Research - (2020) Volume 8, Issue 6
Protective Effects of Trimetazidine Against Bilirubin-Induced Neurotoxicity
Bedri Selim Benek1, Recep Bayram2, Ummugulsum Benek1 and Kenan Gumustekin3*
*Correspondence: Kenan Gumustekin, Department of Physiology, Medical Faculty, Ala-Too International University, Kyrgyzstan, Email:
Abstract
Background and aims: We aimed to research the effects of limiting oxidation and blocking membrane calcium channels on induced-bilirubin neurotoxicity. For this purpose, we used anti-ischemic drugs that Trimetazidine (TMZ) has features as limiting membrane damage induced by reactive oxygen radicals and decreasing overload calcium into the cell.
Methods: In this study, the SH-SY5Y neuroblastoma cell line was used. Cells at a concentration of 3x104 cell/100μl were seeded to a 96-well plate. The toxic doses of bilirubin and the protecting effect of TMZ were determined by the MTT test. To detect the poisonous mechanism of bilirubin, the Muse Cell Analyzer Annexin V&Dead Cell assay kit protocol was applied. Determination of the intracellular free oxygen radicals was made with the flow cytometric oxidative stress assay according to the Muse Cell Analyzer Oxidative Stress assay protocols.
Results: 10 and 100 μM doses of bilirubin were determined as toxic, while for TMZ, 10 and 100 μM treatments were determined as protective doses. In both doses of TMZ, we observed significant positive effects against oxidative stress and apoptosis caused by 10 μM bilirubin.
Conclusion: Trimetazidine should be further studied as a potential protecting molecule in hyperbilirubinemia.
Keywords
Bilirubin, Neurological disorders, Tumor cell line, Trimetazidine, Apoptosis, Oxidative stress
Introduction
Bilirubin is a product of heme catabolism, the oxygen-carrying molecule in hemoglobin. Of the bilirubin pigment, 85% is formed by the lysis of aged erythrocytes in the reticuloendothelial system, while 15% arises from the destruction of myoglobin and hemoproteins (such as catalase, peroxidase, guanylate cyclase, and cytochrome). Due to the shortened erythrocyte lifetime compared to adults (between 70-90 days) and the increased amount of heme released, as well as increased absorption of bilirubin from the intestine, newborns have higher bilirubin levels [1].
Bilirubin is an antioxidant that readily binds to membrane lipids. It can prevent membrane peroxidation, causing changes in the intracellular and extracellular ion equilibrium [2]. Indirect (unconjugated) bilirubin, which is useful for cells at low concentrations, shows toxic effects at high levels. Since it is fat-soluble, bilirubin can easily pass through the blood-brain barrier. In conditions deteriorating the blood-brain barrier, such as anoxia, hyperosmolarity, hypercarbia, and prematurity, bilirubin can pass into the brain at a higher rate. In particular, the competitive binding of sulfonamide-like drugs to albumin and increased permeability of the blood-brain barrier can facilitate the passage of bilirubin [3].
Jaundice is one of the most common problems in newborns. Clinical icterus may develop in 60% of term babies and 80% of preterm babies in the first week of life. When the total bilirubin levels reach 2 mg/dl in adults and children, signs of yellow sclera and skin are observed. However, jaundice is not seen in newborns until it reaches 5-6 mg/dl. It is known that indirect bilirubin is neurotoxic, and its high levels cause encephalopathy and kernicterus. However, it is still unclear why the non-toxic precursor of bilirubin is not removed from the body and converted into the potentially toxic bilirubin molecule [4].
Trimetazidine is considered as a panacea commonly used to treat angina pectoris [5]. It limits H+ ion production and intracellular acidosis, prevents calcium ion accumulation, and reduces the glucose requirement of cells. TMZ also leads to a reduction in cytoplasmic sodium accumulation and free oxygen radical formation in cardiomyocytes [6]. On the other hand, it also reduces the infiltration of neutrophils to the inflammation site. Another mechanism of action of TMZ is to increase the mechanical resistance of the cell membrane. Mechanical stress-induced by excessive oxygenation during reperfusion following acute ischemia can cause tissue edema by targeting the cell membrane. The protection of cells in the reperfusion area by increasing the membrane resistance against apoptosis reduces the infarct area associated with necrosis [7].
In the literature, the effects of TMZ against bilirubin toxicity have not been extensively investigated. Furthermore, we hypothesized that, besides the already known indications, TMZ can be used to prevent bilirubin toxicity. This study aimed to examine the effects of limiting oxidation and blocking membrane calcium channels with TMZ on induced-bilirubin neurotoxicity.
Methods
Trial design
This study employed an experimental design using the human neuroblastoma cell line (SHSY5Y). The cell line was obtained from the Abant İzzet Baysal University Medical Faculty Department of Medical Pharmacology.
Passaging and cell culturing
The -80 C frozen SH-SY5Y cell line was defrosted and passage into 96-well plates. After sufficient cells were obtained by passaging, cells collected by trypsinization were homogeneously planted in 96-well plates according to the test type in standard density. The Neubauer chamber (BLAUBRAND® Neubauer, Germany) was used to count the number of cells. A cell density of 3x104 cells/100 μl was achieved.
The experimental groups
In this study, 7 groups were formed depending on the administered bilirubin and TMZ amounts as follows:
Group I, control (n=3): No intervention.
Group II, Bilirubin10 (n=3): 10 μM bilirubin applied to the SH-SY5Y cell line for 48 hours.
Group III, Bilirubin100 (n=3): 100 μM bilirubin applied to the SH-SY5Y cell line for 48 hours.
Group IV, TMZ10-Bilirubin10 (n=3): 10 μM TMZ applied to the cell line for 3 hours before administering 10 μM bilirubin.
Group V, TMZ100-Bilirubin10 (n=3): 100 μM TMZ applied to the cell line for 3 hours before administering 10 μM bilirubin.
Group VI, TMZ10-Bilirubin100 (n=3): 10 μM TMZ applied to the cell line for 3 hours before administering 100 μM bilirubin.
Group VII, TMZ100-Bilirubin100 (n=3): 100 μM TMZ applied to the cell line for 3 hours before administering 100 μM bilirubin.
Preparation of the bilirubin solution
Of the powdered UCB (Merck KGaA, Darmstadt, Germany), 7.3 mg were weighed and dissolved in 5 ml NaOH+HCl (2.5 ml NaOH+2.5 ml HCl). Thus, the stock solution was prepared to be 2.5 mM. The bilirubin stock solution was diluted with cell medium until the desired bilirubin concentration values were obtained. The bilirubin solution was made ready in a dark environment. The solutions were passed through a 0.2 μm sterile filter before being applied to the cells at different concentrations. Different doses of UCB were prepared, and the determined bilirubin doses were administered on the cells in the SH-SY5Y cell line.
Preparation of the trimetazidine solution
The TMZ solution was prepared by weighing 26.6 mg of powdered TMZ (Merck KGaA, Darmstadt, Germany) on a sensitive scale and producing a stock solution concentration of 10 mM using 10 ml medium. The TMZ stock solution was stored at +4°C. The stock solution was diluted with a medium until the desired concentrations were obtained. Before applying to the groups, the TMZ solutions were passed through a 0.22-μm sterile filter.
Studying the effects of TMZ
Different doses of TMZ were prepared in the cell line, which was applied to the cells 3 hours before the determined bilirubin doses were administered. After 48 hours, protective doses were investigated with the methyl thiazole diphenyl tetrazolium (MTT) test.
The MTT test protocol
Described first by Mossmann (1983) as a colorimetric method, yellow tetrazolium salt is transformed into formazan crystals by breaking the tetrazolium ring by dehydrogenase enzymes in the mitochondria of living cells. The color produced in the medium as a result of dissolving the formazan crystals formed in solvents, such as isopropanol or DMSO, is measured spectrophotometrically, and the measured value is correlated directly with the number of living cells.
In the test protocol, 48 hours after bilirubin was added to the cells, the MTT test was performed adhering to the kit protocol (Roche Diagnostics, Turkey). Of the MTT solution, 10 μl was added to the cells in 90 μl of medium and incubated at 37 °C for 4 hours. To completely dissolve the formazan product, 1 volume of MTT-2 kit solution was added to the samples, and after incubation for a night, the cell viability was evaluated by reading the absorbance values at 570 nm with an ELISA reader.
Apoptosis test
The changes in the cell membrane were investigated using the Muse® Annexin V&Dead Cell Assay Kit (Merck Millipore Muse Cell Analyzer Kit, Turkey) apoptosis marker. Before measuring apoptosis, we kept the bilirubin applied to the cell cultures for 48 hours. At the end of the incubation, the cells collected by trypsinization were transferred to Eppendorf tubes at a density of approximately 1x10 6-1x107 cells/ml for each group. 100 μl of the Muse Annexin V&Dead Cell Reagent solution was added to the samples. Samples were vortexed at medium speed for 3-5 minutes. After incubating for 20 minutes at room temperature, the samples were read on the Muse cell analyzer.
Testing oxidative stress
Before starting the study, the test solutions in the Muse Oxidative Stress Kit (Meck Millipore, Turkey) stored at -20 °C were prepared per the test protocol at room temperature and protected from light. First, Muse oxidative stress reagent intermediate solution was prepared. This intermediate solution was prepared by diluting 1/100 of 1% assay buffer from the oxidative stress reagent stock (198 μl of 1X assay buffer was added to 2 μl of Muse Oxidative Stress reagent solution). This intermediate solution was diluted by 1/80 with 1X assay buffer, and the Muse Oxidative Stress working solution was prepared.
Oxidative stress measurement
After the cell samples were collected, they were prepared as 1x10 6 cells/ml in 1X assay buffer. Of these cell suspension samples, 10 μl was added to each working tube. 190 μl of the prepared Muse Oxidative Stress working solution was added to the cells in each container. Tubes were vortexed at medium speed for 3-5 minutes. After the samples were incubated at 37 °C for 30 minutes, reading was done on a Muse cell analyzer.
Statistical methods
Data analysis was performed with the SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). The difference between the groups was first determined by the ANOVA test and then by using the post hoc Tukey test. p-values of less than 0.05 were accepted as statistically significant.
Results
TMZ significantly protected cells against 10 μM bilirubin-induced toxicity at 10, 50 and 100 μM doses, reducing toxicity by 19 ± 3.9%, 17 ± 5.4% and 17 ± 5.8%, respectively (p=0.838, 0.015, 0.015, 0.007, and 1.000 at 5 μM, 10 μM, 50 μM, 100 μM, and 500 μM TMZ doses, respectively (Table 1 and Figure 1). TMZ reduced the toxicity of 100 μM UCB on average by 10 ± 6%, but this result was not statistically significant (p=0.487) (Table 2 and Figure 2).
| Control | TMZ (µM)+10 µM UCB | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 50 | 100 | 500 | ||
| Well 1 | 0.594 | 0.448 | 0.533 | 0.508 | 0.564 | 0.498 |
| Well 2 | 0.606 | 0.415 | 0.532 | 0.526 | 0.541 | 0.467 |
| Well 3 | 0.628 | 0.452 | 0.574 | 0.572 | 0.495 | 0.428 |
| Mean | 0.609 | 0.464 | 0.533 | 0.535 | 0.546 | 0.438 |
Table 1: ELISA absorbance values of TMZ applied before 10 μM bilirubin toxicity.
| Control | TMZ (µM)+100 µM UCB | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 50 | 100 | 500 | ||
| Well 1 | 0.594 | 0.375 | 0.454 | 0.399 | 0.484 | 0.453 |
| Well 2 | 0.606 | 0.354 | 0.398 | 0.471 | 0.402 | 0.403 |
| Well 2 | 0.628 | 0.428 | 0.369 | 0.446 | 0.383 | 0.378 |
| Mean | 0.609 | 0.411 | 0.423 | 0.439 | 0.407 | 0.386 |
Table 2: ELISA absorbance values of TMZ applied before 100 μM bilirubin toxicity.
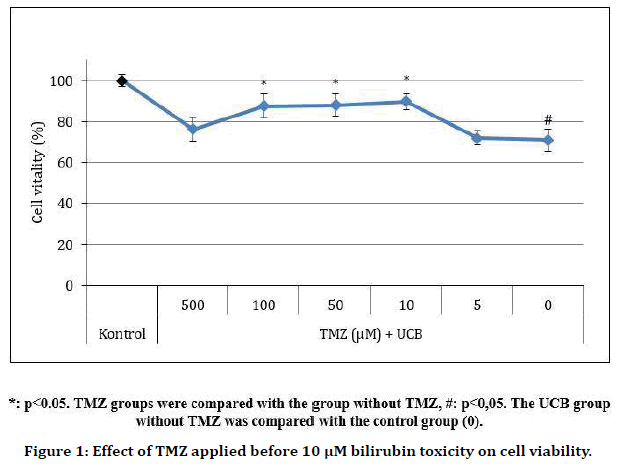
Figure 1. Effect of TMZ applied before 10 μM bilirubin toxicity on cell viability.
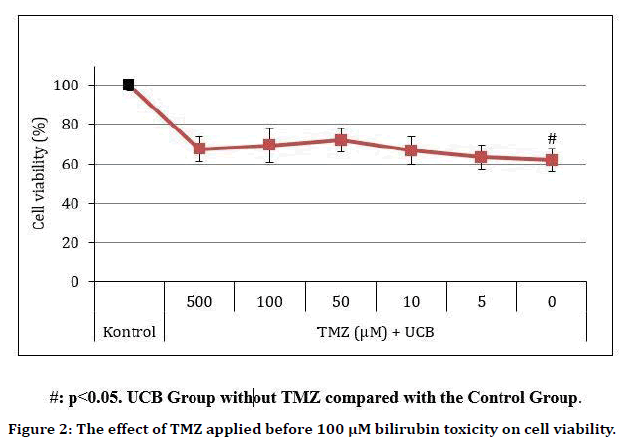
Figure 2. The effect of TMZ applied before 100 μM bilirubin toxicity on cell viability.
Apoptosis
It was observed that both 10 and 100 μM doses of TMZ reduced cellular apoptosis rate against bilirubin (10 μM) toxicity (p=0.004 at 10 μM TMZ; p<0.001 at 100 μM TMZ). In the measurement of apoptosis, live-cell ratios were determined as 99.9 ± 1%, 55.85 ± 1%, and 43.25 ± 2.75% in the control, 10 μM bilirubin and 100 μM bilirubin groups, respectively (p<0.001 in all). Dead cell ratios in these groups were 0.1%, 5.6% and 5.7%, respectively (p=0.004 in 10 μM UCB p=0.004 in 100 μM UCB). Early apoptosis rates were 0%, 26.55 ± 1.67%, and 28.65 ± 1.15% in the control, 10 μM bilirubin and 100 μM bilirubin groups, respectively (p<0.001 in all groups). Late apoptosis rates were 0%, 12 ± 1.45% and 22.4 ± 2.76%, respectively (p=0.002 at 10 μM UCB; p<0.001 at 100 μM UCB) (Figure 3, Table 3).
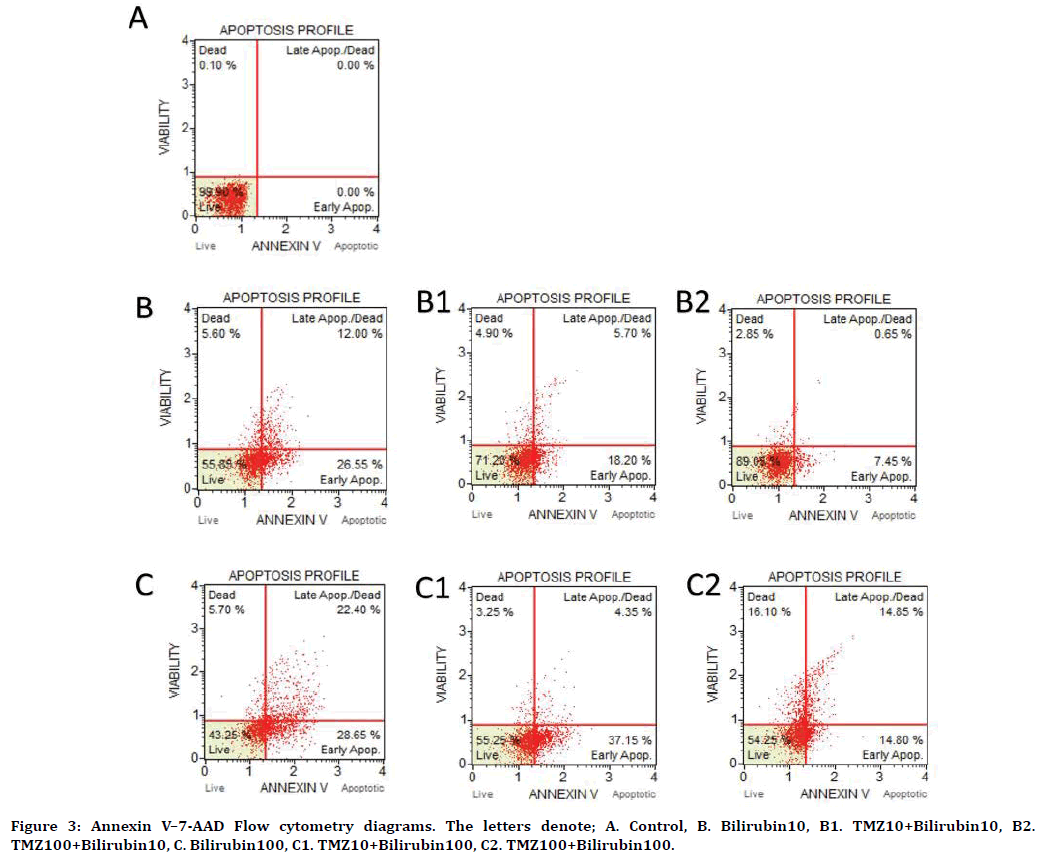
Figure 3. Annexin V–7-AAD Flow cytometry diagrams. The letters denote; A. Control, B. Bilirubin10, B1. TMZ10+Bilirubin10, B2. TMZ100+Bilirubin10, C. Bilirubin100, C1. TMZ10+Bilirubin100, C2. TMZ100+Bilirubin100.
| Live cells | Early apoptosis | Late apoptosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | UCB10 | TMZ+UCB10 | Control | UCB | TMZ+UCB10 | Control | UCB10 | TMZ+UCB10 | |||
| 10 µM | 10 µM | 100 µM | 0 | 10 µM | 10 µM | 100 µM | 0 | 10 µM | 10 µM | 100 µM | |
| 99.9 | 55.85 | 71.2 | 89.05 | 26.55 | 18.2 | 7.45 | 12 | 5.7 | 0.65 | ||
| Control | UCB100 | TMZ+UCB100 | Control | UCB100 | TMZ+UCB100 | Control | UCB100 | TMZ+UCB100 | |||
| 100 µM | 10 µM | 100 µM | 0 | 100 µM | 10 µM | 100 µM | 0 | 100 µM | 10 µM | 100 µM | |
| 99.9 | 43.25 | 55.25 | 54.25 | 28.65 | 37.15 | 14.8 | 22.4 | 4.35 | 14.85 | ||
Table 3: Apoptosis values.
Bilirubin (10 μM) toxicity increased the viable cell ratios of 10 and 100 μM TMZ to 71.2 ± 1.06% and 89.05% ± 1.06%, respectively (compared to the 10 μM UCB group, p=0.004 and p<0.001 for 10 μM TMZ and 100 μM TMZ, respectively). TMZ (10 and 100 μM) was found to reduce early apoptotic cell ratios to 18.2 ± 0.75% and 7.45 ± 0.72%, respectively (compared to the 10 μM UCB group, p<0.001 for both 10 μM and 100 μM TMZ). Late apoptotic cell ratios decreased to 5.7 ± 1.99 and 0.65 ± 0.21%, respectively (compared to 10 μM UCB group, p=0.150 and p=0.003 for at 10 μM and 100 μM TMZ, respectively). When 10 and 100 μM TMZ groups were evaluated concerning cell viability against bilirubin toxicity, there was a statistically significant difference between the TMZ groups (p=0.001) (Figure 2 and Figure 3).
Under 100 μM bilirubin toxicity, 10 and 100 μM TMZ increased the viable cell proportions to 55.25 ± 6.54% and 54.25 ± 7.27%, respectively (compared to the 100 μM UCB group p=0.026 at 10 μM TMZ and p=0.047 at 100 μM TMZ). It increased the rates of early apoptotic cells to 37.15 ± 3.58% and decreased to 14.8 ± 1.31% in the two groups respectively (compared to the 100 μM UCB group, p<0.001 both at 10 μM and 100 μM TMZ). On the other hand, it reduced the rates of late apoptotic cells to 4.35 ± 4.71% and 14.85 ± 4.27%, respectively (compared to the 100 μM UCB group, p<0.001 at 10 μM TMZ and p=0.058 at 100 μM TMZ) (Figure 2 and Figure 3).
Oxidative stress
The antioxidant effects of 10 and 100 μM TMZ doses against bilirubin toxicity (10 and 100 μM) were investigated by flow cytometry. It was observed that both 10 μM and 100 μM TMZ reduce cellular oxidative stress rate against 10 μM bilirubin toxicity. SOR (+) cell ratios in the control, 10 μM bilirubin, and 100 μM bilirubin groups were 0%, 99.07±1.1%, and 99.67±0.27% respectively (p<0.001 both at 10 μM and 100 μM UCB). On the other hand, SOR (-) cell ratios in these groups were 100%, 0.93%, and 0.33%, respectively (Figure 4).
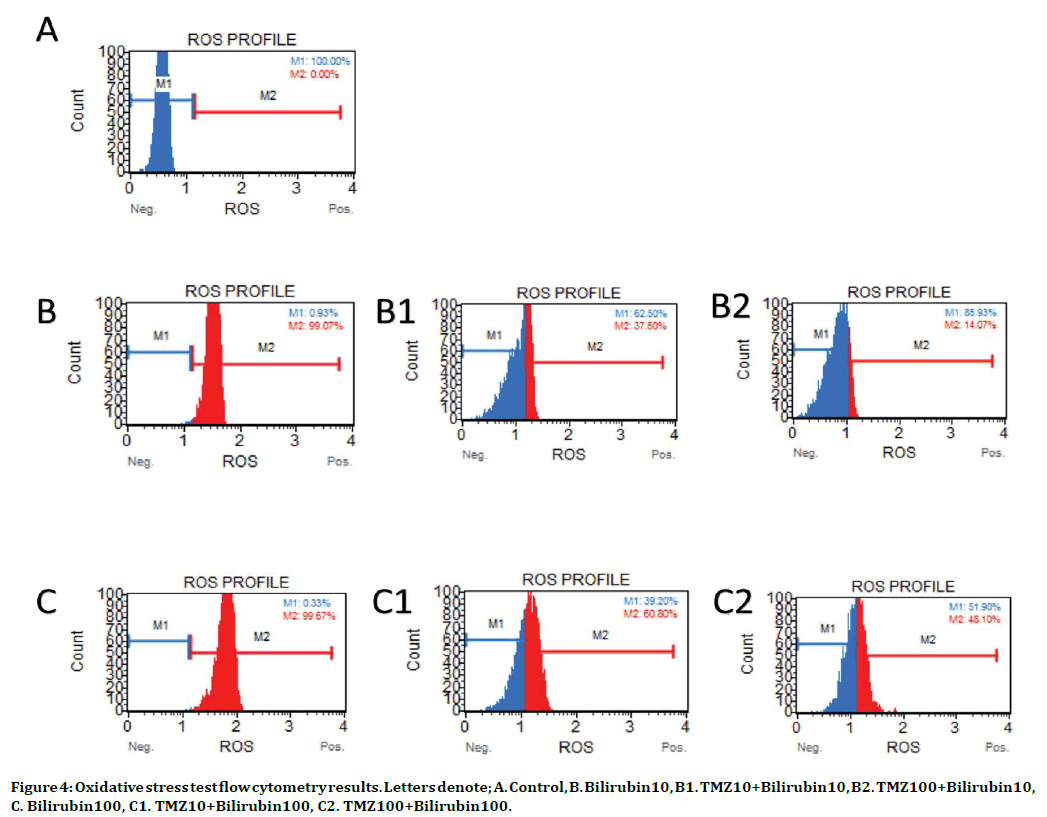
Figure 4. Oxidative stress test flow cytometry results. Letters denote; A. Control, B. Bilirubin10, B1. TMZ10+Bilirubin10, B2. TMZ100+Bilirubin10, C. Bilirubin100, C1. TMZ10+Bilirubin100, C2. TMZ100+Bilirubin100.
In 10 μM bilirubin toxicity, 10 and 100 μM TMZ reduced SOR (+) cell ratios to 37.5±3.18% and 14.07±3.54%, respectively (compared to the 10 μM UCB group, p<0.001 both at 10 μM and 100 μM TMZ), whereas, it increased the SOR (-) cell ratios to 62.5% and 85.93%, respectively (compared to the 10 μM UCB group, p<0.001 both at 10 μM and 100 μM TMZ) (Figure 4).
In 100 μM bilirubin toxicity, 10 and 100 μM TMZ reduced SOR (+) cell ratios to 60.8±16.46% and 48.1±9.9%, respectively (compared to the 100 μM UCB group, p<0.001 both at 10 μM and 100 μM TMZ), whereas it increased the SOR (-) cell ratios to 39.2% and 51.9%, respectively (compared to the 100 μM UCB group, p<0.001 both at 10 μM and 100 μM TMZ) (Figure 4) ).
Discussion
This study demonstrated the potential benefits of TMZ in preventing bilirubin toxicity. TMZ decreased the proportion of apoptosis and oxidative stress in neuroblastoma cell lines exposed to bilirubin toxicity.
At physiological levels, bilirubin has antioxidant properties and is even neuroprotective. In the study of Ostrow et al., [8] free bilirubin has been reported to protect central nervous system cells against oxidative damage at concentrations below 70 nM but showed neurotoxic effects at doses up to 770 nM. Dore and Synder [9] demonstrated that bilirubin at concentrations of 25 nM and 50 nM protects hippocampal cells against hydrogen peroxide. On the other hand, Silva et al. [10] reported that neurons and astrocytes respond differently to bilirubin. Also, Genç et al. [11] found that there was no significant decrease in the cell viability with 10 μM indirect bilirubin at 24, 48, and 72 hours, while a significant cytotoxic effect occurred with 100 μM bilirubin.
Although many studies have been done on hyperbilirubinemia, the molecular mechanism of bilirubin neurotoxicity has not been fully elucidated. However, some theories have been suggested. Neuronal dysfunction in the brain linked to bilirubin is explained by the fact that bilirubin inhibits protein phosphorylation [12]. Besides, bilirubin shows cytotoxic effects by inhibiting oxidative phosphorylation in mitochondria [13]. Furthermore, high bilirubin levels have been shown to induce oxidative stress by increasing free radical formation in the brain [14]. Indirect bilirubin reduces mitochondrial transmembrane potential in nerve cells, increases membrane permeability, and causes cytochrome c release. At the same time, indirect bilirubin causes apoptotic Bax translocation in the mitochondrial membrane and inactivation of Bcl-2, which inhibits apoptosis [15].
SH-SY5Y human neuroblastoma cell line is a widely used and well-known cell to investigate the biological effects of neuroactive substances, drugs, and toxins. Corich et al. and Calligaris et al. emphasized that UCB concentration results in the SH-SY5Y cell line are a model that reflects the clinical picture of hyperbilirubinemia [16]. Although it is limited regarding reflecting the functional disorders that occur in the newborn brain, SH-SY5Y cell-type induced toxicity in our study was an in vitro model for understanding the molecular events of UCB toxicity. In light of this information, we have induced toxicity in neuronal cells, considering that neurons have a crucial role in encephalopathy, and thus, have to be targeted during treatment.
In vitro studies showing the neurotoxic effects of bilirubin often present studies that describe reduced cell proliferation and cell death through apoptosis in addition to macroscopic changes, such as decreased dendritic and axonal branches and decreased neuronal extensions [17,18]. In another study on the SH-SY5Y neuroblastoma cell line, bilirubin has been shown to cause delays in the cell cycle S-phase progression and lead to cell cycle arrest [19]. In our study, even at high concentrations of bilirubin, there was approximately 30% viability, which suggests that it causes dysfunction rather than neuronal death. Additionally, the fact that the cells affected by bilirubin in the apoptotic cell evaluation are seen more early in the apoptotic process suggests that bilirubin directs the cells to apoptosis in 48 hours, but does not cause a high rate of death.
According to research, trimetazidine, which is known to have a very promising and neuroprotective efficacy, shows its effects mostly through regulation of the antioxidant mechanisms and modulation of glutamate receptors. TMZ is generally used in the treatment of angina pectoris [20] but has also been recommended for the treatment of many other diseases [21–23].
Studies on the medical management of hyperbilirubinemia are scarce. Both endogenous and exogenous substances used in the investigations appear to be agents investigated for their protection against bilirubin toxicity without considering their systemic side effects and pharmacokinetics. There are studies in the literature showing that minocycline, an antibiotic of the tetracycline class, is protective against bilirubin toxicity and bilirubin-induced hearing loss [24]. Gaspa et al. [25] reported that ursodeoxycholic acid, which is found in low levels in the human bile, reduces bilirubininduced apoptosis in bone cells by decreasing Bax levels and increasing Bcl-2 levels. In the in-vitro and in-vivo bilirubin toxicity study conducted by Gao et al. [26], they reported that taurine protects cells against ischemia and toxicity by decreasing intracellular calcium and caspase 3 levels. Taştekin et al. [27] reported that L-carnitine prevents bilirubin-induced neuronal death according to the cell activation test results.
Furthermore, Almaas et al. [28] reported that dexamethasone, a potent corticosteroid, reduced IL-8 level, and decreased bilirubin-induced toxicity according to cell activation test results. Park et al. [29] showed that 7-nitroimidazole, a selective nitic-oxide synthase inhibitor, reduces bilirubin toxicity by regulating energy metabolism and cell membrane function in the brain. Our findings suggest that TMZ has very few side effects compared to the literature and is one step ahead of bilirubin toxicity compared to the other agents. Also, since TMZ activates many different cellular protective pathways, it is thought that it may reveal this protective activity at the cell level against bilirubin toxicity.
This is the first study to demonstrate the neuroprotective activity of TMZ in the neuroblastoma cell line against bilirubin toxicity. Our results reveal that glutamatergic stimulation is an essential factor in the pathway of bilirubin toxicity and that the enhancement of antioxidant mechanisms can be limited in this pathophysiological pathway. With this study, the necessity of investigating the combined effects of more potent antioxidant substances, as well as stronger glutamate inhibition in bilirubin toxicity, became prominent.
Conclusion
In the neuroblastoma cell line, the toxic bilirubin concentration was established as 10 μM, leading to 25±5.6% cell death. Bilirubin did not kill cells but caused apoptosis. TMZ was effective against bilirubin toxicity at 10, 50, and 100 μM concentrations. In 10 μM bilirubin toxicity, 10 μM TMZ increased cell viability by 19%, while 100 μM TMZ increased cell viability by 17%. It was seen that both 10 μM and 100 μM TMZ reduced cellular apoptosis rate against the toxicity induced by 10 μM dose of bilirubin. The fact that not all cells die indicates differences in their susceptibility to bilirubin damage. Additional studies on gene and protein expression are needed to elucidate the factors that determine these sensitivities. Agents affecting the determined mechanisms should be tested, aiming to eliminate or reduce the damage. The in vivo efficacy of trimetazidine should be tested in experimental rat hyperbilirubinemia models.
Conflict of Interest
The authors declare no conflict of interest.
Funding
None.
Author Info
Bedri Selim Benek1, Recep Bayram2, Ummugulsum Benek1 and Kenan Gumustekin3*
1Department of Physiology, Medical Faculty, Abant Izzet Baysal University, Bolu, Turkey2Department of Pharmacology, Medical Faculty, Abant Izzet Baysal University, Bolu, Turkey
3Department of Physiology, Medical Faculty, Ala-Too International University, Bishkek, Kyrgyzstan
Citation: Bedri Selim Benek, Recep Bayram, Ummugulsum Benek, Kenan Gumustekin, Protective Effects of Trimetazidine Against Bilirubin-Induced Neurotoxicity, J Res Med Dent Sci, 2020, 8 (6): 191-199.
Received: 31-Aug-2020 Accepted: 21-Sep-2020
