Research - (2023) Volume 11, Issue 1
Pathogenesis, Clinical Characteristics, Quality Of Life And Management Approach Among Women With Polycystic Ovary Syndrome (PCOS)
Deeksha Rana, Neema Acharya* and Ashish Bhatt
*Correspondence: Neema Acharya, Department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (DMIMS), India, Email:
Abstract
Introduction: PCOS is most common hormonal disturbance affecting 6%-20% of reproductive age women, considered multifaceted with spectrum of conditions including menstrual irregularities, acne, hair loss, obesity and hirsutism. Moreover, PCOS is also the cause of adverse cardiovascular, metabolic, reproductive and psychological approaches for reduction in QOL. This review will help females understand responsible factors for PCOS incidence, its health consequences and management needed. Methodology: The literature reviewed in this narrative article is obtained from various databases such as Google, PubMed, Scopus, and Web of Science using MeSH-compliant keywords including PCOS, Rotterdam criteria, QOL, PCOS management and consulting related books. References from year 2003 to 2021 were assessed and relatable information included. Results: Studies conducted across country reflects high PCOS prevalence and irregular menses, hirsutism, acne, obesity, AN, infertility and negative impact on QoL. Discussion: PCOS females present with reproductive and metabolic manifestations along with potential of several co-morbidities. Stigma associated with PCOS remains neglected global issue. PCOS has significant association with short and long-term health conditions, if untreated, lead complications. The goals of PCOS management are menstrual regularity, hyperandrogenism reduction, infertility management and IR resolution through non-pharmacological and pharmacological approaches. Conclusion: Large scale studies across country are necessary for actual status. Accurate diagnosis at young age is the key factor. Counselling young females should be done to spread awareness and remove stigma for better future.
Keywords
Polycystic ovary syndrome, Clinical features, Management
Introduction
Polycystic ovary syndrome (PCOS), is the most common hormonal disturbance which affects 6%–20% of reproductive age women, and has been the most debatable female endocrine disorder worldwide, with India being no exception [1]. It is defined as the association of elevated androgen levels with chronic anovulation without specific underlying disease of the adrenal or pituitary glands [2]. The disorder can be morphological (polycystic ovaries i.e., small sized cysts on one or both ovaries) or biochemical (hyperandrogenism i.e. elevated androgen levels) which is usually presented as a clinical hallmark of PCOS [3]. Typically, PCOS is identified during the early years of adolescence and is considered to be multifaceted with a spectrum of conditions including menstrual irregularities (manifested as amenorrhea, oligo menorrhea, dysfunctional uterine bleeding), cystic acne, seborrhea, hair loss, obesity and hirsutism or excess body hair (or its equivalents such as acne vulgaris or pattern alopecia) [4,5].
Moreover, PCOS, has contribution towards certain significantly adverse multidisciplinary morbid approaches such as Cardiovascular-hypertension, obstructive sleep apnea; Metabolic–early onset of type 2 diabetes, dyslipidaemia, hyperinsulinemia, insulin resistance, obesity, acanthosis nigricans; Reproductive– an ovulatory infertility affecting around 90–95% of women [6], potential endometrial, breast and ovarian cancer. They can also present with a pregnancyrelated complication, such as gestational diabetes or spontaneous abortion [7]. Also, women with PCOS suffer from body dissatisfaction and present with psychological disabilities including increased risk of mood, generalized anxiety, depression and eating disorders which become responsible factors for reduction in quality of life [8].
Historical significance
During early 1700s, Vallisneri reported females who are married but childless having white glossy ovaries with its dimensions similar to eggs of a dove [9].
During late 1800s, bilateral oophorectomy–a surgical intervention for ovary cyst degradation was described through Lawson Tait [10]. In 1935, Irving F. Stein and Michael L. Leventhal presented a group of 7 women with common features: oligo/amenorrhea combined with presence of bilateral polycystic ovaries established during surgery. Three of these seven patients also presented with obesity, whereas five showed signs of hirsutism. On performing bilateral ovary wedge resection as surgery of choice, 90% females achieved regular menses and among them, 65% achieved pregnancy. Hence, PCOS is also termed as Stein – Leventhal syndrome [11,12].
In late 1970s, Swanson demonstrated PCOS ovarian design through USG.
In 1990s, National Institutes of Health (NIH) published “the NIH criteria” which defined PCOS as unexplained hyper androgenic anovulation and confirmed diagnosis if the following criteria are present - symptoms of excess of androgens (clinical or biochemical), rare ovulations, exclusion of other disorders with similar clinical symptoms.
The “Rotterdam criteria,” which included the dimension and shape of the ovaries as measured by USG, were created in 2004. According to this criterion, two out of three of the following are required for a PCOS diagnosis: erratic or absent ovulation, undue androgen activity established through clinical or lab investigation, and USG characteristics of PCOS followed by excluding further pathological changes descriptive of hyperandrogenism. When drugs like clomiphene and follicle-stimulating hormone were accessible, therapeutic intervention became the favored alternative over surgical ovaries excision. Newer expertise like USG imaging came as new innovation on the account of PCOS to make a more straightforward analysis. Though, this had the unintended consequence of various females being labelled with polycystic ovaries while having minor or no other symptoms. This gave rise to the label polycystic ovarian (PCO) morphology, whose magnitude is still debated
Epidemiology
According to World Health Organization (WHO) 2020 statistics, PCOS affected 116 million (3.4%) of women across the world which has progressed to at least 1 in every 10 women to have confirmed diagnosis of PCOS worldwide [13]. In last couple of years, the country has witnessed an increase in the prevalence of disorder, especially in adolescents. Lack of knowledge and lifestyle changes is considered to be the major factors leading to this phenomenon [14]. However, on the basis of recruitment of patients, criteria used and application of screening methods, there are few and mostly convenience based–sampling studies on the prevalence of PCOS in India which may hamper the true status [15].
In a study conducted in Mumbai among adolescents aged 15-24 years, the prevalence was noted to be 22.5%. Cross-sectional study conducted in Tamil Nadu found 18% prevalence in young adolescent females. The prevalence is more in women living in cities comparative to women in towns and villages. Reports from Lucknow showed prevalence in college–going women aged 18-25 years was only 3.7% [7]. In another study on adolescents in Andhra Pradesh, the noted prevalence was 9.13% [15]. Hence, it can be attributed that PCOS prevalence vary between regions of country with an estimated range of 3.7–22.5%.
Etiopathogenesis
PCOS is understood to be multifaceted pathological changes which are still under researches to be proven. Surprisingly, high incidence in recent years can be attributed to multiple hypotheses ranging from hereditary to lack of awareness associated with it both in utero and in postnatal life (Figure 1).
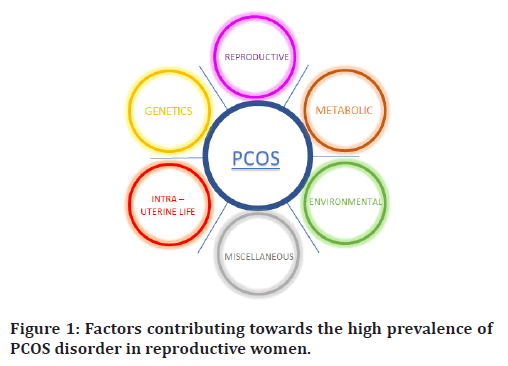
Figure 1. Factors contributing towards the high prevalence of PCOS disorder in reproductive women.
Intra–uterine life (iul)
Evidence of certain experimental studies suggest that certain critical conditions during pregnancy, such as Intrauterine growth restriction (IUGR), can increase fetal exposure to excess androgens or glucocorticoids that might induce the development of symptoms along with deciding the phenotypic expression of PCOS during adolescence. However, the viability of this potential theory is mixed, supported by some research while other researches indicating no such association [16]. In some cases, Congenital Virilizing Disorders contributes premature adrenarche, true sexual precocity, overgrowth due to pseudo acromegaly as responsible factors to development of PCOS.
Genetics
Many susceptibility genes, including cytochrome CYP1A1, CYP17A1, 17-hydroxysteroid dehydrogenase (HSD17B6), androgen receptor (AR), sex hormonebinding globulin (SHBG), insulin receptor substrate 1 (IRS1), follicle stimulating hormone receptor (FSHR), luteinizing hormone receptor have been recognized through candidate gene method, while, genome - wide correlation findings identified a number of susceptibility locus, such as LHCGR, FSHR, and ERBB4, which all play a role in PCOS genetic inheritance. Till date, various literatures have supported facts that the risk of PCOS incidence is largely attributed to family history – 40% siblings and 35% mothers of patients affected, with autosomal dominant pattern, aggravated by various environmental factors.
Reproductive
There are primarily two pathogenic mechanisms which contribute to excess androgen levels, ultimately causing development of PCOS and its manifestations.
Abnormal Steroid genesis majorly influence thecal cytochrome P450c17 (CYP17) work that possess both 17α-hydroxylase as well as 17,20-lyase action, where the second is rate-limiting step of this process. This causes substantial intra ovarian androgen excess resulting in characteristic functional ovarian hyper androgenism condition, which in return, stimulates undue development for primordial follicles though hampering the necessary follicles maturity towards the foremost follicle development, thus, being responsible for ovarian abnormalities [17].
Primary Defect in Hypothalamic–Pituitary Axis includes hampered gonadotropin – releasing hormone (GnRH) regulation leading to reduced FSH levels and low retaliation by follicles in ovaries, excess LH and high AMH levels. This causes premature arrest of follicles and increased secretion of testosterone, estradiol and dehydroepiandrosterone (DHEA) by ovarian thecal cells resulting in androgen excess.
Metabolic
It is also reported that high leptin levels have been positively correlated with serum testosterone levels and plasma leptin: adiponectin ratio (L:A ratio) have positive association to insulin resistance parameters, thus, validating low ratio to be probable biomarker, with central fat accumulation as liable constituent for insulin resistance manifestation as well as for metabolic syndrome. Insulin Resistance (IR) defined as reduced glucose response to a given amount of insulin and consequent compensatory hyperinsulinemia is the most widely accepted explanation for the resulting metabolic defects [18]. In PCOS, IR affects skeletal muscles, adipose tissue and liver, whereas, due to hyperinsulinism, direct stimulation of steroidogenic ovaries and adrenal glands causes androgen secretion and reduced synthesis of sex hormone binding globulin (SHBG) in liver, leading to elevated levels of free, biologically active androgens. This excess of ovarian androgen production causes dysregulation resulting in premature follicular atresia and anovulation.
Environmental
Women during their daily chores, unknowingly, get exposed to certain chemicals known as endocrine disruptors (EDs), most commonly bisphenol-A (BPA) have anti-estrogenic, anti-androgenic properties that interfere with feedback regulation, DNA methylation, and neuroendocrine cells alternation. These EDs in result contribute as a causative factor either to unveil PCOS characteristics in genetically susceptible females or interrupt hormone homeostasis and deteriorate fertility status of women with PCOS. For instance, positive alliance among androgens along with high BPA concentrations in PCOS females is suggestive of this ED as causative agent [19].
Usually, sedentary lifestyle and lack of physical activity causes generalized obesity and abnormal body fat distribution which does play as exacerbating factors for PCOS. Also, fat cells in PCOS females are metabolically different as compared to normal, with former having larger subcutaneous adipocytes that are less responsive to lipolytic effects of catecholamine’s along with lower serum adiponectin production [20].
The available data support that unhealthy habits like smoking, alcohol and diet with high carbohydrates, low fiber content and high trans-fat often act as potential factors for PCOS. In addition, PCOS women with Vitamin D deficiency are likely to have high fasting glucose and insulin resistance which makes body prone to obesity.
Miscellaneous
Certain reports suggested positive correlation between PCOS with high concentration of zinc, copper and calcium and low concentration of manganese levels. The significantly deranged plasma amino acid levels of cysteine, phenylalanine, glycine, lysine, tyrosine etc., along with large concentration of arginine highlighted as parameter for metabolic and oxidative stress in PCOS [21].
During the onset of puberty, when females witness sudden major changes in their menstrual cycles, excessive weight gain, acne, overall physical appearance, it leads to confusion, stress and acts as a contributing factor of psychological dilemma. Lack of awareness, undiagnosed or inappropriate medical therapy often leads ‘PCOS Negative Impact’ resulting in high risk of anxiety, severe mood swings, depression and even sometimes, towards suicidal tendencies.
Histopathology
Under the gross description of PCOS, the ovaries are twice the size of normal, thick capsule with pearly white color and increased ovarian volume more than 10 cm3. There is presence of numerous (i.e., more than 12) subcortical cysts which represent immature follicles, measuring around 2-9 mm in diameter and give a crowded appearance around the cortex. Also, endometrium may show metaplastic changes resembling adenoacanthoma or adenocarcinoma [22].
On microscopic examination, the multiple cystic follicles are covered by dense thickening of tunica albuginea. There is Latinization of the theca interna causing stromal hypertrophy. Follicular cysts at different stages of maturation and atresia are seen.
Types of PCOS
Insulin resistant PCOS
Also known as metabolic syndrome is most common, caused by factors such as smoking, sugar, and trans-fat. In this type, high levels of insulin prevent ovulation and trigger the ovaries to create testosterone. Consistent hyper-insulinism can present itself as symptom of pre – diabetes.
Pill induced PCOS
It is second most common among PCOS females and mainly develops due to birth control pills as withdrawal effects which primarily suppress ovulation and causes sudden androgen surge, for instance, use of drospirenone or cyproterone [23].
Adrenal PCOS
This is usually caused by an exaggerated physiological reaction to stress. Such females have only high levels of DHEA-S in adrenal glands and normal testosterone and androstenedione levels in ovaries [24,25].
Inflammatory PCOS
This occurs when an inflammatory cascade act as primary agent causing hormonal imbalance and prevention of ovulation that would trigger body to release high number of androgens. It is also known as Hidden PCOS and factors include iodine deficiency, thyroid disease, zinc deficiency, autoimmune disorders, environmental toxins, etc. Once the cause is addressed and treated, it takes about 3-4 months to resolve. PCOS can also be classified on the basis of possible phenotypes i.e., observed clinical features in females. PCOS women can have combination of major phenotypic conditions excess androgen levels, ovarian dysfunction and polycystic ovarian morphology. Type A and Type C are noted to be most common phenotypes with Type A being most severe observed in women with PCOS.
Clinical features
Clinical features are mentioned in Table 2.
Females suffering from PCOS mainly present with reproductive and metabolic manifestations along with potential of leading several co-morbidities with it.
Reproductive manifestations
Menstrual Disturbances manifest as oligomenorrhea, amenorrhea or dysfunctional uterine bleeding in around two – thirds of young females with PCOS mainly due to elevated levels of LH coupled with androgens and insulin along with low levels of FSH. Also, this hormonal imbalance by ovaries can lead to presence of tiny cysts on the surface of large-sized ovaries along with hair, skin features and anaemia. Due to menstrual irregularities, PCOS is the causative factor for anovulatory infertility or in some cases of pregnancy in PCOS women, it possesses as risk factor for suffering spontaneous abortion or developing gestational diabetes.
Skin and Hair characteristics are the most visible changes in this syndrome mainly caused due to excessive production of androgens and insulin. Hirsutism defined as appearance of excessive hair growth in male pattern on androgen – dependent sites of body, is recognised as classic feature and present in two-thirds of cases. It is evaluated as per Ferriman - Galleway classification through which the result of hair growth •8 suggest positive sign. Other hirsutism equivalents include seborrhoea, excess acne, alopecia, hyperhidrosis as cutaneous signs of hyperandrogenism. It is believed that irregular menses persist in first few months and acnes are quite common during adolescence, thus, progressive hirsutism regarded as good marker in PCOS diagnosis and it is also suggested that assessment for diagnosis should be done at least two years after the onset of menstrual cycle.
Metabolic manifestations
Android Obesity defined as central fat accumulation with waist circumference more than 88 cm after attainment of puberty is observed in about half of PCOS women and often regarded as risk factor for amplification of metabolic consequences.
Studies have hypothesised that obesity acts synergistically as well as independently leading to development of insulin resistance (IR) which presents Acanthosis Nigricans (AN) as cutaneous sign, defined as dirty looking raised velvety, mossy, hyperpigmented skin condition, commonly found in body folds around neck, armpits, groin and breast and is considered an indicator for the severity of hyperinsulinemia along with hyperkeratosis and papillomatosis as histological characteristics. It also leads to skin tags, rough elbows or rough red hair follicles on upper arms.
Metabolic consequences due to insulin resistance include impaired glucose tolerance with intense craving for carbohydrates, type 2 DM, abnormal lipid profile presenting with elevated levels of LDL and total cholesterol. Certain studies have reported that hyperandrogenism causes sleep – breathing disorders leading to an increased risk of cardiovascular morbidity which is often predicted through levels of plasma gamma glutamyl transferase (GGT) [6]. Psychiatric disturbances such as anxiety and depression are also observed in adolescents.
Differential diagnosis
During puberty, young females go through many physical changes which can be recognized as normal variants. For instance, approximately, 50% of adolescents have anovulatory menstrual cycles during the first two years of post – menarche; 75% develop acne and excessive hair growth and weight gain is observed in over 25%, thus making diagnosis of PCOS quite challenging. Keeping hyperandrogenism as hallmark of PCOS, the distinguishing features of certain conditions should be assessed before confirming diagnosis –
Hirsutism should be differentiated from hypertrichosis, where, the latter is not caused due to excess androgen and has non – sexual distribution i.e., commonly seen at temple region and articulatio humeri.
Most commonly, hyperandrogenism has been regarded as causative factor for congenital adrenal hyperplasia, malignant tumours predominantly producing androgens and PCOS.
Moderate androgen elevations causing disorders like Cushing’s disease, Polycystic ovaries (PCO) along with high prolactin levels can mimic PCOS.
On rare occasions, cortisol resistance or apparent cortisone reductase deficiency can give rise to dexamethasone - resistant functional adrenal hyperandrogenism. Hermaphroditism is also considered rare gonadal androgen excess.
Diagnostic criteria
The onset of diverse characteristics of PCOS start from an early age, hence, timely diagnosis and medical intervention are important to reduce symptoms, preserve fertility and halt complications which can develop throughout a girl’s adolescence till the postmenopausal period. PCOS is regarded as ‘diagnosis of exclusion’, thus, approach to reach correct diagnosis includes screening of suspected individuals, determining the presence and source of excess androgen levels and laboratory investigations (Table 3).
Screening of individuals
Failure to establish normal menstrual cycle even after 2 years of menarche carries two-third risk of oligoovulation.
Pre-pubertal acne being resistant to standard treatment modalities or require isotretinoin management suggest non-classic congenital adrenal hyperplasia.
Hirsutism associated with menstrual irregularities or obesity suggest androgen excess.
Central fat distribution with violaceous striae is suggestive of Cushing’s syndrome.
Acceleration of obesity during puberty, presence of acanthosis nigricans or family history of metabolic syndrome or type 2 DM.
Presence and source of androgen excess
Researches have suggested level of total testosterone above 90 ng/dl reliable for androgen excess. Moreover, free testosterone which is the fraction of plasma testosterone that is bound to albumin or free bioavailable fraction, is recognized as best single indicator because of its suppression by androgen excess and insulin resistance.
The most discriminating criterion for typical PCOS is adrenocorticoid and androgen dominance as the source. Accordingly, dose of 0.5 mg of dexamethasone 4 times meant for four days is given every day and androgen panel along with cortisol levels will be considered after final dose in subsequent morning. Dominance of ACTHdependent adrenal function by dexamethasone results in levels of plasma free testosterone fall below 8 pg/ml, total testosterone below 35 ng/dl, DHEA sulfate below 80 μg/dl and plasma cortisol below 1.5 μg/dl.
Laboratory investigations
Along with complete medical history and physical examination along with biochemical investigations, predominantly blood report for hormone and metabolic assessment provides information that aid diagnosis –
Serum AMH levels can be considered as an investigatory parameter because the levels are 2-4 times more than normal in PCOS with proposed value standing at 4.9 ng/ ml or 35 pmol/L using enzyme immunoassay (AMH-EIA). The major advantage is that assessment is achievable in any stage during menses.
Pelvic examination through transvaginal sonography is performed to scan ovaries cysts, exclude ovarian tumours, and endometrial thickness to rule out hyperplasia changes.
Hormonal profile includes assessment of β-HCG to exclude oligomenorrhea; FSH, LH, PE2 to exclude amenorrhea, premature ovarian failure; thyroid function tests to exclude hypothyroidism; prolactin to exclude hyperprolactinaemia; androgens to exclude congenital adrenal hyperplasia or androgen secreting tumours and subsequently confirm PCOS [19].
Metabolic profiling includes oral glucose tolerance test to exclude type 2 DM; fasting lipid to exclude dyslipidaemia; dexamethasone/cortisol levels to exclude Cushing’s syndrome.
Despite PCOS along with its impact on woman’s health is out of the closet, the stigma associated with it, still, remains a neglected issue in global response. Learning that they have PCOS, women feel shattered and think that they have distorted the definition of womanhood. Emotionally, many women express shame, anger, guilt, blame, denial and in many cases, considering fertility treatment possess moral, ethical and religious dilemmas in their life. There is psychological wrenching through decreased self-confidence, sexual dissatisfaction, loss of feminine identity, imperfect body image, avoidance of social interaction to name a few.
Quality of Life (QoL) is a multidimensional, dynamic concept used to describe physical, psychological and social components of health status associated with particular disease or its treatment. Hence, patient assessment is highly recommended which is achieved through questionnaires that can either be generic, specific or both. Specific tools are more preferred as they provide evaluation of each factor associated with the disease. Generic questionnaires include SF-36, WHOQOL-BREF, CHQ-CF87, while, PCOS disease specific tools are PCOSQ, MPCOSQ and PCOSQ-50.
Late sequlae of PCOS
There is significant association of PCOS with short and long-term health conditions, primarily comprising of cardio-metabolic, psychological and reproductive disorders which if left untreated, can cause aggravation of syndrome leading to serious complications. Recent studies have noted an estimated 4-times augmented peril for NAFLD among PCOS population with underlying pathogenesis of complex interplay between androgen excess, insulin resistance and obesity. NAFLD has a high mortality rate as it causes spectrum of continuous inflammatory morphological changes, starting from hepatic steatosis, continuing to nonalcoholic steatohepatitis (NASH) and ending with stages of either hepatic cirrhosis, liver failure or hepatocellular carcinoma [18].
Insulin resistance with compensatory hyperinsulinemia affects about 70% PCOS population and is associated with a high prevalence of type 2 DM (79%) and central obesity (60%). Triglyceride levels more than 150 mg/ dl, HDL levels less than 50 mg/dl, BP more than 130/80 mm Hg, OGTT level more than 100 mg/dl and Abdominal obesity more than 88 cm., with presence of at least 3 findings is considered diagnostic of metabolic syndrome [23].
Reproductive complications include about 33% chances of infertility and exaggerated features of hyperandrogenism in at least 77% cases. The collaboration of chronic anovulation with hyperinsulinemia might induce aggravation of endometrial proliferation resulting an increased risk of endometrial and ovarian carcinomas [8]. Some other biochemical abnormalities associated with PCOS include hyperlipidaemia, androgenic follicular micromovement, hyperprolactinaemia, hypersecretion of LH and low serum levels of progesterone and FSH levels.
Management
The goals of PCOS management are to resolve its major four components comprising of menstrual regularity, hyperandrogenism reduction, infertility management and resolution of insulin resistance through nonpharmacological and pharmacological approach. Along with this, all patients are needed to be clinically evaluated for improvement in cardinal manifestations and close monitoring of any abnormal drug-drug interactions and adverse effects.
Lifestyle modifications
Dietary Intervention in form of a nutritionally adequate weight loss diet is proven to be beneficial in PCOS, particularly, in improving insulin resistance and reducing excess androgen levels and is regarded as first line treatment. Regular exercise leads to improvement in insulin sensitivity, SHBG and lipid levels and decrease in stress, androgen levels, weight and blood pressure, thus enhancing a healthy physical appearance and mood and ameliorating anxiety and depression.
Medical Nutrition Therapy sheds light on the necessary Nutrition Diagnosis which screens the presence or potential risk of nutritional deficit development that needs to be addressed with immediate action, Nutrition Intervention including collaborative medical and behavioural goals which the patient is comfortable with, as well as, specific remedies such as healthy meal – planning strategy, controlling food size portions etc., and Nutrition Monitoring through follow-ups for evaluating outcomes and modify interventions as required.
Appropriate drug therapy
PCOS treatment is usually directed on ameliorating immediate presenting complaints and reduces the burden of symptoms, however, it does not correct the underlying pathogenesis. Major therapies include oral contraceptive pills (OCPs) for menstrual regularity and hirsutism/acne improvement, anti - androgens in combination with OCPs and insulin sensitising agents such as metformin and inositol [19]. A study was conducted in 2019 among 440 PCOS patients among which, metformin was prescribed to 81%, OCPs to 59.4%, Infertility medication to 44.6%, anti-androgens drugs to 18%, anti-acne treatment to 7.7% and antihirsutism drugs to 4.8%.
Before prescribing treatment, it is necessary to obtain complete history of patient including – menstrual history, onset and progression of symptoms, weight changes, presence of hair loss and acne, change in facial appearance and family history of similar complaints. Also, a thorough physical examination is to be conducted to establish type, pattern and extent of symptoms, signs of abnormalities such as – masculinization, virilization, thyroid enlargement and presence of any pelvic or abdominal mass.
Menstrual regularity
OCPs have been considered primary intervention because these induce regular menses which is beneficial than any further therapeutic result. Commonly used OCPs are oestrogen–progestins which act by inhibiting ovarian function and suppressing free testosterone levels along with lowering DHEA sulfate and elevating levels of SHBG.
Progestin therapy includes medroxyprogesterone acetate (MPA) at dose of 5-10 mg/day for 10-14 days each month is used to treat amenorrhea or dysfunctional uterine bleeding by preventing atypical endometrium rise, however, there is no subdue of ovarian – androgen synthesis. Lipid profiling recuperates, hence, is recommended to women who are not at risk for pregnancy and not ready to conceive. In cases of heavy DUB, Oestrogen can be prescribed with dose of 1 tablet thrice daily for 7 days, with a gap of five days and then continued. These drugs are usually contraindicated in venous thrombosis case and side effects include abdominal pain, mastalgia, cramps, heavy withdrawal bleeding, reduced libido, weight changes, headaches, confusion etc.
OCPs are continued till the attainment of gynaecological maturity i.e., five years post-menarche or when patient had adequate weight loss. Generally, it is advised that treatment should be withhold for some time, so that the physiological pituitary – gonadal axis recovers, also, for checking persistence of any other irregularity for further assessment.
Infertility management
The drug of choice in such cases is Clomiphene Citrate for resolving chronic anovulation with 50 mg/day for 5 days initially. When response not achieved after first cycle, an increased amount with 100 mg daily for 5 days recommended, but after a gap of at least 30 days of previous therapy. In infertility management, usually maximum six cycles of therapy can be attempted before any further assessment is considered. Studies have proven clomiphene drug to induce an estimate of 30% successful pregnancies.
Hyperandrogenism reduction
Therapeutic acne intervention is commonly through benzylperoxide, clindamycin, retinoid creams etc., which are applied on affected skin areas. Although, patients with harsh acne consider antibiotics, mostly, tetracycline or oral isotretinoin management.
Hirsutism can be quite worrisome and needs cosmetic amelioration along with medications to slow down hair growth. Skin dealing for hirsutism involves trimming, waxing, inhibition of hair development by eflornithine hydrochloride topical application and dermal papilla annihilation by electrolysis or laser therapy however, is has consequences like itchiness and tenderness on skin surface. For androgen suppression, most popular are oral contraceptives (OCPs) which suppress LH and FSH levels causing decrease in ovarian androgen production resulting in improvement of acne and hirsutism within 3 months. Most commonly used is Yasmin comprising of ethinyl oestradiol (30μg) and drospirenone (3mg) and its mild anti-mineralocorticoid and anti - androgenic behaviour makes this medication suitable to diminish hyperandrogenic effects. Effects are observed after period of 9 months due to long growth cycles of sexual hair follicles. Usually, antiandrogens and OCPs are prescribed together for strong intervention. Alopecia Rana, et al. treated with topical antiandrogens like minoxidil.
Most commonly used anti-androgen is Spironolactone due to its low cost, availability, high potency and safety. Usually, prescribed dose is 25-100 mg twice daily which lowers the hirsutism score by one-third and then gradually reduced for maintenance therapy. It may also decrease lipid levels, however, side-effects include vaginal/uterine bleeding, reduced libido, dysuria, headaches etc., and contraindicated in adrenal, hepatic or renal insufficiency cases. Other antiandrogen equivalents include cyproterone acetate which is pregestational antiandrogen with weak anti-glucocorticoid effects, 5 α-reductase inhibitors such as finasteride and flutamide. Flutamide has similar efficacy as that of cyproterone and spironolactone, however, high expense and potential hepatocellular toxicity limits its use.
Resolution of insulin resistance
Metformin 500 mg thrice daily acts by reducing the sugar concentration in plasma and recuperating glucose with its necessary usage along with decrease in production of glucose in liver, hence, essential for menstrual regularity, enhancing ovulation rate and pregnancy chances.
Accordingly, Myoinositol 3g/day also improves IR through the use of glucose at periphery, subsequently, improving metabolic and hormonal states and restoring spontaneous ovulation. When a study was conducted regarding the association of inositol and d-chiro-inositol with PCOS, notable significant weight loss, decreased FSH and LH levels and subsequent increase in SHBG level was observed. Also, there was decrease in serum glucose level within 6 months of therapy and improved skin conditions after 3 months of treatment.
Surgical measures
These are usually undertaken when medical treatment fails to restore ovulation. In such cases, pre – operative screening is done and contraindications are excluded such as patient haemodynamically unstable, severe cardiopulmonary disease, generalized peritonitis, significant hemoperitoneum and intestinal obstruction to name few. Informed consent is taken and general anaesthesia is generally preferred.
Wedge Resection is removal of one-third ovarian tissue with base on the surface and apex extending to medulla. Not common surgical operation nowadays.
Laparoscopic Ovarian Drilling is more commonly preferred. It can be done using a needle point monopolar cautery, bipolar cautery or by laser through which cortex is usually punctured up to 3-5 mm depth at 4-6 sites. A retrospective study by Yanamandra and Gundabattula among women who underwent laparoscopy and hysteroscopy followed by ovarian drilling was conducted in response to primary infertility treatment and reported an estimate of 50% enhanced conception.
Adverse Drug Reaction (ADRS)
The above mentioned medications are reported to be effective in providing symptomatic relief, though, findings reported physical health to be affected due to low incidence of adverse effects such as diarrhoea (80%), trailed by sickness (66%), temperament disorders (64%), sore breast (60%), and abdominal discomfort (59%) and back discomfort (43.6%). Significant association was shown between occurrence of diarrhoea with use of metformin, clomiphene, and letrozole along with usage of spironolactone, letrozole and clomiphene with breast tenderness, although, the effects were appeared to be well tolerated by PCOS women. A number of related studies were reviewed.
Future work
After confirmed diagnosis of PCOS, researches show that more than 60% report with insulin resistance, more than 10% develop pre-diabetes or type 2 diabetes by 40 years age. It is also associated with long-term risk of cardiovascular diseases – hypertension, MI; high cholesterol, sleep apnea, endometrial carcinoma, infertility, anxiety, depression etc, which if untreated can lead to serious complications. Hence, parameters such as profiling of lipids and OGTT should be assessed especially, females with excess weight issues. There are certain findings indicating big risk of NAFLD per most probable etiologic factor of hyperandrogenism. Hence, screening test should be considered and future research should be conducted on the same.
Some recommendations can be considered to spread its awareness, avoid the term ‘disease’ and stigma associated with it and improve overall health and quality of life.
Counseling of school–going girls is important so that appropriate treatment and preventive action can be initiated which might become key factor in preclusion of consequences.
The diagnosed females should be counselled for the occurrence of clinical manifestations and use of pharmacological agents. Healthy nutritious food, regular exercise and psychotherapy should be advised.
PCOS websites regarding information and helplines should be developed and kept available 24x7 hours for addressing the uncertainties.
PCOS females who became pregnant should be counselled for risk of miscarriages, diabetes or hypertension in pregnancy, premature baby.
Conclusion
Available studies suggest, PCOS is complex heterogenous endocrinological syndrome seen in women, accompanying life-term morbid conditions. Prevalence in our country ranges from 3.7%-22.5% and no constant treatment is present due to its multifaceted nature. Accurate diagnosis at young age can be the key factor and physicians should be familiar with the PCOS phenotypes. The most commonly preferred screening is plasma free testosterone level, while, ultrasonography helps in differential diagnosis. The major clinical manifestations include chronic anovulation, menstrual irregularities and change in physical appearance such as obesity, acanthosis nigricans, hirsutism, acne and alopecia which adversely leads to development risk of endometrial hyperplasia and carcinoma, infertility and metabolic and psychological distress respectively.
Insulin–lowering treatment, metformin, spironolactone and glucocorticoids, restoring ovulation using hormonal drugs including OCPs, progestins and antiandrogens and use of IVF in refractory scenarios to increase chances of successful pregnancy have been reported on ameliorating symptoms. Review analysis on women’s health created an influence regarding the treatment success, hence, might add necessary information in clinic trials for evaluating the effectiveness of various treatment modalities and also, to the natural history studies. Providing complete knowledge and young females counselling must be assessed to make them aware so as to eliminate the stigma associated with it and to empower and support the PCOS women to take decisions for management and better health in present and future.
Conflict of Interest
No conflict of interest is declared by the authors.
References
- Sidra S, Tariq MH, Farrukh MJ, et al. Evaluation of clinical manifestations, health risks, and quality of life among women with polycystic ovary syndrome. PloS one 2019; 14:e0223329.
- Jones GL, Benes K, Clark TL, et al. The polycystic ovary syndrome health‐related quality of life questionnaire (PCOSQ): A validation. Hum Reprod 2004; 19:371-377.
- Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: A review of treatment options with a focus on pharmacological approaches. Pharm Therap 2013; 38:336.
- Nasiri-Amiri F, Tehrani FR, Simbar M, et al. The polycystic ovary syndrome health-related quality-of-life questionnaire: confirmatory factor analysis. Int J Endocrinol Metab 2018; 16.
- Mehta AV, Shah ZR, Mehta KV. Screening of polycystic ovarian syndrome in young females of Gujarat. Int J Health Sci Res 2021; 11:33-37.
- Toosy S, Sodi R, Pappachan JM. Lean polycystic ovary syndrome (PCOS): An evidence-based practical approach. J Diabetes Metab Disord 2018; 17:277-285.
- Ganie MA, Vasudevan V, Wani IA, et al. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J Med Res 2019; 150:333.
- Dennett CC, Simon J. The role of polycystic ovary syndrome in reproductive and metabolic health: overview and approaches for treatment. Diabetes Spectr 2015; 28:116-120.
- Insler V, Lunenfeld B. Polycystic ovarian disease: A challenge and controversy. Gynecol Endocrinol 1990; 4:51-70.
- Szydlarska D, Machaj M, Jakimiuk A. History of discovery of polycystic ovary syndrome. Adv Clin Exp Med 2017; 26:555-558.
- Stein IF. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935; 29:181-191.
- Stein IF, Cohen MR, Elson R. Results of bilateral ovarian wedge resection in 47 cases of sterility: Twenty-year end results: 75 cases of bilateral polycystic ovaries. Am J Obstet Gynecol 1949; 58:267-274.
- Agarwal S. Development of kap tool and Its application in assessment of young females with Pcos. Indian J Appl Res 2020; 1-4.
- Upadhye JJ, Shembekar CA. Awareness of PCOS (polycystic ovarian syndrome) in adolescent and young girls. Int J Reprod Contracept Obstet Gynecol 2017; 6:2297-2301.
- Radha P, Devi RS, Madhavi J. Comparative study of prevalence of polycystic ovarian syndrome in rural and urban population. JAMDSR 2016; 4:90.
- Merkin SS, Phy JL, Sites CK, et al. Environmental determinants of polycystic ovary syndrome. Fertility Sterility 2016; 106:16-24.
- Buggs C, Rosenfield RL. Polycystic ovary syndrome in adolescence. Endocrino Metabol Clin 2005; 34:677-705.
- Kumarendran B, O’Reilly MW, Manolopoulos KN, et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database. PLoS Med 2018; 15:e1002542.
- Costello MF. Polycystic ovary syndrome: A management update. Aust Fam Physician 2005; 34.
- Kshetrimayum C, Sharma A, Mishra VV, et al. Polycystic ovarian syndrome: Environmental/occupational, lifestyle factors; An overview. J Turk Ger Gynecol 2019; 20:255.
- Tabassum F, Jyoti C, Sinha HH, et al. Impact of polycystic ovary syndrome on quality of life of women in correlation to age, basal metabolic index, education and marriage. PloS one 2021; 16:e0247486.
- Konar H. DC Dutta's textbook of gynecology. JP Medical Ltd 2016.
- Dutta DC. Textbook of gynaecology. New central book agency 2003.
- Konar H. DC Dutta's textbook of gynecology. JP Medical Ltd 2016.
- Ramanand SJ, Ghongane BB, Ramanand JB, et al. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab 2013; 17:138.
- Singh P. Case report on left ovarian torsion: A rare complication in an adolescent PCOS. J Pharm Res Int 2021; 33.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Deeksha Rana, Neema Acharya* and Ashish Bhatt
Department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (DMIMS), Sawangi, Wardha, IndiaReceived: 02-Dec-2022, Manuscript No. JRMDS-22-85228; , Pre QC No. JRMDS-22-85228(PQ); Editor assigned: 05-Dec-2022, Pre QC No. JRMDS-22-85228(PQ); Reviewed: 20-Dec-2022, QC No. JRMDS-22-85228 (QC); Revised: 27-Dec-2022, Manuscript No. JRMDS-22-85228(R); Published: 03-Jan-2023
