Research Article - (2021) Volume 9, Issue 12
Expression of P53 Protein in Premalignant and Malignant Lesions of the Oral Cavity
Bindthiya RC Babu AR Subasree and Ramalakshmi V*
*Correspondence: Ramalakshmi V, Department Pathology, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, India, Email:
Abstract
To determine the overexpression of P53 protein in histological sections of oral cavity lesions. To compare the degree of P53 overexpression in premalignant and malignant lesions of the oral cavity with that of the normal oral mucous membrane. The study group was further divided into 2 equal groups; Group I had 35 histopathologically diagnosed, formalin fixed, paraffin embedded samples of oral leukoplakia. Group II had 35 histopathologically diagnosed, formalin fixed, paraffin embedded samples of oral squamous cell carcinoma. The samples were then subjected to immunohistochemistry by indirect 1mmunoenzyme LSAB method for detection of P53 protein. P53 immunohistochemical analysis 1s strongly recommended in conjunction with histological parameters, to increase the sensitivity of detection of cases that will progress to carcinoma.
For more detailed information visit the following sites:
https://marmarisdentalcenter.com
https://dentalclinicmarmaris.com
https://marmarisdentals.com
Keywords
P53 protein, Malignant lesions, oral cavityIntroduction
Oral cancer constitutes 5.5 % of all malignancies globally and is the sixth most common cancer worldwide. Globally, about 500,000 new oral cancers are diagnosed annually and three fourths of these are from the developing world with about 65,000 cases from India. 1 India tops in the prevalence of oral cancer in the world and is the third most common cancer in India.2 Epidemiological, clinical and laboratory data have been unequivocally established tobacco usage and alcohol consumption as the major sources of intra-oral carcinogens in the global scenario leading to oral and oropharyngeal cancers., 3 4 The increased prevalence of the oral cancer 1n the Indian subcontinent seems to be due to the high exposure to sunlight due to farming, smoking and other smokeless tobacco habits, alcohol, spicy food, and neglect of overall oral health. 2
Tobacco usage can also cause two major clinically recognized precancerous lesions like leukoplakia and erythroplakia. In India and several other Southeast Asian countries, oral submucous fibrosis, primarily associated with areca nut chewing habit has been recognized as a precancerous condition. 5 In India, only less than one-fifth (19%) of tobacco consumed is in the form of cigarettes. Rest is smoked as bidi or in smokeless form, such as chewing tobacco and mishiri (tooth cleaner applied tobacco). 6 Areca nut is used as naturally crude areca nut or as betel quid or paan paraag. Other areca nut preparations ready for immediate consumption are 'panmasala' (without tobacco), 'gutkha' (with tobacco) and supari (natural type ).6
Oral carcinogenesis 1s a complex, multi-step process that includes initiation, promotion, and progression. Interaction between presumed carcinogens and cellular macromolecules such as DNA, proteins, and lipids is the most important and decisive event of the chemical carc1. nogenes1. 7,8 These genetic damages result in tumors by disrupting the normal regulatory patnways that control basic cellular functions. In recent years, it is becoming clear that malignancy anses because of the accumulations of mutations in two major classes of genes, namely proto oncogenes and tumor suppressor genes. 9 Tumor suppressor genes (TSG) are those genes, which act to limit growth of tumors by slowing and halting cell cycle progression. 10 TSG expresses a product that can suppress the expression or function of other genes involved with cell growth and proliferation.11 These genes are vulnerable sites for critical deoxyribonucleic acid (DNA) damage because they normally function as physiologic barriers against clonal expansion or genomic mutability and are able to hinder the growth and metastasis of cells driven to uncontrolled proliferation by oncogenes.
Nearly all human cancers are associated with a loss of function of the p53 tumor suppressor gene, indicating the important role of p53 in cancer development .12 p53 gene produces a protein P53 that acts as a tumor suppressor. Loss of P53 runction causes loss of cell cycle control and presumably accumulates damage - induced mutations that results in malignant transformation of cells. Inactivation of P53 protein occurs either due to the mutation of the p53 gene or by stabilization of P53 protein by binding to other proteins like MDM2.
Expression of P53 protein has been widely investigat ed• in oral cancers and oral premalignancies. A history of tobacco and alcohol use was associated with a high frequency of p53 mutations in patients with squamous- cell carcinoma of the head and neck . Preliminary evidence linked cigarette smoking to p53 mutations at nonendogenous mutation sites. 13 This study aims at demonstrating P53 protein 1n oral leukoplakia and oral squamous cell carcinoma (OSCC) by immunohistochemistry.
Material and Method
A total number of 80 cases of formalin fixed, paraffin Embedded tissue samples from the oral cavity were collected from the Department of Pathology, Sree Balaji Medical College during the period from August 2010 to July 2012. These were then divided into control group and study group. The control group consisted of 10 formalin fixed, paraffin embedded samples of oral cavity which were histopathologically diagnosed as normal oral mucous membrane. The study group consisted of 70 formalin fixed, paraffin embedded samples which were histopathologically diagnosed as oral leukoplakia and oral squamous cell carcinoma. In the study group, equal number of cases were selected i.e.; 35 cases out of the total 69 oral leukoplakia cases received, and 35 cases out of the total 52 oral squamous carcinoma cases were taken, to make a comparison between both the groups. Relevant clinical history was obtained.
The control group composed of 10 formalin fixed, paraffin embedded samples of the oral cavity which were histopathologically diagnosed as normal healthy oral mucosa. The age group of the control group was 23-46 years. The study group consisted of 70 formalin fixed, paraffin embedded samples of the oral cavity which were histopathologicall y diagnosed as oral leukoplakia and oral squamous cell carcinoma. The age range of the study group varies from 28 to 91years.
The samples of the study group were further grouped into 2 sub-groups:
Group I - Consisted of samples of histopathologically diagnosed, formalin - fixed , paraffin embedded tissue samples of oral leukoplakia
Group II - Consisted of 35 samples of histopathologically diagnosed, formalin fixed, paraffin embedded tissue samples of oral squamous cell carcinoma
Inclusion criteria
For Control Group - Formalin fixed, paraffin embedded samples of the oral cavity which were histopathologically diagnosed as normal healthy oral mucosa.
For Study Group -
Group I - Histopathologically diagnosed, formalin fixed paraffin embedded tissue samples of oral leukoplakia.
Group II - Histopathologically diagnosed, formalin fixed paraffin embedded tissue samples of oral squamous cell carcinoma.
Methodology preparation of slides
The paraffin wax blocks of histopathologically diagnosed lesions of oral leukoplakia, and oral squamous cell carcinoma were selected. These were cut into 3-5µ thin tissue sections with the help of a rotary microtome, LEICA. The sections were placed on poly- L-Lyseine coated slides. These slides were subjected to both Hematoxylin and Eosin staining as well as P53 staining.
a. Sections are deparaffinised in xylol hydrated through graded alcohols,brought to water for 5 minutes.
b. Sections are stained in hematoxylin for 10 minutes.
c. Washed well in running tap water.
d. Differentiated in 1% acid alcohol for few seconds. The blue staining of the haematoxylin is changed to red by the action of the acid.
e. Washed in alkaline running tap water until sections are again blue for 5 minutes.
f. Sections are stained in 1% aqueous eos1n for 2 minutes.
g. Washed in running tap water for 2 minutes.
h. Sections are dehydrated with graded ethyl alcohols, cleared 1n xylol and mounted with DPX (dextrenepolysterene xylene).
The slides stained for P53 were observed under light microscope with a magnification of 400x. the tissue samples were thoroughly examined and total number of 100 cells were counted. Then, among the 100 cells, the number of cells which had taken up the stain were counted. A brown precipitate seen within the nucleus confirmed the presence of P53 protein. The P53 positive samples were then evaluated semiquantitatively on a 4- point scale based on the percentage of cells showing P53 staining. The results thus obtained were analyzed statistically by Chi- square (x2 ) test of significance. In this test, the p value of less than 0.05 was accepted as indicating statistical significance.
Results
A The present study was designed to determine the overexpression of P53 protein in oral leukoplakia and oral squamous cell carcinoma. A group of 80 histopathologically diagnosed, formalin fixed,paraffin embedded tissue samples were collected and were grouped into control group and study group. The control group consisted of 10 formalin fixed,paraffin embedded samples which were histopathologically diagnosed as normal oral mucous membrane. The study group included histopathologically diagnosed, formalin- fixed, paraffin embedded samples of oral leukoplakia as Group I and oral squamous cell carcinoma as Group II. The tissue samples obtained were subjected to immunohistochemical methods to detect P53 protein. The technique used was indirect immunoenzyme LSAB method. Control Group:Out of the 10 samples, 6 (60%) were from males and 4 (40%) were from females.
Group I - out of the 35 samples of oral leukoplakia, 21 (60%) were from male patients and 14(40%) were from female patients Group II- out of the 35 samples of oral squamous cell carcinoma, 26 (74.29 %) were from male patients and 9 (25.71%) were from female patients. The gender distribution among the various groups had a p value of 0.407 which was not statistically significant [Table - 4] The mean age was 33.6 ± 6.67 with a minimum age of 23 years and a maximum age of 46 years
Group I - The mean age among the samples of oral leukoplakia was 56.97 ± 18.18 with a minimum of 28 years and a maximum of 88 years. Group II - The mean age among the samples of oral squamous cell carcinoma was 55.51 ± 18.18 with a minimum of 30 years and a maximum of 85 years. The age distribution of the various groups was noted [Table - 5]. 7/35 (20.00%) samples of Group I and 12/35 (34.29 %) of Group II were positive for P53. None of the samples of the control group were positive for P53. 6 samples of group II and none of the samples of group I showed 3+ staining. The p value was 0.028 and was statistically significant. [Table-6]
In Group I, 7 /35 (20%) samples were positive for P53 while none of the samples of the control group were positive for P53. The p value was 0.124 and thus the p value was not statistically significant [ Table - 7] In Group II, 12/35 (34.29 %) samples were positive for P53 while none of the samples of the control group were positive for P53. The p value was 0.197 and thus the p value was not statistically significant [ Table - 8] 7/35 ( 20%) samples of group I and 12/35 (34.29%) samples of group II were positive for P53. 6 samples of group II and none of the samples of group I showed 3+ staining. The p value was 0.025 and was statistically significant [ Table - 9].
In all the study groups P53 was seen as a brown stain within the nucleus. But the pattern of expression of the stain was different among, the groups.[ Figures 2 - 9]. Group I - The brown stain of P53 was seen in the basal and immediate suprabasal layers. Group II - The brown stain of P53 was diffuse involving all the layers of the epithelium. The intensity of P53 stain also varied between both the study groups. Group I - less intense staining of P53 Group II - more intense staining of P5 3.
Table 1: P53 Status in the Control Group.
| P53 status | |||
|---|---|---|---|
| Negative | Positive | Total | |
| Normal tissue sample | 10 (100%) | 0 | 10 |
Table 2: P53 Status in Group I.
| P53 status | Total | ||
|---|---|---|---|
| Negative | Positive | ||
| Leukoplakia | 28 (80%) | 7 (20%) | 35 |
Table 3: P53 Status in Group I I.
| P53 status | Total | ||
|---|---|---|---|
| Negative | Positive | ||
| Squamous cell | 2.,, (65.71%) | 12 (34.29%) | 35 |
| carcinoma | |||
Table 4: Gender Distribution Among the Control Group and the Study Group.
| Group | Sex | Total | |
|---|---|---|---|
| Male | Female | ||
| Normal tissue sample | 6 (60.00%) | 4 (40.00%) | 10 |
| Leukoplakia | 21 (60.00%) | 14 (40.00%) | 35 |
| Squamous cell carcinoma | 26 (74.29%) | 9 (25.71%) | 35 |
| Total | 53 | 27 | 80 |
| Statistic | DF | Value | p |
| Chi - square | 2 | 1.797 | 0.407 |
Table 5: Mean Age Among the Control Group and the Study Group.
| Group | No. | Mean | Std. | Min | |
|---|---|---|---|---|---|
| Observed | Dev | Max | |||
| Normal tissue sample | 10 | 33.6 | 6.67 | 23 | 46 |
| Leukoplakia | 35 | 56.97 | 18.18 | 28 | 88 |
| Squamous cell carcinoma | |||||
| 35 | 55.51 | 18.18 | 30 | 85 |
Table 6: P53 Grading among the Control Group and the Study Group.
| Group | P53 Grade | Total | |||
|---|---|---|---|---|---|
| Negative | l+ | 2+ | 3+ | ||
| Normal | 10 | 0 | 0 | 0 | 10 |
| -1 | 0 | 0 | 0 | ||
| Leukoplakia | 28 | 7 | 0 | 0 | 35 |
| -0.8 | -0.2 | 0 | 0 | ||
| Squamous cell carcinoma | 23 | 4 | 2 | 6 | |
| -0.6571 | -0.1143 | -0.0571 | -0.1714 | 35 | |
| Total | 61 | 11 | 2 | 6 | 80 |
| Statistic | DF | Value | Prob |
| Chi-square | 6 | 14.106 | 0.028 |
Table 7: Comparison of P53 Grading Between Group I and Control Group.
| Group | P53 grade | ||||
|---|---|---|---|---|---|
| Negative | 1+ | 2+ | 3+ | Total | |
| Leukoplakia | 28 | 7 | 0 | 0 | |
| -0.8 | -0.2 | 0 | 0 | 35 | |
| Normal | 10 | 0 | 0 | 0 | |
| -1 | (0 .00%) | (0 .00%) | (0 .00%) | 10 | |
| Total | 38 | 7 | 0 | 0 | 45 |
| Statistic | DF | Value | Prob |
| Chi -Square | 1 | 2.368 | 0.124 |
Table 8: Comparison of P53 Grading Between Group II and Control Group.
| Group | P53 grade | To tal | |||
|---|---|---|---|---|---|
| Negative | 1+ | 2+ | 3+ | ||
| Squamous cell | 23 | 4 | 2 | 6 | |
| ca rcino ma | -0.6571 | -0.1143 | -0.05714 | -0.1714 | 35 |
| Normal | 10 | 0 | 0 | 0 | 10 |
| -1 | 0 | 0 | 0 | ||
| Total | 33 | 4 | 2 | 6 | 45 |
| Statistic | DF | Value | Prob |
| Chi -Square | 3 | 4.675 | 0.197 |
Table 9: T11A Comparison of P53 Grading Between Group and Group II.
| P53 grade | |||||
|---|---|---|---|---|---|
| Group | Negative | 1+ | 2+ | 3+ | Total |
| 28 | 7 | 0 | 0 | ||
| Leukoplakia | -0.8 | -0.2 | 0 | 0 | 35 |
| Squamous | 23 | ||||
| Cell Carcinoma | -0.6571 | 4 | 2 | 6 | 35 |
| -0.1143 | -0.05714 | -0.1714 | |||
| Total | 51 | 11 | 2 | 6 | 70 |
| Statistic | DF | Value | Prob |
| Chi -Square | 9.308 | 3 | 0.025 |
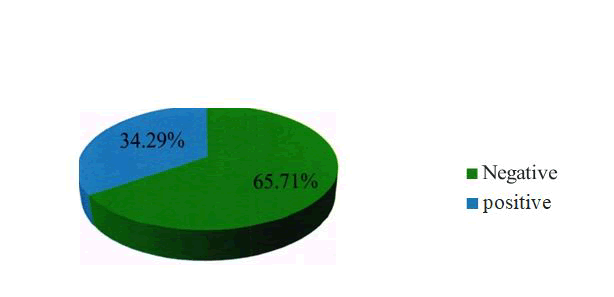
Figure 3: Expression in Group II.
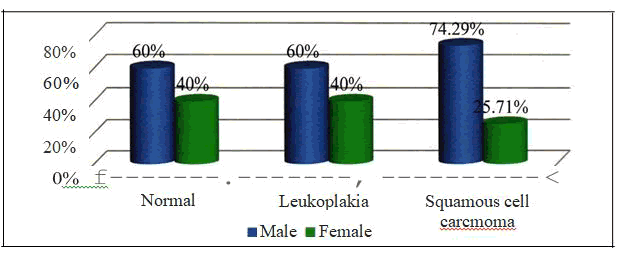
Figure 4: Gender Distribution Among the Study and Control Group.
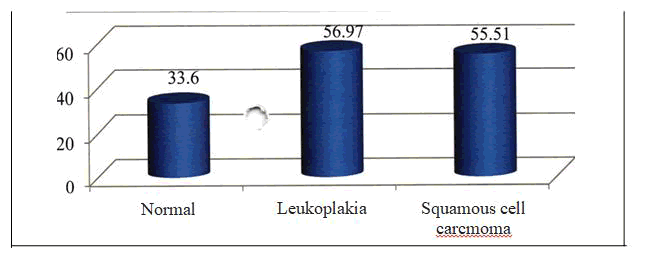
Figure 5: Gender Distribution Among the Study and Control Group.
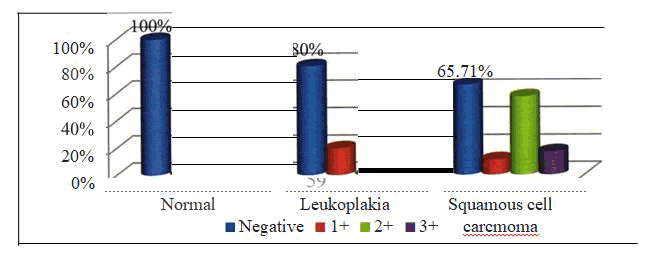
Figure 6: P53 Grade Distribution among the Study Group and Control Group.
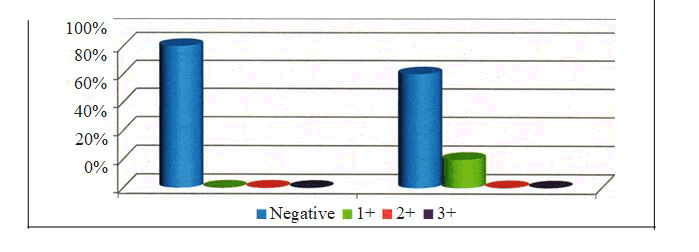
Figure 7: P53 Grade Distribution among the Study Group and Control Group.
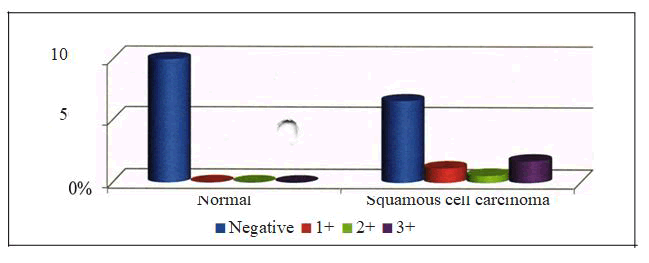
Figure 8: Grading Between Group II and Control Group.
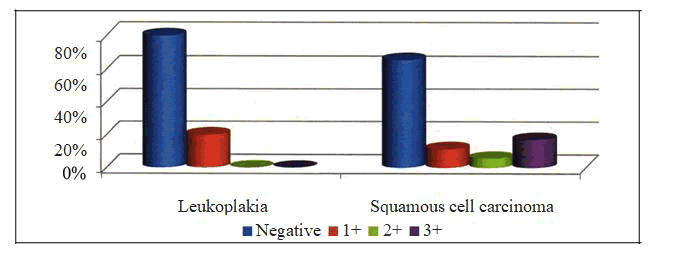
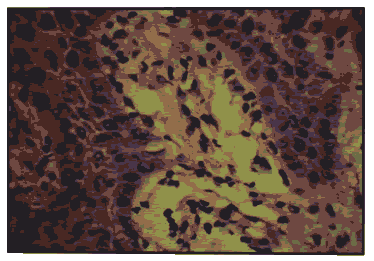
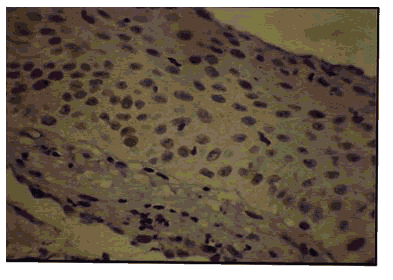
Figure 9: Oral Leukoplakia - H & E X 400.
Pic 1: P53 Grading Between Group I And Group II.
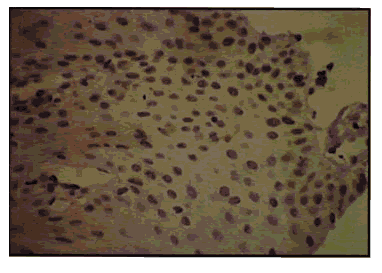
Pic 2: Oral leuh. jplakia showing 1+ p53 expression x 400.
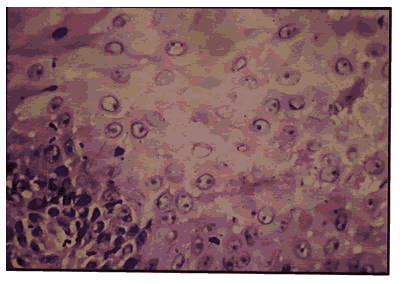
Pic 3: Oral squamous cell carcinoma - h & Ex400.
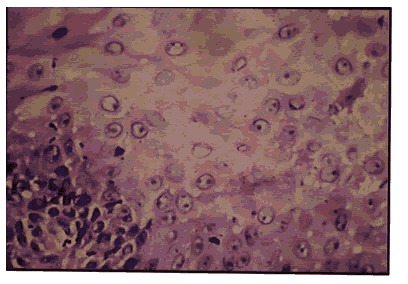
Pic 4: Oral squam.vus cell carcinoma showing 1+ p53 expression x 400.
Pic 5: Oral squamous cell carcinoma - h & ex 100.
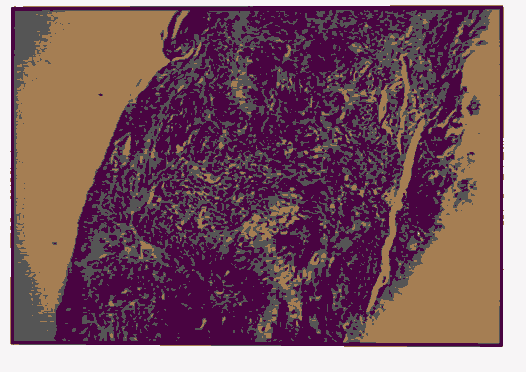
Pic 6: Oral sql. jnmous cell carcinoma showing 2+ p53 expression x 400.
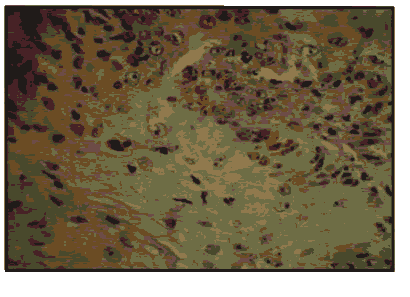
Pic 7: H & E of oral squamous cell carcinoma x 400.
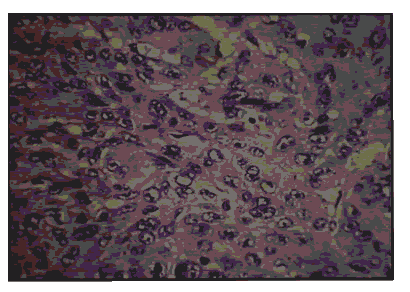
Pic 8: Oral squamous cell carcinoma - showing 3+ p53 expression x 400.
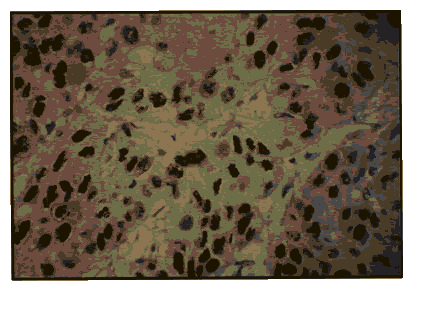
Pic 9: Oral squamous cell carcinoma - showing 3+ p53 expression x 400.
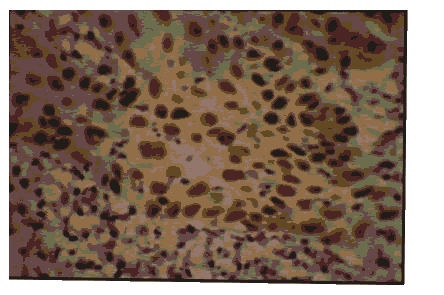
Pic 10: Oral squh I\1. ou s cell carcinoma- showing 3+ p53 expressionx 100.
Discussion
Aberrations of p53 gene are the most frequent molecular events 1n human cancers. The P53 gene 1n chromosome 17p13.1 encodes the P53 protein involved 1n many key events in the cell like regulation of cell cycle and glucose metabolism in cancer cells, DNA-repair, apoptosis, and senescence and induced by various stress signals, including DNA-damage and inflammation. P53 protein acts by causing cell cycle arrest in the G1 phase in response to DNA damage, allowing for repair of the DNA or regulation of apoptosis if the damage is irreparable. Hence, p53 gene is known asa tumor suppressor gene.14,15
In the present study, 80 histopathologically diagnosed, formalin fixed, paraffin embedded tissue samples were considered.These samples were grouped into control group and study group. The control group consisted of 10 normal oral mucous membrane tissue samples.The study group was further divided into two equal groups : Group I consisted of 35 cases of oral leukoplakia. Group II consisted of 35 samples of oral squamous cell carcinoma.The tissue samples were then subjected to immunohistochemical method using indirect immunoenzyme LSAB method for detection of P53 protein. In the present study, all cases of normal oral mucosa assessed were P53 negate, This is in agreement with most reports in the literature, regardless of differences 1n antibodies, IHC procedures and types of specimens used.16
In the present study, 7/3 5 (20%) samples of Group I and 12/35 (34.29%) samples of Group II were positive for P53 protein. None of the normal tissue samples were positive for P53 protein. These results are correlating with the following studies. Saranath et al (1) showed that overexpress1on of P53 protein was seen in 6/22 (27%) samples of leukoplakia and 23/62 (37%) samples of oral cancer. 17 Warnakulasuriya et al 35 reported that 2/12 (17%) of the samples of dysplasia and 13/37 (35%) samples of oral cancer showed positivity for P53 protein. All normal tissue specimens were negative for mutant P53 expression. T.Tsuji et al 38 showed that 2/12 (14.3%) specimens of leukoplakia and 22/50 (44%) specimens of squamous cell carcinoma, were positive £ r P53 protein. Tomohiro Matsumura et al 41 observed that 4/11(36%) cases of leukoplakia and 22/42 (52%) cases of squamous cell carcinoma were positive for P53. Murti et al 46 observed that 21/68 (31%) samples of precancerous lesions that did not progress to cancer and 6/22 (27.23 %) samples of precancerous lesions and conditions that progressed to cancer were positive for P53.18-21
Soni S et al 58 found that while 10/ 90 (11 %) patients with potentially malignant lesions and 40/220(18%) of OSCC patients showed alterations of p53. C. Jane et al 59 found that 36% OSCC were found to be positive for nuclear p53 staining. M. Pillay et al 55 found that 40/110 (36%) cases of oral carcinoma were p53 positive and 6/35 (17%) dysplastic lesions studied showed p53 positive staining. None of the hyperplastic lesions and normal oral lesions showed any evidence of p53 positivity The previous studies showed that P53 express10n 1n OSCC has a range from 4/38 (11%) to 24/32 (75%). In the current study, 12/35 (34.29%) of OSCC were P53 positive. 49 The earlier reported studies showed P53 positivity in premalignant lesions ranges from 2/12 (17%) to 40/45 (89%).49 In the present study, 7 /35 (20%) cases of oral leukoplakia showed positivity for P53.22
The wide range in impressibility of P53 proteins have been stated by Rich et al, 49 Diogene et al, 43 Paterson et al 44 and G. R.O gden.36 The reasons for this are: Variations 1n the etiological factors and ethnic background of the patients, with a generally lower prevalence of P53 alterations in oral squamous cell carcinoma in patients from the Indian subcontinent and a higher prevalence in patients from western societies. 23Variations in the immunohistochemical technique used also explain some of the differences, with the use of microwave antigen retrieval increasing the proportion of positive results. Tumors may have lost both the alleles of the p53 gene. Tumors may have a level of P53 protein that cannot be detected by IHC. The inability to detect P53 protein in the normal tissue samples can be attributed to the short half-life of the normal tissues. 24-26
In the current study, it was observed that none of the samples of Group I showed 3+ staining, whereas 6 samples of Group II showed 3+ staining. This showed that more number of cells contain mutated or stabilized P53 protein in case of oral squamous cell carcinoma. This is similar to the following studies: Kaur et al 27 reported that a progressive increase 1n P53 expression occurs 1n oral. uamous cells as they acquire a more malignant phenotype. Alison M. Rich et al 49 reported that 18/50 (36%) hyperplastic cases, 35/41 (85%) leukoplakia and 45/48 (94%) OSCC were positive for P53 protein and all cases of normal mucosa were negative for P53 protein. The specimens of dysplasia had a greater intensity of P53 staining than hyperplasia and specimens of OSCC had even greater intensity of P53 staining than dysplasia. 28 proportions of cases with positive P53 express 10n increased from hyperplasia to dysplasia to OSCC. Further there was a difference 1n the pattern of P53 expression among the study groups. The intensity of stain was more 1n the samples of group II when compared to group I.
The localization of the P53 stain was different. The P53 staining was diffuse among the samples of group I and group II. Previous researchers Trivedy C. et al, 29 Chun-Pin Chiang et al 54 have repo• ed similar results that correlate with the results of the present study. Rajaram Gopalakrishnan et al 45 found that the staining for p53 protein 1n proliferative verrucous leukoplakia was restricted to the basal and the intermediate suprabasal layers of the epithelium whereas in oral squamous cell carcinoma the staining tended to be diffuse involving all the layers of the epithelium. They also reported that oral squamous cell carcinoma stained more intensely than proliferative verrucous leukoplakia. 45 Santos Garcia A et al 17 found that p53 was negative in all samples of normal epithelium. In dysplasias the number of stained nuclei increased and the distribution was patched. They also found significant differences between normal epithelium and "in situ" and microinvasive carcinomas. Humayun S et al 29 found that all samples studied showed positive staining for p53. The staining was confined to basal layer in most of the cases except OSCC in which it was seen 1n all layers. The intensity of staining was moderate to intense. The percentage of p53-positive cells in normal mucosa was 15-2: -' which was increased to 95% in malignant mucosa.
Isabel B. Cruzet al 48 found that 7/35 (20%) premalignant lesions showed p53 expression clearly above the basal cell layer and 6 of these (86%) developed carcinomas. The results suggest that clear expression of p53 above the basal cell layer is an early event in oral carcinogenesis and an indicator of a developing carcinoma. The suprabasal expression of P53 could indicate that a larger part of the epithelium than normal is dividing .22 The comparison of P53 grading between the study group and control group in the present study was found to be significant while that of the study group with the control group was not significant. This suggests that P53 expression 1s more seen with the progression of premalignant to malignant lesions of the oral cavity. This correlated with the studies done by Warnakulasuriya K.A.A.S et al 35 reported that 35% of squamous cell carcinoma 17% of the dysplastic lesions were positive for mutant P53 protein. Authors concluded that p53 gene mutation in oral malignancy might occur early in the multistep process of malignant transformation. J. D. Langdon et al 18 showed that 2/2 cases of leukoplakia, 12/15 cases of squamous cell carcinoma were positive for P53, while all the samples of erythroplakia and normal oral mucosa were negative for P53. The results of this study have demonstrated that the expression of mutant P53 protein is a common event in the development of oral squamous cell carcinoma.30
S.C.Girod et al 31 reported that 8/39 (21%) premalignant lesions without dysplasia, 9/25 (36%) premalignant lesions with dysplasia, 19/40 (47%) specimens of highly differentiated squamous cell carcinoma, 27/45 (60%) specimens of moderate to poorly differentiated squamous cell carcinoma and 18/27 (66%) specimens of recurrent squamous cell carcinoma were P53 positive. This suggest that mutation of p53 gene is related to increasing dysplasia and loss of diffe re nt ia tior.7 n the carcinogenesis of the oral mucosa. Thus concluding that the mutation of the p53 tumor suppressor gene plays an important role in the carcinogenesis of the oral mucosa. Jasbir Kaur et al 27 found that 15/27 (55%) remalignant les ions and 24/32 (75%) squamous cell carcinoma were positive for P53 protein. It was found that there was no positive staining seen 1n normal oral tissues and 1n non- malignant oral lesions. The higher level of P53 protein in severe and moderate dysplasia than in mild dysplasia indicated that a progressive increase 1n P53 expression occurs in oral squamous cells as they acquire a more malignant phenotype.32-34
T.Tsuji et al 35 showed that 22/50 (44%) specimens of squamous cell carcinoma, 2/12 (14.3%) specimens of leukoplakia and 1/11 (9.1%) pleomorphic adenoma were positive for P53 protein. concluded that p53 is an indicator of the malignant potential of the oral mucosa and that the expression of P53 protein is a reliable indicator of poor prognosis. Regezi J.A. et al 40 reported that 6/13 cases of epithelial dysplasia and 4/6 cases of carcinoma-in-situ were P53 positive. The authors concluded that P53 expression appears to an early event in the development of P53 positive oral squamous cell carcinoma. andhya Agarwal et al 50 reported that 22/33 premalignant lesions, 20/45 cases of oral squamous cell carcinoma and 2/30 normal oral tissues were positive for P53 protein. 39 They also suggest that these alterations are early events and may be involved in preinvasive stages of oral tumorigenesis.
Humayun S et al 40 found that the percentage of p53- positive cells in normal mucosa was 15-25% which was increased to 95% in malignant mucosa. The expression of p53 increases as normal oral mucosa becomes dysplastic and undergoes malignant transformation, This study suggests that P53 may play an important role in oral carcinogenesis. Thus emphasizing the potential use of p53 as potential m24ers of malignant transformation and carcinogenesis in oral premalignant lesions, conditions and OSCC and that in future they may serve as prognostic tools in the early detection of malignant transformation 1n oral.
Conclusion
Expression of P53 protein is one of the most common events in oral carcinogenesis. There is a wide range of expression of P53 protein in premalignancies and squamous cell carcinoma of the oral cavity. The difference in the number of tumors expressing the protein can be attributed to variations in etiologic and ethnic background and variations in immunohistochemical methods employed. In the present study, a significant number of samples of leukoplakia and oral squamous cell carcinoma were positive for P53 protein. But there is wide range of expression of P53 protein.P53 protein detected by immunohisto chemistry may be one of the most important tumor marker.
There was a difference in the pattern of P53 expression with diffuse staining seen in oral squamous cell carcinoma. This implies that there is progressive accumulation of P53 protein as the normal cells change to dysplastic lesons and later to invasive carcinoma. Thus, it can be inferred that P53 may be considered as a tumor marker. Expression of P53 protein may help in determining the prognosis as well as play a key role in the gene therapy for the treatments of oral squamous cell carcinoma in future. However, long term follow up studies with larger sample size would further help to assess the significance of P53 protein in oral carcinogenesis.
Acknowledgements
The encouragement and support from Bharath Institute of Higher Education and Research, Chennai is gratefully acknowledged. For provided the laboratory facilities to carry out the research work.
Declaration of Conflict of Interest
The authors declare no conflict of interest.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
References
- Saranath D, Tandle AT, Teni TR, et al. p53 inactivation in chewing tobacco-induced oral cancers and leukoplakias from India. Oral oncology 1999;35:242-50.
- Brugere J, Guenel P, Leclerc A, et al. Differential effects of tobacco and alcohol in cancer of the larynx, pharynx, and mouth. Cancer 1986;57:391-395.
- Madani AH, Dikshit M, Bhaduri D. Risk for oral cancer associated to smoking, smokeless and oral dip products. Indian J pub healt 2012;56:50-52.
- Levine AJ, Perry ME, Chang A, et al. The 1993 Walter Hubert Lecture: the role of the p53 tumour-suppressor gene in tumorigenesis. BJC 1994;69:409-416.
- Gleich LL, Salamone FN. Molecular genetics of head and neck cancer. Cancer Control 2002 Sep;9:369-378
Author Info
Bindthiya RC Babu AR Subasree and Ramalakshmi V*
Department Pathology, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaCitation: Rawda Aljadar, Mohammed Yunus, Amr Zaher, Sajid Hussain, Expression of P53 Protein in Premalignant and Malignant Lesions of the Oral Cavity, J Res Med Dent Sci, 2021, 9(9): 1-10
Received: 01-Dec-2021 Accepted: 15-Dec-2021 Published: 22-Dec-2021
