Research - (2025) Volume 13, Issue 5
Evaluation of Anti-Hyperlipidemic Activity of Clerodendrum Serratum on High Cholesterol Diet Induced Hyperlipidaemia in Rats
*Correspondence: K Mahammad Areef, Department of Pharmacology, Srilakshmi Venkateshwara Institute of Pharmaceutical Sciences Kothapeta, Proddatur, Kadapa, Andhra Pradesh, India, Email:
Abstract
Obesity has turned up as one of the major health concerns in the 21st century and is one of the leading causes of preventable death. Obesity is a term applied to excess body weight with an abnormally high proportion of body fat. Thermo dynamically speaking, imbalance between energy intake (feeding) and energy expenditure. Development of obesity is, however, more complicated than that; sedentary life style, genetic factors, medical illness, microbiological aspects, social factors and neurobiological mechanisms are also involved. Pre-clinical evaluator study of compound can be done by using three animal models i.e., drug induced obesity, food induced obesity and genetically modified C57BL/6J female mice. The neuro active steroid, progesterone is a female reproductive hormone. Its level increases during the later phase of the menstrual cycle and controls the secretory phase of the endometrium. Substantial evidence links progesterone excess in pathophysiology of eating and affective disorders. Some reports suggest the use of progesterone containing preparations as contraceptive or for the hormone replacement therapy to cause sufficient weight gain by causing hyperphagia and increased fat deposition in the body.
Keywords
Clerodendrum serratum, Hyperlipidaemia, High cholesterol diet, Diabetes, Hypothyroidism, Cushing's syndrome, Kidney failure
Introduction
Obesity has reached epidemic proportions globally, with more than 1 billion adults overweight - at least 300 million of them clinically obese - and is a major contributor to the global burden of chronic disease and disability. Often coexisting in developing countries with under-nutrition, obesity is a complex condition, with serious social and psychological dimensions, affecting virtually all ages and socioeconomic groups WHO (2009). Increased consumption of more energy-dense, nutrient-poor foods with high levels of sugar and saturated fats, combined with reduced physical activity, have led to obesity rates that have risen three-fold or more since 1980 in some areas of North America, the United Kingdom, Eastern Europe, the Middle East, the Pacific Islands, Australasia and China. The obesity epidemic is not restricted to industrialized societies; this increase is often faster in developing countries than in the developed world WHO (2009). A growing public health concern is that the prevalence of obesity among children aged 6–19 is up to 16.5% in the USA [1] and has also increased in Europe, Asia, Africa and South American countries [2].
Despite increased attention given to overweight and obesity by virtually every major body concerned with public health, including the National Institutes of Health (NIH) [3] the Centre’s for Disease Control, the United States Department of Agriculture and the World Health Organization primary and secondary prevention efforts have generally been disappointing. Obesity impacts many facets of society. For example, it is economically costly to society increases mortality rate reduces quality of life and increases the risk of various morbidities. Extreme obesity has been estimated to truncate the lifespan of young adults by 5–20 years. The medical problems caused by obesity begin at the head and end at the toes and involve almost every organ in between. Several of these problems contribute to the earlier mortality associated with obesity and include coronary artery disease, severe hypertension that may be refractory to medical management, impaired cardiac function, adult-onset (type 2) diabetes mellitus, obesity hypoventilation and sleep apnea syndromes, cirrhosis, venous stasis and hypercoagulability with an increased risk of pulmonary embolism, and necrotizing panniculitis [4].
The association of obesity with T2DM (Type 2 DM) has been observed in comparisons of different populations and within populations. Approximately 10% of the obese population develop T2DM. This may be because of “glucotoxicity” (expression of glucose allostasis and increase in allostatic load), “lipotoxicity” fatty acid supply to peripheral tissues impairs glucose uptake and storage in the muscle and adipokines (adipose tissue) hormones that are secreted by the adipose tissue (TNF-a, IL-6, complement C3, MIF, adiponectin) are associated with insulin resistance, often independently of the degree of adiposity. Unfortunately, with the exception of surgery (a procedure appropriate for only a minority of obese individuals), available treatments for obesity are, at best, of modest efficacy. With regard to nonsurgical treatments for obesity, Ayyad and Andersen, in a quantitative synthesis of the literature, found that even in the best of conditions, the median percentage of patients who achieved and maintained clinically meaningful weight loss for at least 3– 5 years was only about 20%, whereas others have reported that of those who lose weight, 90– 95% eventually regain it [5].
Review of Literature
Disease profile
Introduction
Definition
Obesity is a chronic disease that causes risks ill health, impaired quality of life, and premature mortality. Obesity results from a complex interaction of genetic predisposition, environmental, societal and individual psychological factors that all summate to produce a chronic positive energy balance. Obesity results from a positive energy balance i.e., when caloric intake chronically exceeds energy expenditure. This in turn causes excess of adipose tissue mass with body mass index (BMI >30 kg/m2) (Caterson, 1999).
The classification of body weight according to WHO is as follows:
• BMI<18.5=Underweight
• BMI 18.5-24.9=BMI 18.5 - 24.9
• BMI 25-29.9=Overweight
• BMI 30-39.9=Obese
• BMI 40 or higher=Severely obese
Although there is a correlation between obesity and cardiac disease, BMI is not a precise predictor of cardiovascular disease; absolute waist circumference or waist to hip ratio are more precise measures of central obesity and correlate better to health risks than BMI [6].
Obesity and insulin resistance diabetes
The medical problems caused by obesity begin at the head and end at the toes and involve almost every organ in between. Several of these problems contribute to the earlier mortality associated with obesity include adult-adultonset (type 2) diabetes mellitus, coronary artery disease, severe hypertension that may be refractory to medical management, impaired cardiac function, obesity hypoventilation and sleep apnea syndromes, cirrhosis, venous stasis and hypercoagulability with an increased risk of pulmonary embolism, and necrotizing panniculitis [7].
Epidemiology
Globally, more than 1.1 billion adults worldwide are overweight, and 312 million of them are obese. In addition, at least 155 million children worldwide are overweight or obese, according to the International Obesity Task Force. This task force and the World Health Organization (WHO) have revised the definition of obesity to adjust for ethnic differences, and this broader definition may reflect an even higher prevalence with 1.7 billion people classified as overweight worldwide.
About 18 million people die every year from cardiovascular disease, for which diabetes and hypertension are major predisposing factors. Propelling the upsurge in cases of diabetes and hypertension is the growing prevalence of overweight and obesity.
Etiology of obesity
The exact Etiology of obesity is unclear. The multiple causative factors like genetic, environmental, nutritional, physiological, psychological, social and cultural factors have been linked to its development and progression [8].
Environmental factors
The current environmental risk factors include over consumption of energy (increase in fat to carbohydrate ratio) and decrease in physical activity. These factors offer more reasonable explanation for the recent dramatic surge in the prevalence of obesity.
Nutritional factors
Numerous metabolic studies have shown that high fat diets may lead to a high energy intake and hyperphagia. The reason may be that fat has a weaker effect on the satiety Centre and on heat production (diet-induced thermogenesis) and it possesses a higher energy density compared to carbohydrates. Also, fats are highly palatable and heighten the flavour of food stuffs which leads to their passive overconsumption. This ultimately increases fat deposits and causes obesity and related problems [9].
Physiological factors
These involve the impairment of the central mechanism regulating appetite and food intake which is thought to be regulated by a complex interplay of neurotransmitters in the hypothalamic region of the brain. Approximately 1-2% of obesity can be ascribed to lesions in hypothalamic regulatory centers. Such lesions may be due to trauma, tumours, inflammatory processes, or carotid artery aneurysms.
Psychological factors
The psychogenic theory of obesity long held that obesity resulted from an emotional disorder in which food intake, relieved the anxiety and depression to which obese persons are usually susceptible. Stress associated with traumatic emotional events has been held responsible for certain cases of obesity and has been implicated in the pathogenesis of eating disorders such as night-eating syndrome and bulimia [10].
Role of Genes versus Environment
Obesity is commonly seen in families, and the hereditability of body weight is similar to that for height. Inheritance is usually not Mendelian, however, and it is difficult to distinguish the role of genes and environmental factors. Adoptees usually resemble their biologic rather than adoptive parents with respect to obesity, providing strong support for genetic influences. Likewise, identical twins have very similar BMIs (Body Mass Index) whether reared together or apart, and their BMIs are much more strongly correlated than those of dizygotic twins. These genetic effects appear to relate to both energy intake and expenditure.
Specific Genetic Syndromes
For many years obesity in rodents has been known to be caused by a number of distinct mutations distributed through the genome. Most of these single-gene mutations cause both hyperphagia and diminished energy expenditure, suggesting a link between these two parameters of energy homeostasis. Identification of the ob. gene mutation in genetically obese (ob/ob) mice represented a major breakthrough in the field [11].
Other Specific Syndromes Associated with Obesity
Insulinoma Patients with insulinoma often gain weight as a result of overeating to avoid hypoglycaemia symptoms. The increased substrate plus high insulin levels promotes energy storage in fat. This can be marked in some individuals but is modest in most.
Cushing’s syndrome
Although obese patients commonly have central obesity, hypertension, and glucose intolerance, they lack other specific stigmata of Cushing‘s syndrome. Nonetheless, a potential diagnosis of Cushing‘s syndrome is often entertained. Cortisol production and urinary metabolites (17OH steroids) may be increased in simple obesity. Unlike in Cushing ‘s syndrome, however, cortisol levels in blood and urine in the basal state and in response to corticotrophin-releasing hormone (CRH) or ACTH are normal; the overnight 1-mg dexamethasone suppression test is normal in 90%, with the remainder being normal on a standard 2-day low-dose dexamethasone suppression test. Obesity may be associated with local reactivation of cortisol in fat by 11 ß hydroxyl steroid dehydrogenase 1, an enzyme that converts cortisone to cortisol [12].
Hypothyroidism
The possibility of hypothyroidism should be considered, but it is an uncommon cause of obesity; hypothyroidism is easily ruled out by measuring thyroid-stimulating hormone (TSH). Much of the weight gain that occurs in hypothyroidism is due to myxoedema [13].
Craniopharyngioma and other disorders involving the hypothalamus
Whether through tumors, trauma, or inflammation, hypothalamic dysfunction of systems controlling satiety, hunger, and energy expenditure can cause varying degrees of obesity. It is uncommon to identify a discrete anatomic basis for these disorders. Subtle hypothalamic dysfunction is probably a more common cause of obesity than can be documented using currently available imaging techniques. Growth hormone (GH), which exerts lipolytic activity, is diminished in obesity and is increased with weight loss. Despite low GH levels, insulin-like growth factor (IGF) I (somatomedin) production is normal, suggesting that GH suppression is a compensatory response to increased nutritional supply [14].
Types of obesity
Central (android) (apple) versus Peripheral (gynoid) (pear)
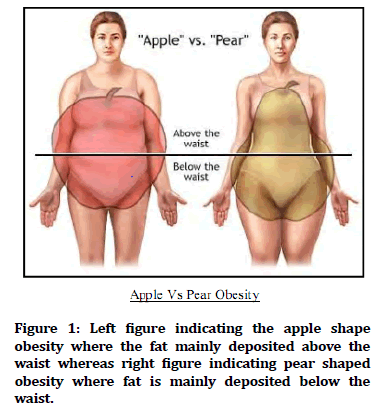
It has been noted that central (android, more prevalent in men) obesity is associated with a higher mortality than peripheral (gynoid, more prevalent in women) obesity. This discrepancy has been attributed to the fact that visceral adipose tissue is metabolically more active than subcutaneous fat, causing a greater rate of glucose production, type II diabetes mellitus, and hyper insulinism. Increased insulin secretion is thought to increase sodium reabsorption and result in hypertension. Central obesity is also associated with increased production of cholesterol, primarily in the form of lowdensity lipoprotein, leading to increased incidence of atherosclerotic cardiovascular disease and gallstones. The increased visceral fat has been related to an increased waist: hip ratio or, in more common terms, as the appleâ?? versus pearl distribution of fat (Figure 1). Computed tomography (CT) scans, however, have noted a much better correlation between anterior-posterior abdominal diameter and visceral fat distribution than the waist: hip ratio, especially in women with both central and peripheral obesity. In this situation the peripheral obesity dilutesâ?? the central obesity as measured by the waist: hip ratio, so either waist circumference alone or sagittal abdominal diameter should be used as a measurement of central obesity [15].
Figure 1: Left figure indicating the apple shape obesity where the fat mainly deposited above the waist whereas right figure indicating pear shaped obesity where fat is mainly deposited below the waist.
The adipocyte and adipose tissue
Adipose tissue is composed of the lipid-storing adipose cell and a stromal/vascular compartment in which preadipocytes reside. Adipose mass increases by enlargement of adipose cells through lipid deposition, as well as by an increase in the number of adipocytes. The process by which adipose cells are derived from a mesenchymal preadipocyte involves an orchestrated series of differentiation steps mediated by a cascade of specific transcription factors. One of the key transcription factors is peroxisome proliferator activated receptor γ (PPARγ), a nuclear receptor that binds the thiazolidinedione class of insulin-sensitizing drugs used in the treatment of type 2 diabetes [16].
Management of obesity
Currently three options are available for the treatment of obesity and associated complications are nonpharmacological treatment, pharmacotherapy and surgical procedures, each of these are having their own advantageous and drawbacks.
Non-Pharmacological approach
Non-pharmacological measures are preferred for many reasons for many reasons including adverse effects of anti-obesity drugs, contraindications or allergic reactions to drugs, perceptions of adverse effects of drugs, or personal preference for natural or alternative therapies. A more aggressive integrative approach to the management of obesity is recommended to improve outcomes, minimize adverse effects, and reduce health care costs. Non-pharmacological treatment consists of lifestyle modification, reduction of total caloric intake and regular aerobic exercise [17].
Lifestyle Modifications
The primary approach for achieving weight loss, in the vast majority of cases, is lifestyle modification, including a reduction in energy intake and an increase in physical activity [18].
Diet
There is no evidence to suggest that specific components of the diet (i.e., carbohydrate, fat, protein, vitamins, and micronutrients) influence the ways in which food energy is absorbed or used. Therefore, the main dietary approach for reducing weight is to reduce the total amount of calories consumed, and this is best achieved by a reduction in the amount of fat in the diet and calories from soft drinks. A moderate decrease in caloric balance (500-1000 kcal/d) will result in a slow but progressive weight loss. In addition, evidence suggests that the components of diet currently recommended as healthy--including low consumption of saturated and trans fats, intake of carbohydrates that are rich in dietary fiber, high fruit and vegetable intake, and the inclusion of low-fat dairy foods—are likely to protect against metabolic syndrome.
Exercise
Most obesity studies have not adequately measured physical activity and functional capacity, and the independent contributions of "fitness" versus "fatness" to health risks associated with obesity are still being debated [19]. Nonetheless, the role of physical activity as a treatment and/or preventive strategy for combating obesity has been the subject of substantial research. A systematic review of the literature concluded that limited evidence from a number of studies that used imaging techniques to quantify changes in abdominal obesity suggests a beneficial influence of physical activity on reduction of abdominal fat and VAT in overweight and obese subjects [20]. Reductions in VAT and total abdominal fat may occur in the absence of changes in body mass and waist circumference.
Pharmacological measures of obesity
Obesity therapies include reducing nutrient absorption and applying anorectic drugs, thermo genic drugs or drugs that affect lipid mobilization and utilization. With the exception of Orlistat, a recently approved gastrointestinal lipase inhibitor, all drugs approved for the treatment of obesity are either catechol aminergic or serotonergic CNS-active (activating the sympathetic nervous system) anorectic agents. Since some of these drugs may lead to dependency, they are recommended for short-term use like amphetamine-like drugs. Upon termination of therapy with these drugs, weight is rapidly regained in many cases.
Surgical approach
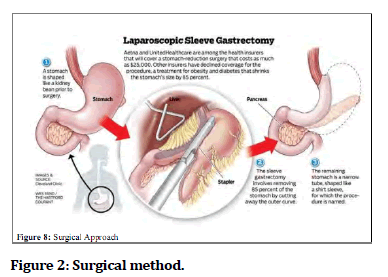
The contrasting effects of various surgical procedures on the metabolic profile have underscored the crucial role of intra-abdominal adipose tissue (i.e., VAT) rather than subcutaneous abdominal adipose tissue (Figure 2). Indeed, whereas large volume liposuction that reduces abdominal subcutaneous fat depots by 8 to 10 kg has almost no favourable effects on the metabolic profile, Omental fat reduction (corresponding to only 0.8% of total body fat) in connection with adjustable gastric banding results in a dramatic improvement in insulin resistance and associated glucose disturbances. Bariatric surgical procedures (i.e. gastro plasty, gastric bypass) are the only procedures that provide marked and sustained weight reduction in morbidly obese patients, leading to improvements in associated metabolic disorders, especially type 2 DM, and a more favourable long-term prognosis, including a reduction in total mortality [21]. However, considering the risk/benefit ratio of bariatric surgery, it may not yet be considered an early option in the management of the abdominally obese patient.
Figure 2:Surgical method.
Literature review of plant
A number of studies have been carried out to investigate the ant obesity effects of polyphenols like apigenin and luteolin, kaempferol, myricetin and quercetin, genistein and diadzein, cyaniding grape seed proanthocyanidin extract (GSPE), xanthohumol and epigallocatechin gallate (EGCG). Likewise, studies involving the effects on lipid metabolism have been carried out with carotenoids like fucoxanthin, Coumarin derivatives like aesculetin and phytoalexins like resveratrol. Other bioactive components of food with ant obesity effects include phytosterols, polyunsaturated fatty acids and organosulfur compounds.
Plant profile
Indian system of health care involves a holistic view of human health. Ayurvedic treatment of a disease consists of salubrious use of crude drugs, diet and certain practices. Plants are being used in medicine from the time immemorial because they have fitted the immediate personal needs, they are accessible and inexpensive. Around 1400 plants are currently used in various Ayurvedic preparations [22]. Plants have long been a very important source of new drugs and many plant species have been screened to see if they contain substances with therapeutic activity. Clerodendrum Serratum (L.) Moon belong to family Verbenaceae is a small perennial shrub growing in moist deciduous forests and occasionally in plains of peninsular India and the Western and Eastern Himalayas up to 1,400 feet above sea level. The leaf and root of this plant have great medicinal value. Root bark contains mainly sapogenins, while leaves contain flavonoids and phenolic acids. The root has been traditionally used in Ayurveda and Siddha systems of medicine for treatment of chronic bronchial asthma and other respiratory diseases, different types of fevers and skin infections (Figure 3). It is one of the ingredients of the Ayurvedic drug. ‘‘Kasadamana,’’ an effective expectorant and antitussive remedy. Plantderived drugs occupy an important place in both traditional and modern medicine [23].
Figure 3:Clerodendrum serratum.
Classification
• Kingdom: Plantae
• Phylum: Tracheophyta
• Class: Magnoliopsida
• Order: Lamiales
• Family: Verbenaceae
• Genus: Clerodendrum
• Species: Serratum
Ayurvedic properties
• Rasam: Tikta, Katu
• Gunam: Lakhu, Rooksha
• Viryam: Ushna
Plant description
A slightly woody shrub with bluntly quadrangular stems and branches, leaves usually three at a node, sometimes opposite oblong or elliptic, serrate; flowers blue, many in long cylindrical thyrsus; fruits 4 lobed purple drupe, somewhat succulent with one pyrene in each lobe.
Medicinal properties
Clerodendrum Serratum (Verbenaceae), shrub distributed though out country, especially common in Assam, Bengal and western gates of India. It is commonly known as Blue flowered glory, Beetle Killer (English), Gantu bharangi (Kannada, Telugu) cherutekku (Tamil). Bharangi (Sanskrit) is bitter in taste and pungent, the pungent taste is used for the post digestive effect. It improves tridosas, vata, pita and kapha. It is used for treatment of conditions as asthma, cough, fever, etc. Plant pacifies vitiated kapha, pitta, inflammations, hypolipidemia, dyspepsia, flatulence, helminthiasis, cough, asthma, bronchitis, hiccough, chronic skin diseases, leucoderma, leprosy and fevers. The leaves can be used or external application in headache. As per the traditional claims roots are the potential source of drugs for aliments such as, body ache, bronchitis, cholera dropsy, eye disease, inflammation, malaria, snake bite, rheumatism, tuberculosis wounds and ulcer [24].
Aim and Objectives
The Goal of the present study is to investigate:
• Anti- Hyperlipidemic activity of Clerodendrum sativum on high cholesterol diet induced hyperlipidaemia in rats.
The following objectives were set to achieve the goal of the present study:
• To perform the phytochemical identification of ethanolic extracts of Clerodendrum sativum.
• To perform the acute toxicity studies of ethanolic extracts of Clerodendrum sativum on mice.
• To investigate the biochemical estimations of various doses of Clerodendrum sativum on lipid profile ( TC, TG, HDL, LDL, VLDL) and kidney parameters urea, uric acid and creatinine in all the groups.
• To investigate the effect of various doses of Clerodendrum sativum on antioxidant enzymes (SOD, GSH and catalase) in all the groups
• Histopathology studies of heart.
Materials and Methods
Materials
Animals
Wistar rats (120-125gms)
Swiss albino mice (20-25gms)
Methods
Identification, collection and authentication of plant material
The aerial parts of Clerodendrum Serratum were collected from tirumala hills belong to Thirupathi, Andhra Pradesh, India. in the year of 2014 (January). The plant specimen was authenticated by Dr. K. Madhava Chetty, Assistant Professor, Botanical survey of medicinal plant unit, Sri Venkateshwara University Thirupathi, Andhra Pradesh. After cleaning parts of plant from foreign particles they spread over trays and separately dried in shade, pulverized by a mechanical grinder and passed through 40-mesh sieve to get the fine powder, finally subjected to extraction [25] (Tables 1and 2)
| S.NO | Chemical | Manufacture |
|---|---|---|
| 1 | Cholesterol | Himedia |
| 2 | Rosvastatin | Ranbaxy |
| 3 | Formalin | Sd-fine chem. Limited |
| 4 | Ethanol | Changshuyangyuan chemicals |
| 5 | Cholesterol kit | Span diagnostics |
| 6 | Triglyceride kit | Span diagnostics |
| 7 | HDL kit | Span diagnostics |
| 8 | Urea kit | Span diagnostics |
| 9 | Creatinine kit | Span diagnostics |
| 10 | Uric acid kit | Span diagnostics |
| 11 | Antioxidant studies kit | Span diagnostics |
Table 1: List of chemicals
| S.NO | Equipment | Manufacture |
|---|---|---|
| 1 | Semi auto analyser (CL380 biochemistry analyser) | Elico |
| 2 | Centrifuge (RM 12C BL) | Remi |
| 3 | Micropipette | Thermo scientific |
| Procedure for 1ml | Procedure for 3ml | |||||
|---|---|---|---|---|---|---|
| B | S | T | B | S | T | |
| Enzyme reagent | 1 ml | 1 ml | 1 ml | 3 ml | 3 ml | 3 ml |
| Standard | - | 10µl | - | - | 30µl | - |
| sample | - | - | 10µl | - | - | 30µl |
Ethanolic extraction of aerial parts of Clerodendrum Serratum
Introduction
The commonly employed technique for the separation of the active constituents from the crude drug is called extraction which involves the use of different solvents. Many of the complex substances metabolized by the plants have therapeutic importance. But these are always found in association with other substances. Therefore, in order to study these active constituents alone it has to be separated from other unwanted substances produced.
Preparation of extract
The aerial parts of Clerodendrum Serratum were collected, washed, dried in shade and pulverized in a grinder- mixer to obtain a coarse powder and then passed through 40 mesh sieves. The powdered drug was subjected to solvent extraction by Soxhlet apparatus [26].
Extraction procedure
About 100g of powdered drug was extracted successively with 70% ethanol using Soxhlet apparatus. The extraction was carried out for 72 hours until the extract becomes colourless. Then the solvent was completely removed by evaporating in rotatory flask evaporator. The dried extract thus obtained was kept in refrigerator until the further experiment [27].
Percentage yield
Percentage yield of 100gms of ethanolic extract of aerial parts of Clerodendrum sativum was found to be 14.6% w/w.
I. Preliminary phytochemical screening:
The Ethanolic extract of aerial parts of Clerodendrum sativum (EECS) was subjected to preliminary phytochemical screening for the detection of various phytochemical constituents such as carbohydrates, alkaloids, Saponins, phenolic compounds, gums, tannins and flavonoids. The detailed study about the phytochemical test procedure as follows
II. Acute toxicity studies
Experimental Animals
Swiss albino mice (20-25gm) and Wistar albino rats (120-125gm) of male rtas were purchased from Sri Venkateshwara Enterprises, Bangalore, India. All animals were maintained in an air-conditioned room at 25°C ± 2°C, with a relative humidity of 75% ± 5%, and a 12-h light/dark cycle. A basal diet and tap water were provided ad libitum. Male and female rats were assigned to each dose group by stratified random sampling based on body weight. The animals were kept under laboratory conditions for an acclimatization period of 7 days before carrying out the experiments [28].
Experimental procedure
Male albino mice weighing 20-25gm were used for the study. The starting dose level of Asparagus gonoclados. Baker was 5, 50, 300, 2000 and 5000 mg/kg body weight p.o. Dose was administered to overnight fasted mice. Food was withheld for a further 3-4 hours after administration of Clerodendron sattivum and observed for signs for toxicity. The body weight of the mice’s before and after administration were noted that changes in eyes and mucous membranes, skin and fur, respiratory, circulatory, autonomic, and central nervous systems, and also motor activity and behaviour pattern. Special attention was directed to observations of convulsions, tremors, diarrhoea, salivation; lethargy, sleep, and coma were noted. The onset of toxicity and signs of toxicity of LD50 values are noted [29].
Pharmacological screening of Hyperlipidemic activity
Cholesterol literally means “solid alcohol from bile”. Cholesterol and triglycerides are two forms of lipid/fat. Cholesterol is made primarily in the liver (about 1,000 mg per day), but it is also created by cells lining the small intestine and by the individual cells in the body.
Cholesterol and other fats cannot dissolve in blood. They have to be transported to and from the cells by special carriers called “lipoproteins” [30]. Important lipoproteins are LDL and HDL14.
The enzymes involved in the cholesterol synthesis are found in the cytosol and microsomal fractions of the cell. Acetate to acetyl Co A provides all the carbon atoms in the cholesterol. Cholesterol synthesis takes place in 5 stages, which includes synthesis of HMG CO A, formation of mevalonate (6c), production of isoprenoid units (5c), synthesis of squalene (30c) and conversion of squalene to cholesterol (27c).
Mechanism of action
Biosynthesis of cholesterol is directly regulated by the cholesterol levels present, though the homeostatic mechanisms involved are only partly understood. A higher intake from food leads to a net decrease in endogenous production, whereas lower intake from food has the opposite effect. The main regulatory mechanism is the sensing of intracellular cholesterol in the endoplasmic reticulum by the protein SREBP (sterol regulatory element-binding protein 1 and 2). In the presence of cholesterol, SREBP is bound to two other proteins: SCAP (SREBP cleavage activating protein) and Insig1. When cholesterol levels fall, Insig-1 dissociates from the SREBP-SCAP complex, which allows the complex to migrate to the Golgi apparatus. Here SREBP is cleaved by S1P and S2P (site-1 and -2 protease), two enzymes that are activated by SCAP when cholesterol levels are low. The cleaved SREBP then migrates to the nucleus, and acts as a transcription factor to bind to the sterol regulatory element (SRE), which stimulates the transcription of many genes. Among these are the lowdensity lipoprotein (LDL) receptor and HMG-CoA reductase. The LDL receptor former scavenges circulating LDL from the bloodstream, whereas HMG-CoA reductase leads to an increase of endogenous production of cholesterol. Then, cholesterol can be filled in arteries which lead to plaque formation and causes insufficient supply of oxygen, B.P increases leads to coronary heart diseases. [American heart association]
Causes
High cholesterol and other lipid disorders can be inherited (genetic) or associated with:
Fatty diets, Diabetes, hypothyroidism, Cushing's syndrome, and kidney failure, certain medications, including birth control pills, estrogen, corticosteroids, certain diuretics, and beta-blockers, Lifestyle factors, including habitual, excessive alcohol use and lack of exercise, leading to obesity.
People who smoke and also have high cholesterol are at even greater risk for heart disease. Lipid disorders are more common in men than women.
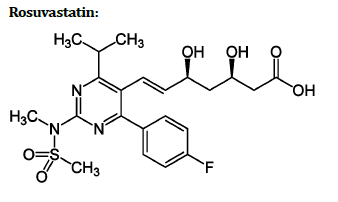
Systematic (IUPAC) name:
(3R, 5S, 6E)-7-[4-(4-fluorophenyl)-2-(Nmethylmethanesulfonamido)- 6-(propan-2-yl) pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid.
Rosuvastatin (marketed by AstraZeneca as Crestor) is a member of the drug class of statins, used in combination with exercise, diet, and weight-loss to treat high cholesterol and related conditions, and to prevent cardiovascular disease. The primary uses of rosuvastatin are for the treatment of dyslipidaemia. It is recommended to be used only after other measures such as diet, exercise, and weight reduction have not improved cholesterol levels. Like all statins, Rosuvastatin works by inhibiting HMG-CoA reductase, an enzyme found in liver tissue that plays a key role in production of cholesterol in the body [31].
Mechanism of action
Rosuvastatin inhibits cholesterol synthesis via the mevalonate pathway by inhibiting 3-hydroxy-3- methylglutaryl-coenzyme A (HMG-CoA) reductase. HMGCoA reductase is the enzyme responsible for the conversion of HMG-CoA to malonic acid, the rate-limiting step of cholesterol synthesis by this pathway. The active form of statins bears a chemical resemblance to the reduced HMG-CoA reaction intermediate that is formed during catalysis. Structure-activity relationship studies have demonstrated that statins bind to HMG-CoA reductase at the same site as the reduced intermediate and are held in place by similar chemical interactions. Unlike Lovastatin and simvastatin, which undergo in vivo hydrolysis to their active form rosuvastatin is synthetically produced in active form. Cholesterol biosynthesis accounts for approximately 80% of cholesterol in the body; thus, inhibiting this process can significantly lower cholesterol levels [32].
Dyslipidaemia
Hypercholesterolemia and mixed dyslipidaemia to reduce total cholesterol, LDL-C, apo-B, triglycerides levels, and CRP as well as increase HDL levels.
• Heterozygous familial
hypercholesterolemia in paediatric patients
• Homozygous familial hypercholesterolemia
• Hypertriglyceridemia (Fredrickson Type IV)
• Primary dys beta lipoproteinemia (Fredrickson Type III)
• Combined hyperlipidaemia
Methodology
Animals
Healthy male wistar albino rats were procured from animal house of Sri Venkateshwara Enterprises, Bangalore, India and weigh about 120-125gms were used for the study. Every experimental animal was clinically examined pre-operatively for any disease. The animals were kept under observation in laboratory and allowed acclimatize, for 7 days before experimentation [33]. Animals kept in separate spacious clean cages under controlled room temperature (25 ± 1) °c & relative humidity (50 ± 15) %; in a 12hrs light-dark cycles. They fed with a standard diet and water ad-libitum. Before the experiment the rats were divided into groups.
Groups
• Group I: Control (Normal control)
• Group II: Positive control (5% cholesterol diet for 3months)
• Group III: Standard (5% cholesterol diet + ROSVASTATIN (20mg/kg))
• Group IV: Test I (Plant Extract-250mg/kg B.W of EECS + 5% cholesterol diet)
• Group V: Test II (Plant Extract-500mg/kg B.W of EECS + 5% cholesterol diet)
Procedure
Rats were divided into five groups each group contain six animals. Group I serves normal which receives normal saline (5ml/kg B.W) for last 28 days orally; Group II serves as positive control which receives 5% cholesterol diet for 3 months continuously; Group III serves as standard which receives Rosuvastatin (20mg/kg B.W; I.P route) for last 28 days and Group IV, Group V serves as test which receives EECS at dose of 250 and 500 mg/kg B.W, through oral route throughout the study period and also receives 5% cholesterol diet for last 28 days [34]. After the study period animals were sacrificed by collecting blood through cardiac puncture and collected blood was centrifuged and serum was collected and biochemical analysis were performed lipid profiles for serum levels TC, TG, HDL, LDL, VLDL, Urea, Creatinine, uric acid and antioxidant studies SOD, GSH and Catalase.
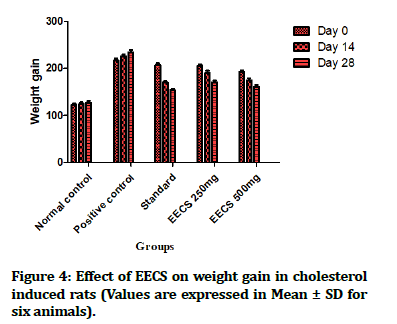
Estimation of weight gain
During the experimental period, the high cholesterol diet consumed and weight gained by rats was recorded on 0th, 14th and 28th day of EECS treatment. The preweighed food pellets (approximately 30g) were placed inside the hopper of the cage. The food consumed by individual rat was quantified by weighing leftover food in the hopper [35].
Estimation of total cholesterol (TC): (CHOD-PAP Method)
The reagents kits intended for the in-vitro quantitative determination of cholesterol in serum/plasma.
Principle
The cholesterol esters are hydrolysed by enzyme cholesterol esterase to give free cholesterol and fatty acid molecules. This free cholesterol gets oxidized in presence of cholesterol oxidase to liberate cholset 4en-3one and H2O2. Liberated H2O2 by this reaction combines with phenol and 4 amino antipyrine in presence of peroxidase to form red colour quinonimine complex, the intensity of which is measured at 505 nm. (490-530nm) it is directly proportional to the cholesterol conc. Present in sample [36].
General system parameters
• Wave length: 505 nm (490-530nm)
• Incubation : 5 min
• Sample volume: 10μl
• Reagent volume: 1.0ml
• Standard concentration: 200mg/dl
Procedure
Bring all the reagents of assay to room temperature.
Mix well and incubate for 5 min at room temperature. Mix well and measure the absorbance of standard and test against the reagent blank at 505 nm. (490-530nm)
Estimation of superoxide dismutase (SOD)
Known amount of tissue was weighed and washed in ice cold saline and homonized in ice cold 0.1M Tris HCl buffer for estimations.
The assay of SOD was based on the ability of SOD to inhibit spontaneous oxidation of adrenaline to adrenochrome. 0.05 ml supernatant was added to 2.0 ml of carbonate buffer and 0.5 ml of 0.01mM EDTA solution. The reaction was initiated by addition of 0.5 ml of epinephrine and the auto oxidation of adrenaline (3× 10-4 M) to adrenochrome at pH 10.2 was measured by following change in OD at 480 nm. The change in optical density every minute was measured at 480 nm against reagent blank. The results are expressed as units of SOD activity (mg/wet tissue). One unit of SOD activity induced approximately 50% inhibition of adrenaline [37-42].
Estimation of catalase activity (CAT)
Catalase activity was determined spectrophotometrically according to previously published method. Briefly, to 1.95ml of 10mM H2O2 in 60mM phosphate buffer (pH=7.0), 0.05ml of the liver homogenate was added and rate of degradation of H2O2 was followed at 240nm per min. Catalase content in terms of U/mg of protein was estimated from the rate of decomposition of H2O2 using the formula k=2.303/ Δt × log (A1/A2) s-1 (A unit of catalase is defined as the quantity which decomposes 1.0 μmole of H2O2 per min at pH=7.0 at 25°C, while H2O2 concentration falls from 10.3 to 9.2 mM) [43,44].
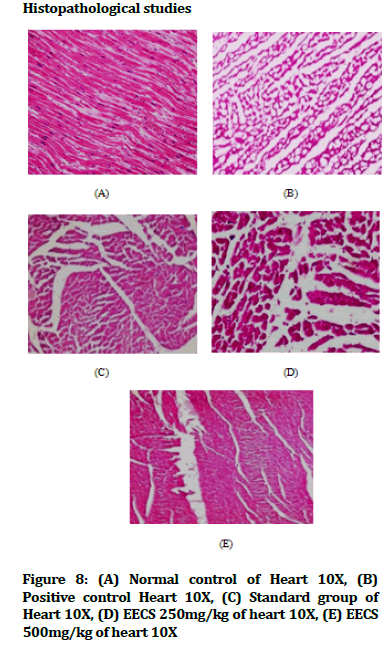
Histopathological studies
The tissues were washed immediately with saline and then fixed in 10% formalin solution. After fixation, the heart tissues were processed in alcohol- xylene series and then embedded in paraffin. The serial sections were cut and each section was stained with haematoxylin and eosin. The slides were examined under microscope and photographs were taken [45].
Statistical analysis
The result was expressed as mean ± SD and analysed statistically using one way ANOVA followed by Dunnet test; Data were computed for statistical analysis using the graph pad software.
Results
Extract
Phytochemical Analysis
The preliminary phytochemical analysis revealed the presence of carbohydrates, alkaloids, flavonoids, tannins, steroids, Saponins, proteins, gums and phenolic compounds in ethanolic extract of aerial parts of Clerodendrum Serratum (Table 3 and 4).
| S.No | Extract | Result |
|---|---|---|
| 1 | Yield | 12.80% |
| 2 | Colour | Yellowish brown |
| 3 | Nature | Semisolid |
Table 3: Ethanolic extract of aerial parts of Clerodendron Serratum shows yield, colour, and nature.
| S.No | Compounds | EECS |
|---|---|---|
| 1 | Carbohydrates | + |
| 2 | Alkaloids | + |
| 3 | Glycosides | + |
| 4 | Fixed oils | - |
| 5 | Flavonoids | + |
| 6 | Tannins | + |
| 7 | Steroids | + |
| 8 | Phenolic compounds | + |
| 9 | Amino acids | - |
| 10 | Saponins | + |
| 11 | Gums | + |
| 12 | Proteins | + |
| +: Presence of compounds | ||
| -: Absence of compounds | ||
Table 4: Preliminary phytochemical analysis of ethanolic extract aerial parts of Clerodendrum Serratum.
Acute toxicological study
The acute toxicity of the ethanolic extract of EECS was carried out and the results are tabulated below according to procedure was followed by using OECD 423 (Acute Toxic Class Method) (Table 5).
| S.NO | Groups | Dose/kg (body weight) p.o. | Signs of Toxicity | Onset of Toxicity | Duration of Study |
|---|---|---|---|---|---|
| 1 | EECS | 5mg | No signs of Toxicity | Nil | 14 days |
| 2 | EECS | 50mg | No signs of Toxicity | Nil | 14 days |
| 3 | EECS | 300mg | No signs of Toxicity | Nil | 14 days |
| 4 | EECS | 2000mg | No signs of Toxicity | Nil | 14 days |
| 5 | EECS | 5000mg | No signs of Toxicity | Nil | 14 days |
Table 5: Acute toxicity studies of ethanolic extract of EECS.
The therapeutic dose was calculated as 1/10th of the lethal dose for the purpose of antihyperlipidemic activity. The dose of EECS was further reduced to 2000 mg/kg and observed for 14 days. This particular dose was found to be safe and no toxicity was observed. One-tenth of the upper limit dose was selected as 250 mg/kg b.wt for test group. The LD50 value determined by the method 423 as operation Development (OECD) was found to be 5000mg/kg b.w. by oral route of EECS which show no toxicity at this particular dose. Therefore, 1/10 and 1/20 of lethal dose has been selected for the experiment. i.e 250 mg and 500 mg/kg.
Hyperlipidaemia Activity: High cholesterol diet induced Hyperlipidemic activity
The values are mentioned in Tables (Tables 6-9) (Figures 4–8)
| Parameter | Test group | Day 0 | 14th day | 28th day |
|---|---|---|---|---|
| Weight gain | Normal control | 122.7 ± 3.10 | 125 ± 3.36 | 128.5 ± 3.12 |
| Positive control | 215.5 ± 7.00 | 224.8 ± 8.21 | 235.8 ± 7.22 | |
| Standard | 200.3 ± 4.79*** | 171.3 ± 5.03*** | 156 ± 4.55*** | |
| EECS250mg/kg | 204.5 ± 5.36* | 190 ± 7.05*** | 174.7 ± 5.01*** | |
| EECS500mg/kg | 193.3 ± 3.78*** | 175.2 ± 4.68*** | 163 ± 4.87*** | |
| Values are expressed as mean ± SD (n=6). Values were significant when compared with cholesterol group. * P<0.05, ** P<0.01, ***P<0.001 (one way ANOVA followed by Dunnett test). | ||||
Table 6: Effect of EECS on weight gain in hyperlipidaemia-induced rats for 12 weeks.
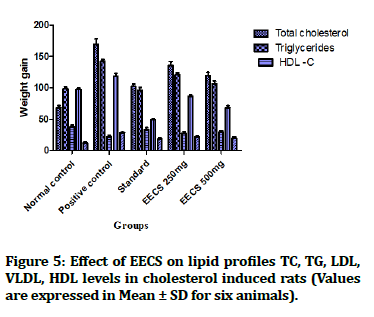
| Groups | Serum cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | VLDL-C (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 67.33 ± 3.98 | 57.17 ± 2.40 | 38.67 ± 4.8 | 18.00 ± 2.60 | 11.33 ± 2.16 |
| Positive control | 171.00 ± 10.57 | 144.8 ± 8.18 | 22.67 ± 2.80 | 119.3 ± 7.16 | 29.67 ± 3.733 |
| Standard | 103.5 ± 3.73*** | 94.83 ± 3.31*** | 34.83 ± 3.18*** | 48.90 ± 3.55*** | 19.83 ± 2.51*** |
| EECS 250mg/kg | 137.8 ± 4.16*** | 120.00 ± 4.74*** | 26.67 ± 2.60ns | 88.00 ± 3.23*** | 22.50 ± 2.739** |
| EECS-500mg/kg | 120.5 ± 4.88*** | 105.8 ± 3.76*** | 30.5 ± 2.73* | 69.33 ± 2.58*** | 20.83 ± 3.18*** |
| Values are expressed as mean ± SD (n=6). Values were significant when compared with cholesterol group. * P<0.05, ** P<0.01, ***P<0.001 (one way ANOVA followed by Dunnet test) | |||||
Table 7: Effect of EECS on serum lipid profile.
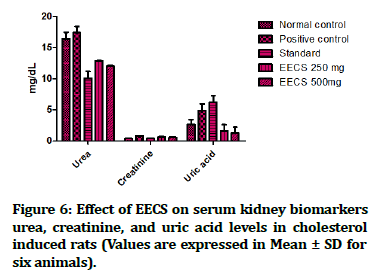
| GROUPS | UREA (mg/dL) | CREATININE (mg/dL) | URIC ACID (mg/dL) |
|---|---|---|---|
| Normal control | 16.2 ± 2.30 | 0.44 ± 0.101 | 2.2 ± 0.50 |
| Positive control | 17.5 ± 3.2 | 0.85 ± 0.12 | 4.80 ± 1.4 |
| Standard | 10.6 ± 1.70*** | 0.45 ± 0.108*** | 1.30 ± 0.10*** |
| EECS-250mg/kg | 13.2 ± 0.80* | 0.65 ± 0.09* | 1.70 ± 0.51*** |
| EECS-500mg/kg | 12.8 ± 0.60* | 0.62 ± 0.07* | 1.40 ± 0.23*** |
| Values are expressed as mean ± SD (n=6). Values were significant when compared with positive control group. * P<0.05, **P<0.01, ***P<0.001 (one way ANOVA followed by Dunnet test). | |||
Table 8: Effect of EECS on serum kidney biomarkers in normal and cholesterol rats.
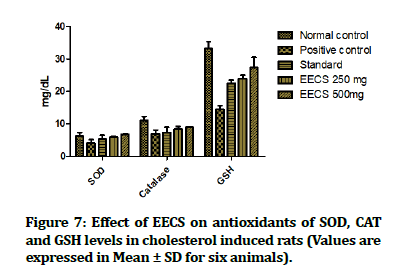
| GROUPS | SOD | CATALASE | GSH |
|---|---|---|---|
| Normal control | 6.90 ± 0.60 | 11.38 ± 0.81 | 34.76 ± 1.16 |
| Positive control | 4.00 ± 0.61 | 6.31 ± 0.69 | 13.39 ± 0.56 |
| Standard | 5.37 ± 0.72* | 7.36 ± 0.64ns | 22.12 ± 0.98*** |
| EECS 250mg/kg | 6.08 ± 0.87*** | 8.41 ± 0.75** | 24.19 ± 1.16*** |
| EECS 500mg/kg | 6.61 ± 0.63*** | 8.83 ± 0.60*** | 28.57 ± 1.87*** |
| Values are expressed as mean ± SD (n=6). Values were significant when compared with cholesterol group. * P<0.05, **P<0.01, ***P<0.001 (one way ANOVA followed by Dunnet test). | |||
Table 9: Effect of EECS on antioxidant enzymes in control and experimental rats.
Figure 4:Effect of EECS on weight gain in cholesterol induced rats (Values are expressed in Mean ± SD for six animals).
Figure 5:Effect of EECS on lipid profiles TC, TG, LDL, VLDL, HDL levels in cholesterol induced rats (Values are expressed in Mean ± SD for six animals).
Figure 6:Effect of EECS on serum kidney biomarkers urea, creatinine, and uric acid levels in cholesterol induced rats (Values are expressed in Mean ± SD for six animals).
Figure 7:Effect of EECS on serum kidney biomarkers urea, creatinine, and uric acid levels in cholesterol induced rats (Values are expressed in Mean ± SD for six animals).
Figure 8:(A) Normal control of Heart 10X, (B) Positive control Heart 10X, (C) Standard group of Heart 10X, (D) EECS 250mg/kg of heart 10X, (E) EECS 500mg/kg of heart 10X.
Discussion
Hyperlipidaemia means presence of a high amount of cholesterol in the body has been demonstrated to elevate total cholesterol and may increase the risk of atherosclerosis and lead to cardiovascular disease. Herbs play a key role in the management of various CVD. Numerous medicinal plants and their formulations are used for hyperlipidaemia in ethno medical practices and in traditional system of medicine in India. However, we do not satisfactory remedy for hyperlipidaemia; most of the herbal drugs speed up the reduction of cholesterol by healthy dietary intake. So, the search for anti- Hyperlipidemic activity of high cholesterol induced rats. The extraction by soxhlet method for 100gms of powder yielded 12.8%. The preliminary phytochemical screening revealed the presence of Carbohydrates, Alkaloids, Glycosides, Flavonoids, Tannins, Phenolic compounds, Amino acid, Saponins, Steroids, Gums and Proteins. The extract was found safe up to a dose of 5000mg/kg body weight. The dried extract was suspended in 1% CMC at dose levels 250mg/kg and 500mg/kg body weight for oral administration. Cholesterol powder was purchased and used with normal diet shows changes in serum cholesterol levels differ significantly between rats and humans. To clarify these confusions, a 12 week’s feeding study with rats was conducted to evaluate the influence of high fat diet on serum lipid profiles, kidney parameters and antioxidant studies.
The use of rats as experimental animals for Hyperlipidemic activity is mainly due to the synthesis and structural similarities in human and rats. The present study carried out inducing 5% cholesterol for the experimental induction in rats. Cholesterol will deposit in the endothelial cells and transport with lipoproteins in blood stream which leads to increase in the TC, TG, LDL, VLDL levels and decreases the HDL levels in the body. It is synthesised in the liver and converted into bile acids and excreted through faeces and due to hyper cholesteremia urine failure will occur due to oxidative stress.
Summary and Conclusions
The aim of the study is to evaluate the antihyperlipidemic activity of the ethanolic extract of aerial part of Clerodendron Serratum in cholesterol diet induced Hyperlipidemic rats. Wistar albino rats were randomly divided into five groups of six each. Group-I served as normal control. Groups II to V were given 5% cholesterol diet for 3 months to induce hyperlipidaemia, and for last 28 days were administered either: 0.5ml water/saline for Group- I; cholesterol diet (5%) for Group-II; Standard drug Rosuvastatin (20mg/kg body weight) for Group-III; EECS at 250 mg/kg bodyweight for Group-IV and 500mg/kg body weight for Group-V. The effects of EECS on Body Weight, serum lipid profile, kidney parameters and antioxidant enzymes (superoxide dismutase, GSH and catalase) were assessed and compared. Cholesterol diet induced hyperlipidaemia rats showed a significant (P<0.001) increase in the plasma concentration of Total Cholesterol (TC), Triglycerides (TG), Low-Density Lipoprotein cholesterol (LDL-c), Very Low-Density Lipoprotein cholesterol (VLDL-c), Body weight and kidney parameters; it decreases in High Density Lipoproteins Cholesterol (HDL-c) and antioxidant enzymes when compared to normal control rats. Coadministration of EEAG and standard drug Rosuvastatin with cholesterol diet caused a significant decrease (p<0.001) in the concentration of serum TC, VLDL, TG, body weight and kidney parameters; increase in the HDLc and antioxidant enzymes when compared with cholesterol fed control rats. The result suggests lipid lowering effects of Clerodendron Serratum which serves as a new potential natural product for preventing hyperlipidaemia.
References
- Ayinla MT, Dada SO, Shittu ST, et al. Anti-hyperlipidemic effect of aqueous leaf extract of Ocimum gratissimum in alloxan induced diabetic rats. Int J Med Med Sci 2011; 3:360-3.
- Anreddy RN, Porika M, Yellu NR, et al. Hypoglycemic and hypolipidemic activities of Trianthema portulacastrum Linn. plant in normal and alloxan induced diabetic rats. Int J Pharma 2010; 6:129-33.
- Ahmed SM, Clasen ME, Donnelly JF. Management of dyslipidemia in adults. Am Family Phys 1998; 57:2192.
- Aissaoui A, Zizi S, Israili ZH, et al. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J Ethnopharm 2011; 137:652-61.
- Trovato A, Monforte MT, Barbera R, et al. Effects of fruit juices of Citrus sinensis L. and Citrus limon L. on experimental hypercholesterolemia in the rat. Phytomed 1996; 2:221-7.
- Akila M, Devaraj H. Synergistic effect of tincture of Crataegus and Mangifera indica L. extract on hyperlipidemic and antioxidant status in atherogenic rats. Vascular Pharm 2008; 49:173-7.
- Bachorik PS, Denke MA, Stein EA, et al. Lipid and Dyslipoproteinemia in clinical diagnosis and management by laboratory methods. 29th Edn 2001;224-248.
- Baron RB. Lipid abnormalities, in current medical diagnosis and treatment. The McGraw-Hill Company 44th Edn 2005; 1202-1213.
- Ghule BV, Ghante MH, Saoji AN, et al. Antihyperlipidemic effect of the methanolic extract from Lagenaria siceraria stand. Fruit in hyperlipidemic rats. J Ethnopharm 2009; 124:333-7.
- Priyanka B, Joy JM, Kumar GA, et al. Comparative antioxidant activity of Asparagus racemosus with its alternative source Asparagus gonoclados. Int J Pharma 2012; 2:51-6.
- Condell RA, Tappel AL. Evidence for suitability of glutathione peroxidase as a protective enzyme: studies of oxidative damage, renaturation, and proteolysis. Arch Biochem Biophy 1983; 223):407-16.
- Drug Evaluation Annual. Published by the American Medical Association. 1995; 2455-2500.
- El-Desoky GE, Aboul-Soud MA, Al-Numair KS. Antidiabetic and hypolipidemic effects of Ceylon cinnamon (Cinnamomum verum) in alloxan-diabetic rats. J Med Plants Res 2012; 6:1685-91.
- Engelmann B, Streich S, Schönthier UM, et al. Changes of membrane phospholipid composition of human erythrocytes in hyperlipidemias. I. Increased phosphatidylcholine and reduced sphingomyelin in patients with elevated levels of triacylglycerol-rich lipoproteins. BBA Lipid Lipid Metabol 1992; 1165:32-7.
- Gayathri V, Ananthi S, Vasanthi HR. Antihyperlipidemic potential of polyphenol and glycoside rich Nerium oleander flower against triton WR-1339-induced hyperlipidemia in experimental Sprague Dawley rats. J Chem 2013; 2013.
- https://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192288734
- Vogel HG. Drug discovery and evaluation: pharmacological assays. Springer Science & Business Media 2002.
- GM, Chatterjee SS, Singh PN, et al. Hypolipidemic and antiobesity-like activity of standardised extract of Hypericum perforatum L. in rats. Int Scholarly Res Notices 2011; 2011.
- Hossain MS, Ahmed M, Islam A. Hypolipidemic and hepatoprotective effects of different fractions of methanolic extract of Momordica charantia (Linn.) in alloxan induced diabetic rats. Int J Pharma Sci Res 2011; 2:601.
- Harnafi H, Caid HS, el Houda Bouanani N, et al. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem 2008; 108:205-12.
- Hermann M, Bogsrud MP, Molden E, et al. Exposure of atorvastatin is unchanged but lactone and acid metabolites are increased several‐fold in patients with atorvastatin‐induced myopathy. Clin Pharmacol Therap 2006; 79:532-9.
- Lee JS, Bok SH, Jeon SM, et al. Antihyperlipidemic effects of buckwheat leaf and flower in rats fed a high-fat diet. Food Chem 2010; 119:235-40.
- Kumari K, Augusti KT. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J Ethnopharma 2007; 109:367-71.
- https://www.bookganga.com/Preview/BooksPreview.aspx?BookId=4865347198497725612
- Kamalraj R. Antihyperlipidemic studies on leaf extract of Erythrina indica lam. Int J Res Pharma Chem 2011; 1:488-90.
- Kumari SS, Menon VP. Changes in levels of lipid peroxides and activities of superoxide dismutase and catalase in isoproterenol induced myocardial infarction in rats. Indian J Exp Biol 1987; 25:419-23.
- Ludwig PW, Hunninghake D, Hoidal J. Increased leucocyte oxidative metabolism in hyperlipoproteinaemia. Lancet 1982; 320:348-50.
- Lakshmi V, Sonkar R, Khanna AK. Antihyperlipidemic and antioxidant activities of Bruguiera cylindrinca (L). Chronicles Young Scient 2012; 3.
- Czakó L, Szabolcs A, Vajda Á, et al. Hyperlipidemia induced by a cholesterol-rich diet aggravates necrotizing pancreatitis in rats. Eur J Pharma 2007; 572:74-81.
- Feng LJ, Yu CH, Ying KJ, et al. Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res Int 2011; 44:404-9.
- Mccormack T, Harvey P, Gaunt R, et al. Incremental cholesterol reduction with ezetimibe/simvastatin, atorvastatin and rosuvastatin in UK General Practice (IN‐PRACTICE): Randomised controlled trial of achievement of Joint British Societies (JBS‐2) cholesterol targets. Int J Clin Practice 2010; 64:1052-61.
- Maniyar Y, Bhixavatimath P. Antihyperglycemic and hypolipidemic activities of aqueous extract of Carica papaya Linn. leaves in alloxan-induced diabetic rats. J Ayurveda Integrative Med 2012; 3:70.
- Mallick C, Maiti R, Ghosh D. Comparative study on antihyperglycemic and antihyperlipidemic effects of separate and composite extract of seed of Eugenia jambolana and root of Musa paradisiaca in streptozotocin-induced diabetic male albino rat. Iranian J Pharmacol Therapeut 2006; 5:27-0.
- Makwana MV, Pandya NM, Doshi BD, et al. Evaluation of antioxidant and antihyperlipidemic potential of Sida cordifolia linn. in experimental animals. Int J Pharma 2012; 2:520-5.
- Zulet MA, Barber A, Garcin H,et al. Alterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. J Am College Nutr 1999; 18:36-42.
- https://www.ahajournals.org/doi/pdf/10.1161/01.HYP.23.3.308?download=true
- https://journals.sagepub.com/doi/pdf/10.1177/000456329102800222
- https://accesspharmacy.mhmedical.com/content.aspx?bookid=1613§ionid=102160735.
- https://accessmedicine.mhmedical.com/book.aspx?bookid=2957
- Madhava Chetty K. Flowering plants of Chittor district, student offset printers, tirupati, Andhra Pradesh, India. 2008; 505.
- Nainwal P, Dhamija K, Tripathi S. Study of antihyperlipidemic effect on the juice of the fresh fruits of Lagenaria siceraria. Int J Pharma Pharma Sci 2011; 3:88-90.
- Punitha DA, Thandavamoorthy AN, Arumugasamy KA, et al. Anti-hyperlipidemic effect of ethanolic leaf extract of Gmelina arborea in streptozotocin induced male wistar albino rats. Pharma Toxicol 2012; 2:46-51.
- Pragda SS, Kuppast I, Mankani K, et al. Evaluation of antihyperlipidemic activity of leaves of Portulaca oleracea Linn against dexamethasone induced hyperlipidemia in rats. Int J Pharm Pharm Sci 2012; 4:279-83.
- Verma PR, Deshpande SA, Kamtham YN, et al. Hypolipidemic and antihyperlipidemic effects from an aqueous extract of Pachyptera hymenaea (DC.) leaves in rats. Food Chem 2012; 132:1251-7.
- Vijayaraj P, Muthukumar K, Sabarirajan J, et al. Antihyperlipidemic activity of Cassia auriculata flowers in triton WR 1339 induced hyperlipidemic rats. Exp Toxicolog Pathol 2013; 65:135-41.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Department of Pharmacology, Srilakshmi Venkateshwara Institute of Pharmaceutical Sciences Kothapeta, Proddatur, Kadapa, Andhra Pradesh, IndiaCitation: K Mahammad Areef, Evaluation of Anti-Hyperlipidemic Activity of Clerodendrum Serratum on High Cholesterol Diet Induced Hyperlipidaemia in Rats, J Res Med Dent Sci, 2021, 10(1): 227-241
Received: 13-Dec-2021, Manuscript No. JRMDS-21-45046; , Pre QC No. JRMDS-21-45046 (PQ); Editor assigned: 15-Dec-2021, Pre QC No. JRMDS-21-45046 (PQ); Reviewed: 29-Dec-2021, QC No. JRMDS-21-45046; Revised: 03-Jan-2022, Manuscript No. JRMDS-21-45046 (R); Published: 10-Jan-2022