Research Article - (2022) Volume 10, Issue 8
Evaluating Three Different Gels in Treating and Protecting the Primary Teeth Enamel Surface Erosion
Ali Jameel Abdulsahib1* and Zain Alabdeen Abdul Abbas Noaman2
*Correspondence: Ali Jameel Abdulsahib, Department of Prosthodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Aim: To compare the effect of NHA gel, HA gel and NaF gel in treating the eroded enamel surface of the primary teeth.
Objectives: Detecting the structure changes of primary enamel surface (surface roughness analysis and surface nanostructure) following erosive exposure to Pepsi, after treatment with NHA gel, HA gel and NaF gel and for the treated.
Material and methods: NHA, HA gels will be prepared and FTIR test to evaluate it. Thirty sound buccal segment of primary first or second molar will be exposed to Pepsi for 60 minutes with shaking 3 times daily for 10 days. Then, the erosive effect of the acid beverage on the primary tooth surface enamel will be studied. After that, different treating gels will be applied to study the remineralization effects on the primary surface enamel. In order to evaluate the preventive effect of the NHA-gel, HA-gel and NaF gel on the treated teeth, a second acid beverage exposure will be conducted. At the end, the primary surface enamel of the sample will be studied again to evaluate the protective effect of applied gel. Spectroscopic morphology (2D, 3D picture) will be studied in the three phases.
Results: Ra, Rq, Rz decrease in remineralization phase and at least in (NHA, NAF, HA) respectively, with p<0.05 between (NHH, HA) but p>0.05 between (NHA, NAF) also (HA, NAF). Ra, Rq, Rz slightly increase in preventive phase p<0.05 between (NHA, NA) also (NHA, NAF) but p>0.05 between (HA, NAF).
Conclusion: 10% NHA gel better than HA, NAF gels in treatment and prevention the effect of erosion cause by Pepsi cola.
Keywords: Sickle cell disease, RBC, HBB, Hydroxyurea, Turmeric
Introduction
In advanced societies, healthy changes in the lifestyle have contributed to an expanded rate of dental disintegration, particularly in children and adolescents [1,2]. For example, the reduced intake of acidic beverage (such as Pepsi) results in decreasing the demineralization of tooth enamel because it was reported that the most common cause of dental erosion was the consumption of acidic food or beverages [3,4]. Enamel repair happens through the precipitation of drained minerals within the demineralized defected area, which can be studied by using the Atomic Force Microscopy (AFM) [5]. Nano-Hydroxyapatite (NHA) utilizes in oral care treatment because of similarity with enamel and dentine in size and structure [6]. In last years, an increasing number of studies have shown that NHA has the potential to remineralization erosion caused by acid or other causes after addition NHA particles to tooth pastes, mouth washes, gel, etc. [7,8]. Teeth remineralization happens daily; hence, teeth remineralization should be enhanced by adding remineralization products such as the NHA to home care products or professionally applied products in order to treat or prevent erosive effect by acid. As the NHA products role in treating and preventing the erosive effect of acidic beverage have not been studied thoroughly in the literature, this study will investigate the NHA effect on the acidly eroded primary teeth enamel.
Materials and Methods
The sample: This study was carried out in in the Department of Paediatric and Preventive Dentistry, after being ethically approved by the ethical committee of the College of Dentistry/University of Baghdad. The study was taken by selecting 30 posterior primary teeth (primary first or second molar) from 50 teeth removed for various purposes at was it governorate specialist health centre using G power 3.1.9.7 to achieve representative sample. To achieve a block of sound tooth construction, the chosen teeth should have a buccal surface free of caries, fillings, and cracks. The preparation began with a visual examination and using Stereomicroscope. The teeth were then automatically placed in universal glass tubes and preserved in distilled water after extraction. To avoid bacteria growth, the bottled water was replaced every 72 hours before the experiment was started.
After that the teeth were polished with pumice containing no fluoride by using a polishing cup in a low velocity hand piece to remove any remaining residue on the tooth surface prior to use. They were then cleaned and dried with cotton pads after being washed with distilled water.
The samples were prepared using a micro-motor hand piece with water cooling and the roots were sectioned from crown using Straight hand piece with a double-sided diamond disk of 4 × 4 mm diameter and 2 mm thickness, and were calibrated using an electronic digital calliper to obtain correct measurements. The specimens were separated horizontally in the cervical area to extract crown from root and split vertically for the labial part applying diamond disc with a water spray. The labial segments of tooth specimens were measured by digital calibrated. Measuring length 4 mm × width 4 mm × depth 2 mm in dimension. Except for the outer enamel surface that was examined, all of the sample surfaces were covered with adhesive tape all over, so that variations in texture of enamel could be completely determined from the uncovered surface by using AFM and the groups studied as follows:
- Group (I): in which, the teeth will be treated with (NHA) gel.
- Group (II): the teeth will be treated with HA gel while in.
- Group (III) the teeth will be treated with NaF gel.
Each group will be exposed to Pepsi for 60 minutes with shaking 3 times daily for 10 days. Then, the erosive effect of the acid beverage on the primary tooth surface enamel will be studied. After that, different treating gels will be applied to study the remineralization effects on the primary surface enamel. To review the preventive level of the 10% NHA-gel, 10% HA-gel and 1.23% NaF gel on the treated teeth, a second acid beverage exposure will be conducted. At the end, the primary surface enamel of the sample will be studied again to evaluate the preventive effect of applied gels.
Materials and equipment
Artificial saliva, cotton pads, De-ionized water, Cast no. (7732-18-5), Disposable cup, Disposable gloves, Distilled water, Gelling agent (carboxymethyl cellulose) cast no. (9000-11-7), HA cast no. 1306-06-5 microscale (sigma Aldrich), micro-motor hand piece NaF gel. 1, 23% Cast no. (7681-49-4), NHA cast no. (12167-74-7) Nano scale (ALDRICH), Non-fluoridated pumice Pepsi cola (Iraq), PH meter (ph-20), Plastic container, rubber cup, Electrical scale, Glycerol cast no 56-81-5 (made in the United Kingdom), digital Vernier, Straight hand piece, Engine (stronger 90), Double-sided diamond disk, Magnetic stirrer.
Pre-prepared artificial saliva
Different materials in different concentrations were dissolved in de-ionized water to prepare 1 L of artificial saliva according to method obtained from the digital PH-meter was calibrated using buffering solution before use.
Pepsi cola: Before measuring the PH of Pepsi cola, the digital PH-meter was calibrated by using buffering solutions. At beginning, buffering solutions of known pH (4,7 and 10.01) were used to calibrate the glass electrode. The PH was estimated by entirely soaking the combined PH sensor for every sample vail, shaking until ten seconds and afterwards documenting the pH in the range. The sensor was cleaned with deionized water and preserve dried between measurements [9]. Then, 25 mi of Pepsi cola was placed in a glass beaker at room temperature at 37 and glass electrode was inserted in it and PH level was displayed on meter pH=2.6. Pepsi cola contains carbonated water, sugar, colour (caramel), caffeine, natural flavour, and phosphoric acid. Nutrition information per 300 ml are energy 126 kcal, sodium less than 10 mg, carbohydrate 10.6 gm, total sugars 10.6 g, protein 0.0 gm, and fat 0.0 gm
Preparation of treatment agents
All agents include as Nano Hydroxyapatite (NHA) and Micro Hydroxyapatite (HA) used were prepared. The therapy is 2 minutes after that the hydrogels were investigated by using reflectance Fourier Transform Infrared Spectroscopy (FTIR) in the ministry of science and technology [10,11].
The evaluative assays
Surface roughness assessment: To measure the changes in primary enamel roughness and surface morphology in the three phases (after first exposure to Pepsi, after treatment to different gels and after second exposure to Pepsi), the samples will be attached to a double sided tape and tested individually by Atomic Force Microscope (AFM). AFM is used to read a surface of the specimen with a very tiny tip on the base of an elastic cantilever while delivering a minimal, normal load [12]. To quantify Nano-roughness, three nanometre roughness parameters will be calculated: Average roughness value (Ra), this is the summation of the hills' heights and valleys' depths from just a mean line [13]. This value indicates the actual roughness of the surface. Root mean square roughness (Rq), which is the overall height variation as a percentage of the mean line. Average maximum height of the profile (Rz) [14].
To create a topographic image of the surface, the AFM was used in tapping mode with a line scan in the centre of the sample surface. The photographs of each sample were captured at a scan size of 10 × 10 um to create two-dimensional (2D) and three-dimensional (3D) pictures [15]. Measurement of the surface height at each pixel or point in a picture yielded the produced pictures. In order to create multi-coloured pictures of surface topography, these heights were often depicted as colours, with low heights or valleys being represented by a dark hue and high regions or peaks being represented by a shining or white hue [13]. Roughness is often defined as closely spaced imperfections on a surface. The vertical separation from a surface's ideal shape is used to calculate it. The surface is rough when the gap is big and smooth when the gap is small.
Statistical analysis
Statistical Package For Social Science (SPSS-22, Chicago, Illinois, USA) was used as descriptive statistics were minimum, maximum, mean and standard deviation (SD) while the inferential statistics were Shapiro Wilk, Levene test, repeated measure ANOVA with Bonferroni posthoc test and partial eta square as small 0.01-0.059, medium 0.06-0.139, large>=0.14 [16].
Results
FTIR test
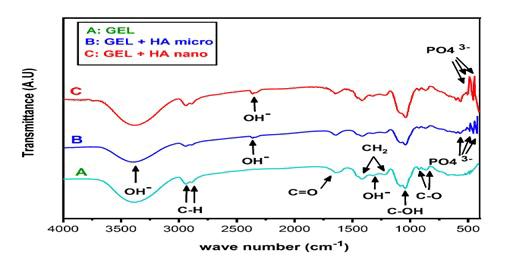
Figure 1 show all sample FTIR spectra were taken in the range of 400 to 4000 cm-1.
Figure 1: All sample FTIR spectra were taken in the range of 400 to 4000 cm-1. IN (A) the stretch vibration of the O-H group was detected in a broad range extending from 3000 to 3600.
AllsampleFTIRspectraweretakenintherangeof400to 4000cm-1. IN (A) the stretch vibration of the O-H group was detected in a broad range extending from 3000 to 3600 which belong to sorbitol, glycerol, NHA and HA. Carbon-hydrogen bonding stretching vibrations (C-H) caused the band from 2940 to 2960 which belong to sorbitol and glycerol. The wavelengths of the C=O, CH2, C-O and OH functional groups were 1709, 1410, 950 and 1320 correspondingly which belong to carboxymethyl cellulose. IN (A) all bands was founded in (B and C). Around 603,470, peaks were visible, which were consistent with po4 which belong to NHA and HA.
All studied variables as Ra, Rq and Rz are normally distributed among phases and groups using Shapiro Wilk at p>0.05. Results below in Tables 1-3 show that in remineralization phase and 2nd exposure to pepsi cola Ra, Rq and Rz values are more in HA followed NAF while the lowest is in the NHA with significant difference, further more using multiple comparisons, in remineralization phase only between NHA and HA is significant while in 2nd exposure, NHA-HA and NHA-NAF are significant, when compared between phases by groups, results show that Ra, Rq and Rz in all groups is decreased then increased when using 2nd exposure to Pepsi cola with significant difference, further using multiple pairwise comparisons, all results between phases by groups are significant.
| Groups | 1st exposure | Remineralization | 2nd exposure | F | P value | ES | |
|---|---|---|---|---|---|---|---|
| NHA gel | Min. | 123.775 | 24.572 | 33.236 | 85.837 | 0.000* | 0.876 |
| Max. | 220 | 49.266 | 77.597 | ||||
| Mean | 159.893 | 39.797 | 58.543 | ||||
| ± SD | 32.273 | 7.966 | 12.63 | ||||
| HA gel | Min. | 132.079 | 40.212 | 82.06 | 91.767 | 0.000* | 0.863 |
| Max. | 194 | 89.969 | 126.774 | ||||
| Mean | 155.585 | 63.506 | 102.349 | ||||
| ± SD | 20.408 | 16.324 | 14.909 | ||||
| NAF gel | Min. | 126.73 | 25.878 | 43.213 | 81.928 | 0.000* | 0.868 |
| Max. | 196 | 103 | 140.578 | ||||
| Mean | 152.008 | 53.003 | 84.011 | ||||
| ± SD | 19.657 | 22.037 | 32.795 | ||||
| F | 0.254 | 5.191 | 9.963 | ||||
| P value | 0.778^ | 0.012* | 0.001* | ||||
| ES | 0.018 | 0.278 | 0.425 | ||||
| ^=not significant at p>0.05, *=significant at p<0.05,ES=Effect size | |||||||
Table 1: Descriptive and statistical test of Ra by groups and periods.
| Groups | 1st exposure | Remineralization | 2nd exposure | F | P value | ES | |
|---|---|---|---|---|---|---|---|
| NHA gel | Min. | 123.775 | 24.572 | 33.236 | 85.837 | 0.000* | 0.876 |
| Max. | 220 | 49.266 | 77.597 | ||||
| Mean | 159.893 | 39.797 | 58.543 | ||||
| ± SD | 32.273 | 7.966 | 12.63 | ||||
| HA gel | Min. | 132.079 | 40.212 | 82.06 | 91.767 | 0.000* | 0.863 |
| Max. | 194 | 89.969 | 126.774 | ||||
| Mean | 155.585 | 63.506 | 102.349 | ||||
| ± SD | 20.408 | 16.324 | 14.909 | ||||
| NAF gel | Min. | 126.73 | 25.878 | 43.213 | 81.928 | 0.000* | 0.868 |
| Max. | 196 | 103 | 140.578 | ||||
| Mean | 152.008 | 53.003 | 84.011 | ||||
| ± SD | 19.657 | 22.037 | 32.795 | ||||
| F | 0.254 | 5.191 | 9.963 | ||||
| P value | 0.778^ | 0.012* | 0.001* | ||||
| ES | 0.018 | 0.278 | 0.425 | ||||
| ^=not significant at p>0.05, *=significant at p<0.05, ES=Effect size | |||||||
Table 2: Descriptive and statistical test of Rq among groups and periods.
| Groups | 1st exposure | Remineralization | 2nd exposure | F | P value | ES | |
|---|---|---|---|---|---|---|---|
| NHA gel | Min. | 335.284 | 92.377 | 108.704 | 45.095 | 0.000* | 0.776 |
| Max. | 890 | 201.67 | 269.469 | ||||
| Mean | 513.024 | 152.752 | 201.91 | ||||
| ± SD | 165.153 | 33.102 | 47.004 | ||||
| HA gel | Min. | 363.851 | 128.951 | 175.993 | 40.475 | 0.000* | 0.757 |
| Max. | 487 | 283.802 | 396.935 | ||||
| Mean | 426.945 | 208.346 | 326.191 | ||||
| ± SD | 47.895 | 58.281 | 74.863 | ||||
| NAF gel | Min. | 308 | 102.458 | 214 | 36.607 | 0.000* | 0.738 |
| Max. | 506 | 260 | 389.535 | ||||
| Mean | 419.96 | 176.908 | 277.685 | ||||
| ± SD | 69.493 | 58.071 | 61.648 | ||||
| F | 2.343 | 3.478 | 10.134 | ||||
| P value | 0.115^ | 0.045* | 0.001* | ||||
| ES | 0.148 | 0.18 | 0.429 | ||||
| ^=not significant at p>0.05, *=significant at p<0.05, ES=Effect size | |||||||
Table 3: Descriptive and statistical test of Rz among groups and periods.
The AFM 2D, 3D images analysis
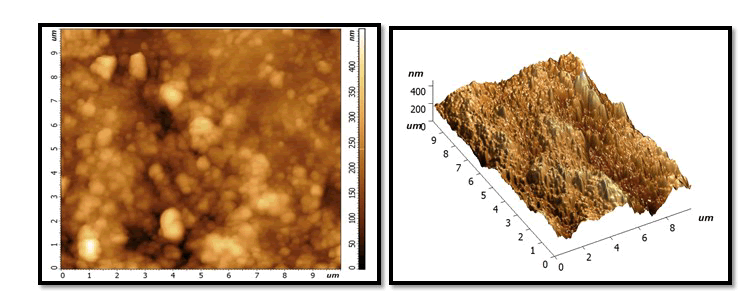
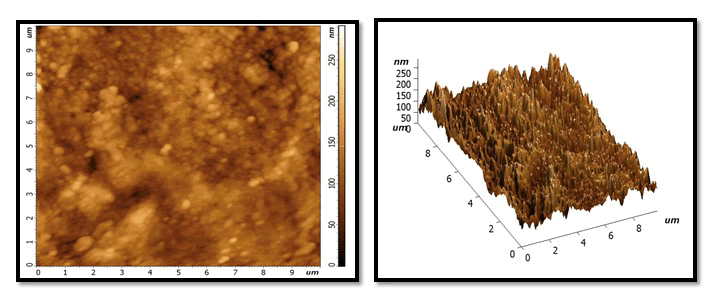
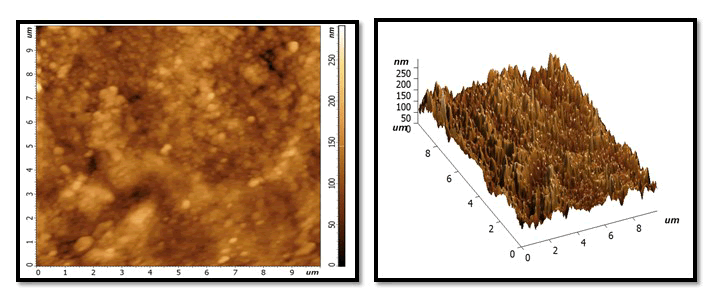
So according, the quantity and distribution of grains, highest elevations, lowest depressions, scratches, and voids were assessed visually for each stage by the number and distribution of grains, largest elevations, least depressions, scratches, and gaps.
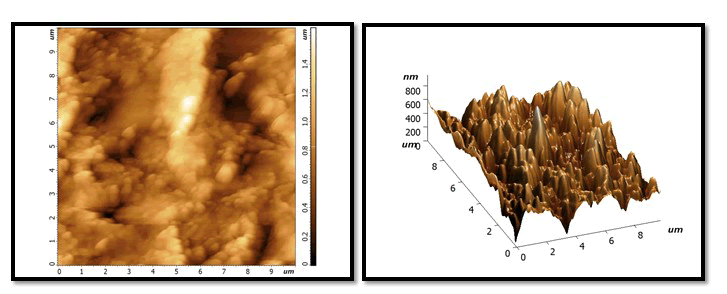
Demineralization phase: With extremely abnormalities, irregularities and grains of tooth enamel, very high peaks, and varied high depressions, the size and number of holes and voids has grown. Dark colour on surface cause by stain of Pepsi cola and also due to loss large amount of minerals (Figures 2-7).
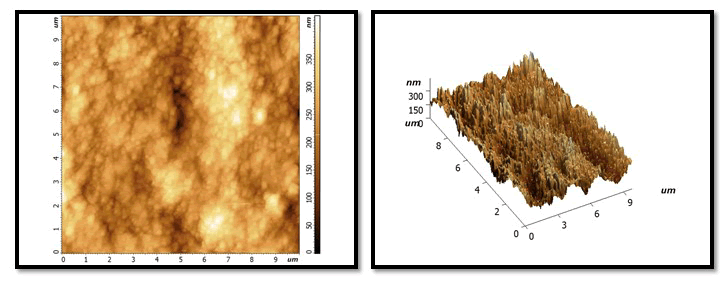
Figure 2: After treatment with NHA GEL: A uniform and homogeneous coating with a smooth surface and more particles and grain, with some lightness and dark area visible in on surface locations. But whiter than the same sample in the previous stage.
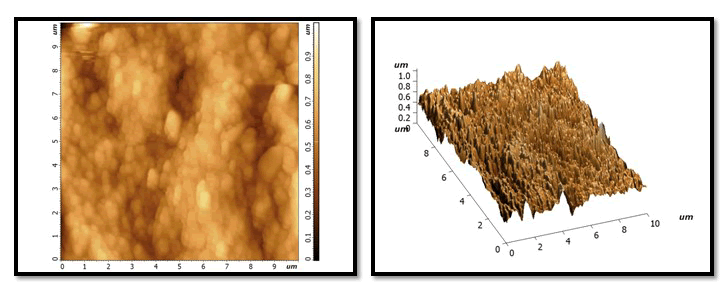
Figure 3: After treatment with HA gel: it will show few homogenous layer with slight flattened surface and little of granules and grains distributed at the surface less than in NHA picture. Whiter colour than the same sample in previous stage but less than the samples colour in NHA group of treatment phase.
Figure 4: After treatment NAF gel: show globular crystal in which has non uniform shape on surface with dark colour more than NHA and HA.
Figure 5: After second exposure to Pepsi cola.
NHA GROUP: still homogenous layer but little darkness than NHA in remineralization phase. No large change notes on valley and picks compare with previous stage and still characterized feature as uniform layer.
Figure 6: HA group: increase in height of peaks and depression of Valles and increase darkness more than previous stage and more than NHA in the same stage.
Figure 7: NAF group: similarity between the characterized feature between it and HA in same stage according to shape of valley and depression and colour.
Discussion
Because of lifestyle modifications and nutritional habits, erosion, a dynamic process involving periods of demineralization and remineralization, has become a prevalent concern in modern civilizations. Therefore, the best strategy for Erosion management is to focus on methods of improving remineralization process with the aid of best remineralization agents. Commercially, a variety of remineralization agents are available such as Nano hydroxyl apatite, fluoride [18]. Pepsi cola more feverous drinking for child among acidic beverages. The link between acidic soft drinks and the development of tooth erosion is well-established. Acidic drinks have been studied as a cause of enamel and dentin erosion in a number of researches. Furthermore, the erosiveness of the identical drinks created controversial in erosive activity in various lands [19]. Acids will demineralize, soften, and reduce the micro hardness of the enamel surface when the pH in the oral cavity falls to its critical value of 5.5 [20]. Phosphoric acid (Pepsi Cola ingredient) has a huge effect since it has a greater ionic strength and calcium binding than citric acid [21]. Pepsi Cola which contains phosphoric acid had more erosive activity than orange juices which contain citric acid [22]. The current study show Pepsi cola pH=2.6 increase in Ra Rq Rz after first erosion this agree with Barac who report roughness of the enamel surfaces increase more after exposed to Coca-Cola than other beverages for 60 min. The rise in surface roughness of enamel associated with Pepsi Cola has been extensively established [23]. The larger roughness can be seen in picture of demineralization phase in result. While fluorides have been added to toothpastes that claim to prevent demineralization and aid remineralization, their capacity to do so is restricted by the low concentrations of calcium and phosphate ions in saliva. Furthermore, NHA material could prevent enamel microscale specimens from irritation, erosions, wear, and cavities by repairing those [24]. There is controversial comparison between Nano Hydroxyapatite and fluoride effect in remineralization erosive enamel. Also there is no study investigation new gel to treatment and prevents erosion. Hence, the aim of this study was to evaluate the remineralization efficacy of three different agents using surface roughness analysis. As available there is no Iraqi study for my design study for using Pepsi cola as in erosive agent with remineralization agents of NHA, HA, NAF so this study used as baseline data for other Iraqi studies to show this agent and effects by erosive agent for preventive strategies.
FTIR test: The FTIR in (A) shows no change in bands and no reaction between materials in gel. The FTIR (B) and (C) spectra revealed that no reaction between materials and the CMC hydrogels were completely blended and that HA, NHA materials had been effectively integrated into the material.
NHA GEL: The result show that in remineralization phase to Pepsi cola Ra, Rq, Rz are less in NHA than NAF and HA. NHA is more effective (decrease roughness) because of strongest affinity of it, biocompatibility, bioactivity and small particle size which lead NHA particles best adsorption on enamel surface and give more essential adequate amount of minerals (calcium and phosphate)[36]. It able to enter and repair defects and cavities of enamel surface and form uniform layer of crystals which decrease roughness [25,26]. This study agree with who repot use NHA significantly remineralization incipient enamel lesions [27,28]. The results of current study were agreement with Andrea [29]. who report efficacy of a biomimetic and activity of Nano-hydroxyapatite remineralizationsolution in a hypo mineralized enamel surface.
Preventive phase: Increase the mineral content of remineralization enamel by supplying enamel surface with sufficient minerals to form a new uniform layer of protection or shield against second exposure to Pepsi cola [27]. The current study is agreed with Maurizio et al 2019 who report of remineralization of the enamel of deciduous teeth, becoming more prevention measure against cavities especially for high-risk individuals [30,31]. Also who report NHA gels provide surface enamel resistance to remineralization which effectively prevented demineralization of enamel.
NAF Gel: The result NAF gel show that in remineralization phase and 2nd exposure to Pepsi cola Ra, Rq, Rz are decrease also(decrease roughness) because formation Flouroapatite crystal increases the stability of Ca+ and PO4+. Thus, filling surface defect cause by Pepsi cola without adding extra minerals [32]. The current study agree with Masatoshi Ando show decrease roughness enamel after apply topical sodium fluoride.
Preventive phase: A fluoride reservoir on enamel surface owing to CaF2 deposition; which affords extra minerals to be added to enamel following NaF treatment this agreement by Philipp Korner [33,34]. Who report fluoride gels are able to significantly reduce gastro oesophageal reflux induced loss of enamel.
Ha gel: The result HA gel show that in remineralization phase and 2nd exposure to Pepsi cola Ra, Rq, Rz are decrease, and has a wide range of applications in this field. Specifically, It has been shown to remineralization both enamel and dentin. The current study agrees with Sonja who report the investigated toothpaste in this study contains micro-hydroxyapatite. It might be suspected that the micro-hydroxyapatite particles are less able to penetrate the lesion body of demineralized enamel but at the same time might form a sealing deposition on the porous surface [35-38].
This study agree with Korner who report Brushing with the micro-HTP showed significantly less remineralization than brushing with 1,400 ppm FTP [39].
In prevention of enamel is agree Kate Hornby who report calcium ions from the HA particles are available to be involved in enamel remineralisation processes that are relevant to help protection from dental erosion in vivo [40].
In de mineralization phase, although the same erosive agent and same exposure time but show less effective of erosion action because of increase calcium and phosphate content in hydroxyapatite act as strongest crystal in addition new layer coated the erosive surface act as buffering agent against Pepsi cola. In NAF group show caf release fluoride when exposure to Pepsi cola and act as buffering agent also flourohydroxyapaptie act as reservoir for calcium and phosphate from saliva use it as protective defence as buffering when second exposure to Pepsi cola.
Comparison between NHA, HA, NAF gels
In current study show three different gels have remineralization effect on erosive enamel in which NHA gel more effective in remineralization of erosive enamel than NAF and HA.
In remineralization period NHA, HA significant difference this result because NHA have a high affinity to bind to substances due to an increased surface area which may improve remineralisation and matches the Nano-sized defects due to acidic erosion at the enamel surface [41]. Micro-HA particles are about 5-10 microns in size, and this is significantly larger than enamel HA and dentinal tubules. Therefore these are not as effective in remineralisation and in reducing sensitivity [42]. The protective effect of NHA is agree with Ayse Dundar, Meyer, Srujana Karumuri who report NHA repair porosities and replace calcium and phosphate and create sacrificial layer which resistance to the acid NHA has large surface energy, more solubility, and high active biologically than HA [43,44].
No different in between NHA, NAF in remineralization Enamel surface this current study agree with AyÅ?e Dundar who report not anti-erosive different between NHA and NAF effect on enamel surface [43].
Significant difference between NHA,NAF in preventive erosion this study agree by 2014 who report compared fluoride varnish and n-HAP paste in their study n-HAP was more protective than fluoride varnish by using AFM.
HA –NAF no significant different remineralisation and prevention. The current study agree by 2019 who report both fluoride and HA Have same effect in remineralization and preventive demineralization of human primary teeth [45].
From the result above from current study, it has been concluded that NHA gel better than NAF gel and HA gel for both treatment and protection erosive enamel. NHA can be used safety in pregnant women and child without risk of dental fluorosis or acute or chronic toxicity related to fluoride over dose. For this reason can be use NHA as daily or professional application without risk.
Conclusion
Based on the outcomes of this research, it can be concluded that:
- Exposure to Pepsi cola show by AFM leads to rise in the roughness of the primary enamel surface.
- Following exposure to Pepsi cola, a novel formula of 10% NHA gel was shown to have higher remineralization efficiency than 10% HA gel and 1.23% NaF gel on the enamel surface of primary teeth by formation strong flourapatite.
- NHA was shown to be more effective than HA and NAF gels in preventing second exposure to Pepsi cola by forming a new protective, uniform layer that resists erosion in this research.
- NHA gel when administrated to oral gel suitable as professional application for child and pregnant women than HA and NAF to avoid risk of dental fluorosis or toxicity.
References
- Lussi A, Jaggi T. Erosion diagnosis and risk factors. Clin Oral Investig 2008; 12:5-13.
[Crossref] [Google Scholar] [Pubmed]
- Mantonanaki M, Koletsi-Kounari H, Mamai-Homata E, et al. Dental erosion prevalence and associated risk indicators among preschool children in Athens, Greece. Clin Oral Investig 2013; 17:585-593.
[Crossref] [Google Scholar] [Pubmed]
- Touyz LZ, Touyz SJ, Nassani LM. Acidic and alcoholic beverages and Teeth and Clinical Advisories. EC Dent Sci 2018;17:1759-1765.
- Zero DT. Etiology of dental erosionâ??extrinsic factors. Eur J Oral Sci 1996; 104:162-177.
[Crossref] [Google Scholar] [Pubmed]
- Barbour ME, Rees JS. The laboratory assessment of enamel erosion: a review. J Dent 2004; 32:591-602.
[Crossref] [Google Scholar] [Pubmed]
- Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater 2009; 4:034104.
[Google Scholar] [Pubmed]
- Hannig M, Hannig C. Nanomaterials in preventive dentistry. Nat Nanotechnol 2010; 5:565-569.
[Crossref] [Google Scholar] [Pubmed]
- Pepla E, Besharat LK, Palaia G, et al. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol 2014; 5:108.
[Google Scholar] [Pubmed]
- Lussi A, Kohler N, Zero D, et al. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci 2000; 108:110-114.
[Crossref] [Google Scholar] [Pubmed]
- Sadiasa A, Franco RA, Seo HS, et al. Hydroxyapatite delivery to dentine tubules using carboxymethyl cellulose dental hydrogel for treatment of dentine hypersensitivity. J Biomed Eng 2013.
- Moosavi H, Darvishzadeh F. The influence of post bleaching treatments in stain absorption and micro hardness. Open Dent J 2016; 10:69.
[Google Scholar] [Pubmed]
- Russel P, Batchelor D. SEM and AFM: complementary techniques for surface investigations. Microscopy and Analysis 2001; 15:9-12.
- Ajami S, Pakshir HR, Babanouri N. Impact of nanohydroxyapatite on enamel surface roughness and colour change after orthodontic debonding. Prog Orthod 2016; 17:1-8.
[Crossref] [Google Scholar] [Pubmed]
- Tocolini DG, Dalledone M, Brancher JA, et al. Evaluation of the erosive capacity of childrenâ??s beverages on primary teeth enamel: An in vitro J clin exp dent 2018; 10:383.
[Google Scholar] [Pubmed]
- Onwubu SC, Mdluli PS, Singh S, et al. An in situ evaluation of the protective effect of nano eggshell/titanium dioxide against erosive acids. Int J Dent 2018.
[Crossref] [Google Scholar] [Pubmed]
- Ferguson CJ. An effect size primer: a guide for clinicians and researchers.
- Taher NM. Atomic force microscopy and tridimensional topography analysis of human enamel after resinous infiltration and storage in water. Saudi Med J 2013; 34:408-414.
[Google Scholar] [Pubmed]
- Arifa MK, Ephraim R, Rajamani T. Recent advances in dental hard tissue remineralization: a review of literature. Int J Clin Pediatr Dent 2019; 12:139.
[Google Scholar] [Pubmed]
- Murrell S, Marshall TA, Moynihan PJ, et al. Comparison of in vitro erosion potentials between beverages available in the United Kingdom and the United States. J dent 2010 ;38:284-289.
[Crossref] [Google Scholar] [Pubmed]
- Schlossman M, Montana M. Preventing damage to oral hard and soft tissues. Prevention Across the Lifespan: A Review of Evidence-Based Interventions for Common Oral Conditions 2017; 97.
- Shellis RP, Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. Erosive Tooth Wear 2014; 25:163-79.
- Jensdottir T, Holbrook P, Nauntofte B, et al. Immediate erosive potential of cola drinks and orange juices. J Dent Res 2006; 85:226-230.
[Crossref] [Google Scholar] [Pubmed]
- Barac R, Gasic J, Trutic N, et al. Erosive effect of different soft drinks on enamel surface in vitro: application of stylus profilometry. Med Princ Pract 2015; 24:451-457.
[Crossref] [Google Scholar] [Pubmed]
- Lelli M, Marchisio O, Foltran I, et al. Different corrosive effects on hydroxyapatite nanocrystals and amine fluoride-based mouthwashes on dental titanium brackets: a comparative in vitro Int J Nanomedicine 2013; 8:307.
[Google Scholar] [Pubmed]
- Schwendicke F, Al-Abdi A, Moscardó AP, et al. Remineralization effects of conventional and experimental ion-releasing materials in chemically or bacterially-induced dentin caries lesions. Dent Mater J 2019; 35:772-779.
[Crossref] [Google Scholar] [Pubmed]
- Nozari A, Ajami S, Rafiei A, et al. Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: an in vitro J Clin Diagn Res JCDR 2017; 11:ZC97.
[Google Scholar] [Pubmed]
- Biria M, Iranparvar P, Fatemi SM, et al. In Vitro Effects of Three Fluoride-Free Pastes on Remineralization of Initial Enamel Carious Lesions. Pediatr Dent 2021; 43:389-95.
[Google Scholar] [Pubmed]
- Ramesh N, Ratnayake JT, Moratti SC, et al. Effect of chitosan infiltration on hydroxyapatite scaffolds derived from New Zealand bovine cancellous bones for bone regeneration. Int J Biol Macromol 2020; 160:1009-1020.
[Crossref] [Google Scholar] [Pubmed]
- Scribante A, Dermenaki Farahani MR, Marino G, et al. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: visual, adhesion strength, and hardness in in vitro tests. BioMed Res Int 2020.
[Crossref] [Google Scholar] [Pubmed]
- Bossù M, Saccucci M, Salucci A, et al. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: an effective option to fluoride toothpaste. J Nanobiotechnology 2019; 17:1-3.
[Crossref] [Google Scholar] [Pubmed]
- Niwut Juntavee, Apa Juntavee, And Preeyarat Plongniras. Effectiveness of Nanohydroxyapatite on Demineralization of Enamel and Cementum Surrounding Margin of Yttria-Stabilized Zirconia Polycrystalline Ceramic Restoration. Sci World J 2021.
[Crossref] [Google Scholar] [Pubmed]
- Harris NO, Garcia-Godoy F. Nielsen Nathe Ch. Primary preventive dentistry. 8th ed. The United States of America: Pearson. 2014; 54.
- Scaramucci T, Borges AB, Lippert F, et al. Sodium fluoride effect on erosionâ??abrasion under hyposalivatory simulating conditions. Arch Oral Biol 2013; 58:1457-1463.
[Crossref] [Google Scholar] [Pubmed]
- Körner P, Georgis L, Wiedemeier DB, et al. Potential of different fluoride gels to prevent erosive tooth wear caused by gastroesophageal reflux. BMC Oral Health 2021; 21:1-7.
[Crossref] [Google Scholar] [Pubmed]
- Schlagenhauf U, Kunzelmann KH, Hannig C, et al. Impact of a nonâ?fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly cariesâ?susceptible orthodontic patients: A randomized, controlled 6â?month trial. J Investig Clin Dent 2019; 10:12399.
[Crossref] [Google Scholar] [Pubmed]
- Cieplik F, Rupp CM, Hirsch S, et al. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC Oral Health 2020; 20:1-8.
[Google Scholar] [Pubmed]
- Cieplik F, Rupp CM, Hirsch S, et al. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC Oral Health 2020; 20:1-8.
[Google Scholar] [Pubmed]
- Steinert S, Kuchenbecker J, Meyer F, et al. Whitening Effects of a Novel Oral Care Gel with Biomimetic Hydroxyapatite: A 4-Week Observational Pilot Study. Biomimetics 2020; 5:65.
- Körner P, Schleich JA, Wiedemeier DB, et a. Effects of additional use of bioactive glasses or a hydroxyapatite toothpaste on remineralization of artificial lesions in vitro. Caries Res 2020; 54:336-42.
[Crossref] [Google Scholar] [Pubmed]
- Hornby K, Evans M, Long M, et al. Enamel benefits of a new hydroxyapatite containing fluoride toothpaste. Int dent J 2009; 59:325-3231.
- Enax J, Fabritius HO, Fabritius-Vilpoux K, et al. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health careâ?? state of the art. Open Dent J 2019; 13:274-87.
- Enax J, Epple M. Synthetic hydroxyapatite as a biomimetic oral care agent. Oral Health Prev Dent 2018; 16:7-19.
[Google Scholar] [Pubmed]
- Dündar A, Å?engün A, BaÅ?lak C, et al. Effects of citric acid modified with fluoride, nano-hydroxyapatite and casein on eroded enamel. Arch Oral Biol 2018; 93:177-186.
[Crossref] [Google Scholar] [Pubmed]
- de Carvalho FG, Vieira BR, Santos RL, et al. In vitro effects of nano-hydroxyapatite paste on initial enamel carious lesions. Pediatr Dent 2014; 36:85-89.
[Google Scholar] [Pubmed]
- Amaechi BT, AbdulAzees PA, Alshareif DO, et al. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ open 2019; 5:1-9.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Ali Jameel Abdulsahib1* and Zain Alabdeen Abdul Abbas Noaman2
1Department of Prosthodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq2Department of Pedodontic, College of Dentistry, University of Baghdad, Baghdad, Iraq
Citation:
Zain Alabdeen Abdul Abbas Noaman, Ali Jameel Abdulsahib, Evaluating Three Different Gels in Treating and Protecting the Primary Teeth Enamel Surface Erosion, J Res Med Dent Sci, 2022, 10 (7):000-000.
Received: 02-Jun-2022, Manuscript No. JRMDS-22-52419; , Pre QC No. JRMDS-22-52419; Editor assigned: 07-Jun-2022, Pre QC No. JRMDS-22-52419; Reviewed: 21-Jul-2022, QC No. JRMDS-22-52419; Revised: 02-Aug-2022, Manuscript No. JRMDS-22-52419; Published: 11-Aug-2022