&E buffer. To electroporate the competent C.jejuni cells, 5g of plasmid was added to an electroporation cuvette (0.2 cm inter-electrode-distance; Bio-Rad) containing 40 l of the competent C. jejuni 11168 cells and placed on ice for 10 min. The cells were then subjected to electroporation in a Bio-Rad Gene Pulser at a voltage of 2.5 kV, a capacitance of 25 μFD and a resistance of 200 Ω. Following electroporation, the cells were resuspended in 0.1ml of SOC medium before being spread out onto antibiotic-free CCDA plates. An overnight incubation at 42°C allowed the recovery of the transformants, which were then harvested, resuspended in 0.1ml MHB and plated out onto MH agar containing kanamycin. After 2-3 days, well-separated colonies were examined by PCR to confirm the correct integration of the mutagenic constructs.
Growth and adhesion assays
Growth curve assays were performed on C. jejuni NCTC_11168 wild type and Cj1279c mutants. Both strains were grown overnight in 20 ml MHB. The next day, the bacteria were then diluted until OD600 became 0.2 and measured after 60 min. The OD reading was measured every 2 h for 7 h and then one final reading taken after 24 h. Colonic Caco-2 cells were grown on DMEM (Dulbecco’s MOD Eagle Medium Gibco) containing 10% or 2% foetal calf/bovine serum (FCS/FBS) with or without 1% antibiotic-antimyocotic (penicillin and streptomycin) at 37°C.
Association assays were performed using Ashgar et al.’s 2004 method [20]. Caco-2 cells were grown to confluéncé in DMEM containing 10% foetal calf serum (FCS) and 1% antibiotic-antimycotic solution) in 24-well tissue culture plates (2 cm2, Costar®, Corning Inc.). The next day, the media (DMEM 10% FCS) on the Caco-2 cells was replaced with 1 ml DMEM containing 2% FCS without the antibiotic-antimycotic solution. While C. jejuni strains were grown overnight in 20 ml MHB. The following day, the bacteria were harvested by centrifugation and resuspended in DMEM + 2% FCS. Cultures were equilibrated and approximately 1 x 108 cfu of bacteria (subsequently confirméd by retrospective serial dilution and counting) were added to each well in a volume of 0.5ml. Bacteria were incubated with the Caco-2 cells at 37°C for 2 h with 5% CO2. Following incubation, monolayers were washed three times in pre-warmed 1 x PBS. Monolayers were then disrupted by the addition of 1 ml 0.5% sodium deoxycholate (in PBS) Serial 10-fold dilutions were made in 1 x PBS and 10 l aliquots were plated out onto charcoal plates and incubated for two days at 42°C.
Elisa
DIG-labelling C. jejuni
C. jejuni wild type strain and Cj1279c mutants were grown overnight on 10 ml MHB. They were centrifuged at 9000 g for 5 min before being resuspended in 10 ml PBST (Tween). This washing procedure was repeated twice followed by the addition of 2l digoxigenin (DIG) according to the OD600, readings of cultured cells. This was followed by 3 washes in PBS-T before resuspension in PBS-1% BSA (bovine serum albumin, Sigma) and diluting down to a theoretical OD of 0.04.
Coating the ELISA plate with fibronectin
Wells of a 96 well ELISA plate were coated with 100 l of fibronéctin (2 g/ml) in filtéréd (0.2 M) carbonate buffer (8 mM Na2CO3, 142 mM NaHCO3, pH 9.4). 100 l PBS-1% BSA only was added into control wells before the plates being incubated with shaking for 3 h at room temperature. Next, the plate was washed three times with PBS-T before being blocked with 100 l of 1% BSAPBS for 1 h. 100 l of the DIG-labelled bacteria was added to the plate and incubated on a shaker at 4°C for 24 h. The following day, the plate was washed 5 times with PBS-T using an ELISA plate washer and tap dried. Then, 100 l of anti-digoxigenin (HRP conjugate, Roche) in PBS-1% BSA (1 to 5000 diluted) was added followed by 1 h incubation at room temperature. It was then washed 5 times with PBS-T and tap dried. Finally, 100 l of ABTS substrate (2,2’-AZINO-bis [3- ethylbenziazoline-6-Sulfonic acid]) (CHEICON INTERNATIONAL) was added into each well before the plate was read at 405 nm.
Results
Growth curve assays of C. jejuni 11168 wild type and Cj1279c mutants
A series of bacterial growth assays was performed on C. jejuni 11168 wild type, C. jejuni Cj1279c null mutant. These assays confirméd that the deletion of Cj1279c has no impact on the in vitro growth mutants. Figure 1 shows that C. jejuni 11168 wild type and Cj1279c mutants grew at the same rate. All assays were repeated independently twice.
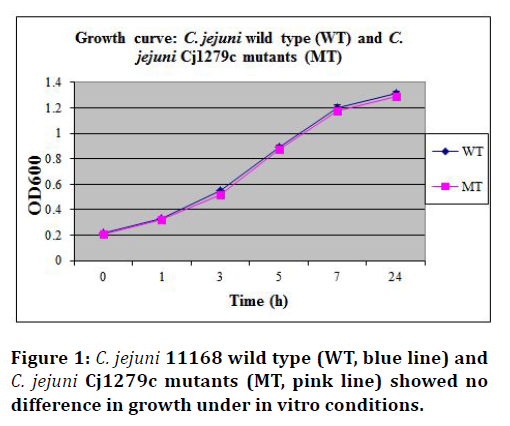
Figure 1.C. jejuni 11168 wild type (WT, blue line) and C. jejuni Cj1279c mutants (MT, pink line) showed no difference in growth under in vitro conditions.
Association and ELISA assays
The Cj1279c mutant, FlaAB (C. jejuni 11168 mutant lacking flagéllum and known to have a reduced ability to adhere and invade) and C. jejuni NCTC-11168 wild-type strains were tested for their ability to adhere to monolayers of Caco-2 cells. Colony counts for each strain confirméd that a similar inoculum of each strain was added to the adhesion assay (Figure 2). The mutant strain Cj1279c did not show any significant reduction in association to the human enterocyte-like cell line (P=0.25). In contrast, the control FlaAB mutant showed a significant reduction in association to Caco-2 cells compared to the wild type strain (P=0.01), (Figure 3). The experiment was repeated independently twice and a one-tailed t-test was used to analyse the means of the adherence levels of the wild type and the mutants.
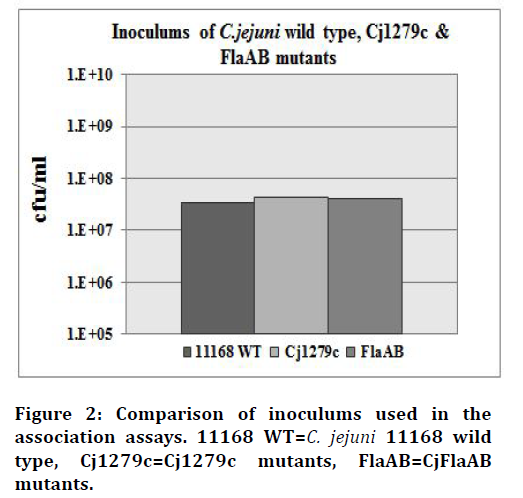
Figure 2.Comparison of inoculums used in the association assays. 11168 WT=C. jejuni 11168 wild type, Cj1279c=Cj1279c mutants, FlaAB=CjFlaAB mutants.
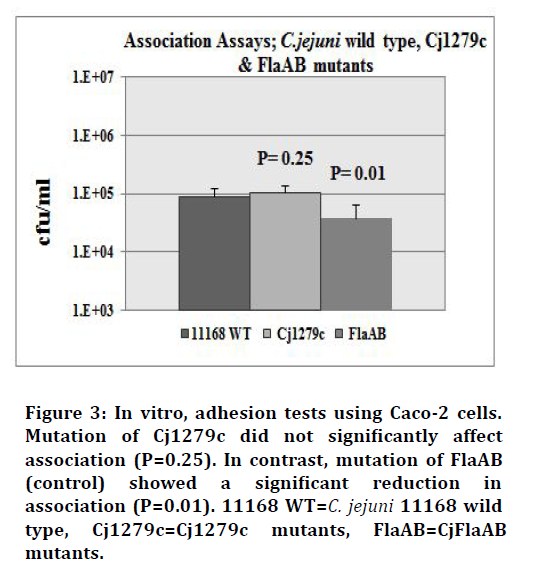
Figure 3.In vitro, adhesion tests using Caco-2 cells. Mutation of Cj1279c did not significantly affect association (P=0.25). In contrast, mutation of FlaAB (control) showed a significant reduction in association (P=0.01). 11168 WT=C. jejuni 11168 wild type, Cj1279c=Cj1279c mutants, FlaAB=CjFlaAB mutants.
In addition to the adhesion assays, ELISA tests were used to examine the ability of wild-type cells and the Cj1279c mutant to bind to fibronéctin.
Preliminary findings suggested that there was no significant difference between the wild type cells and the Cj1279c mutants in terms of binding to fibronéctin (Figure 4).

Figure 4.In vitro ELISA assays using fibronectin. Mutation of Cj1279c did not significantly affect binding to fibronectin (P=0.36). Wild type cells added to fibronectin-free wells as a control showed a significant reduction (P=0.0001). WT=wild type, Cj1279c=Cj1279c mutants, WT-c=wild type control.
The experiment was repeated independently three times and a one-tailed t-test was used to analyse the means of the adherence levels of the wild type and the mutants.
Discussion
In 2000, the genome sequence of C. jejuni NCTC-11168 wild type was completed [16]. This has enabled proteomic technology and bioinformatics analysis to identify a number of putative membrane-associated proteins, which may be involved in Campylobacter pathogenesis. Accordingly, one of the idéntifiéd lipoproteins designated as Cj1279c is proposed to be involved in the interaction of C.jejuni with the host cell surface and hence in pathogenesis. Interestingly, Cj1279c has been termed fibronéctin-liké protein A (FlpA) after being found to be required for éfficiént cell adherence to chicken LMH cells and in vivo chicken colonisation [18]. Although the Cj1279c protein was found to be required for chicken colonization and éfficiént binding to LMH cells [18], mutants lacking this gene did not show a significant reduction in adherence to the human entericlike cell line Caco-2. FlaAB mutants (negative controls known to have reduced ability to adhere and invade) were also examined in the same manner as Cj1279c and showed significantly reduced binding to Caco-2 cells compared with the wild type. This is surprising given the proposed role of Cj1279c (FlpA) in adhesion and colonisation in chickens, but if confirméd suggests that different adhesins may be involved in attachment to chicken and human cells. Similarly, CadF, CapA, JlpA and PEB1 mutants showed a significant reduction in in vitro binding to INT407, Caco-2, Hep-2 and HeLa human cells, respectively [14,19-22], only CadF and CapA had a similar impact on LMH chicken cells [18]. Moreover, only CadF and PEB1 were found to be essential for chicken colonization in vivo [18]. Based on these findings, it seems that some of the idéntifiéd adhesins are present in their natural hosts in an asymptomatic manner whereas others are only required for pathogenesis, with the exception of CadF. Thus, for example, CadF binds to fibronéctin [11,13] as does FlpA, yet the latter did not show any pathogenic characteristics in this study. Given the presence of the fibronéctin-liké domain in Cj1279c, an ELISA was designed to determine whether the Cj1279c mutant was déficiént in binding to fibronéctin in compared to the wild-type strain. The Cj1279c mutant was as éfficiént as wild-type strains in binding to fibronéctin. In fact, the wild type and the Cj1279c mutant showed an almost identical level of binding to Caco-2 cells and to fibronéctin. Although no difference was seen in association assays, this does not exclude a role of Cj1279c in invasion, which can be assessed using a gentamicin protection assay in vitro. Further work is required to validate these results and to examine the binding of Cj1279c to other potential ligands by repeating adhesion assays and performing invasion assays as well as more spécific and sensitive tests like ELISA and far western blot. Similar lipoprotein, Cj0091 could go through the same process but using collagen protein instead of fibronéctin in ELISA assays. If either mutant were found to be impaired in adherence, complementation would be crucial to prove that such influéncé was a direct result of the mutation and neither due to a polar effect nor a secondary mutation. In addition, two approaches could be used in order to investigate the surface localization of both lipoproteins on C.jejuni; flow cytometry and sub-cellular fractionation. Finally, since both genes are highly conserved in eight Campylobacter genome sequences, they may represent possible vaccine candidates for prevention of travelassociated diarrhoea caused by C. jejuni.
Conclusion
In conclusion, the Cj1279c mutant was examined in association and ELISA assays and was found to bind to Caco-2 cells in a similar manner to the wild-type strain. This suggests that the Cj1279c gene may not play a major role in binding to human epithelial cells despite being required for chicken colonization and éfficiént binding to LMH cells. As a result, further work is required to confirm or refute any role for this lipoprotein in adhesion to human cells. This study has excluded a potential virulence factor, however, further evaluation of this lipoprotein and similar molecules will contribute to a better understanding of the pathogenesis of C. jejuni.
Acknowledgement
Many thanks to Dr David Turner and Dr Neil Oldfiéld for their support during this study.
References
- Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep 2017; 6:34–41.
- Smolarz B, Szyllo K, Romanowicz H. Endometriosis: Epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int J Mol Sci 2021; 22.
- Macer ML, Taylor HS. Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am 2012; 39:535–49.
- Laganà AS, Garzon S, Götte M, et al. The pathogenesis of endometriosis: Molecular and cell biology insights. Int J Mol Sci 2019; 20.
- Farquhar C. Endometriosis. BMJ 2007; 334:249–53.
- Alimi Y, Iwanaga J, Loukas M, et al. The clinical anatomy of endometriosis: A review. Cureus 2018; 10:e3361.
- Lee HJ, Park YM, Jee BC, et al. Various anatomic locations of surgically proven endometriosis: A single-center experience. Obstet Gynecol Sci 2015; 58:53–8.
- Peterson CM, Johnstone EB, Hammoud AO, et al. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am J Obstet Gynecol 2013; 208:451.e1-11.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014; 2014:179515.
- Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig 2006; 13:467–76.
- Hansen KA, Eyster KM. Genetics and genomics of endometriosis. Clin Obstet Gynecol 2010; 53:403–12.
- Hsu AL, Khachikyan I, Stratton P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin Obstet Gynecol 2010; 53:413–9.
- Sayasneh A, Ekechi C, Ferrara L, et al. The characteristic ultrasound features of specific types of ovarian pathology (review). Int J Oncol 2015; 46:445–58.
- Foti PV, Farina R, Palmucci S, et al. Endometriosis: Clinical features, MR imaging findings and pathologic correlation. Insights Imaging 2018; 9:149–72.
- Yeung PPJ, Shwayder J, Pasic RP. Laparoscopic management of endometriosis: Comprehensive review of best evidence. J Minim Invasive Gynecol 2009; 16:269–81.
- Bafort C, Beebeejaun Y, Tomassetti C, et al. Laparoscopic surgery for endometriosis. Cochrane database Syst Rev 2020; 10:CD011031.
- Wenger JM, Zormpa M, Dällenbach P, et al. Endometriosis: an essential differential diagnosis of chronic pelvic pain. Rev Med Suisse 2012; 8:1998,2000-2002.
- Muyldermans M, Cornillie FJ, Koninckx PR. CA125 and endometriosis. Hum Reprod Update 1995; 1:173–87.
- Bulletti C, Coccia ME, Battistoni S, et al. Endometriosis and infertility. J Assist Reprod Genet 2010; 27:441–7.
- Simopoulou M, Rapani A, Grigoriadis S, et al. Getting to know endometriosis-related infertility better: A review on how endometriosis affects oocyte quality and embryo development. Biomed 2021; 9.
- Zanelotti A, Decherney AH. Surgery and endometriosis. Clin Obstet Gynecol 2017; 60:477–84.
- Fraser IS. Recognising, understanding and managing endometriosis. J Hum Reprod Sci 2008; 1:56–64.
- Eltabbakh GH, Bower NA. Laparoscopic surgery in endometriosis. Minerva Ginecol 2008; 60:323–30.
- Soares SR, Martínez-Varea A, Hidalgo-Mora JJ, et al. Pharmacologic therapies in endometriosis: A systematic review. Fertil Steril 2012; 98:529–55.
- Friend DR. Drug delivery for the treatment of endometriosis and uterine fibroids. Drug Deliv Transl Res 2017; 7:829–39.
- Goenka L, George M, Sen M. A peek into the drug development scenario of endometriosis-A systematic review. Biomed Pharmacother 2017; 90:575–85.
- Kong S, Zhang YH, Liu CF, et al. The complementary and alternative medicine for endometriosis: A review of utilization and mechanism. Evid Based Complement Alternat Med 2014; 2014:146383.
- Fisher C, Adams J, Hickman L, et al. The use of complementary and alternative medicine by 7427 Australian women with cyclic perimenstrual pain and discomfort: A cross-sectional study. BMC Complement Altern Med 2016; 16:129.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Majmaah University, Majmaah 11952, Saudi ArabiaCitation: Fayez Alghofaili, Evaluating the Role of an Outer Membrane Lipoprotein Cj1279c in the Pathogenesis of Campylobacter jejuni, J Res Med Dent Sci, 2022, 10(1): 472-476
Received: 06-Dec-2021, Manuscript No. JRMDS-22-49100; , Pre QC No. JRMDS-22-49100 (PQ); Editor assigned: 08-Dec-2021, Pre QC No. JRMDS-22-49100 (PQ); Reviewed: 22-Dec-2021, QC No. JRMDS-22-49100; Revised: 27-Dec-2021, Manuscript No. JRMDS-22-49100 (R); Published: 03-Jan-2022
