Research - (2022) Volume 10, Issue 2
Study Level of Some Biochemical Markers in Male and Female Osteoporosis Patients
Shaimaa Hussein Ali1*, Hussein Jasim Obaid Al-Harbi2 and Hussein Jasim Obaid Al-Harbi2
*Correspondence: Shaimaa Hussein Ali, College of Science, Al-Qasim Green University, Iraq, Email:
Abstract
Osteoporosis and consequent fracture are not limited to postmenopausal women. There is increasing attention being paid to osteoporosis in older men. Men suffer osteoporotic fractures about 10 years later in life than women, but life expectancy is increasing faster in men than women. Thus, men are living long enough to fracture, and when they do the consequences are greater than in women. The main purpose of this study is the measuring serum level of (CX3C, Bone sialoprotein, Gelsolin, Cathepcin K, Vitamin D, Calcium, Phosphorous and Alkaline phosphatase) in 100 patients (20 men and 80 women) and 50 as control group (19 men and 31women). The statistical analysis of results showed that the serum level of (CX3C, Gelsolin and Cathepcin K) increase in female osteoporosis patients and no significant increase in male osteoporosis patients compared to control group. The results also showed a rise serum level of Bone sialoprotein and Alkalin phosphatase in male and female osteoporosis compared to control group. The results also showed no significant difference in serum level of (vitamin D, Calcium and Phsphorouse) in male and female osteoporosis patients compared to control group. In conclusion The Cathepcin K, Gelsolin and Bone sialoprotein play an important role in bone metabolism and increase level in osteoporosis patients.
Keywords
Osteoporosis, CX3C, Cathepcin K, Gelsolin, Bone sialoprotein
Introduction
Osteoporosis is a progressive skeletal disorder whereby the bone strength (bone density and quality) is compromised thereby predisposing an individual to an increased risk of fractures which could occur spontaneously or after minor injuries. It is associated with low bone mineral density (BMD) and loss of structural and biomechanical properties that are vital for the maintenance of bone homeostasis [1]. Osteoporosis is the most prevalent bone disorder in humans and is a major global public health issue [2]. Osteoporotic fractures are associated with increased mortality [3]. In addition, fractures are associated with increased disability, reduced physical functions, and poor quality of life besides an increased financial burden [4]. Globally, approximately 9 million new and 56 million prevalent cases of osteoporotic fractures were estimated in the year 2000.5 In Taiwan, the prevalence of osteoporosis between 2001 and 2011 increased by about 7.6%.6. BMD is a valuable clinical diagnostic index for osteoporosis and the best tool for osteoporotic fracture prediction. Osteoporosis is associated with several genetic and nongenetic factors [1]. some of which include age [5], sex [2], Menopausal status [6], educational level [7], coffee drinking [5], smoking, exercise, alcohol consumption, diet [1], and body mass index (BMI) [8].
Subjects and Methods
This study was carried out on patients attended to Bone Density unit in one hospital which is Marjan Medical City in Babylon Governorate. This study includes 150 females and males. They had been divided into two groups, the first group included 100 patients (80 females and 20 males) with OP and the second group included 50 relatively healthy (females and males) ,the age was of both groups was matched and ranged between (20- 80) years. Venous blood samples were drawn from patients and control subjects by using disposable syringes. Five ml of blood was obtained from each subject, 2 ml was placed into EDTA tubes and the remaining (3ml) pushed slowly into disposable gel containing tubes. Blood in the EDTA tubes was stored in( -20˚C) in order to be used later in genetic part of the study, while blood in the gel containing tubes was allowed to clot at room temperature for 15minutes and then centrifuged at 3000 rpm for approximately 10-15.minutes , after that sera was obtained (Barbara and Anna,2012)and stored at -20˚C.
Biomarkers analysis
Quantitative detection of CXC3, gelsolin, cathepcin K and bone sialoprotein, in serum was done according to the industrial company (Bioassay Technology Laboratory (China) , that depended on the technique of the quantitative sandwich enzyme immunoassay (ELISA) ) and (vitamin D) was assayed in serum according to the industrial company AccuBind ELIS Microwells (USA).that also depended on the technique (ELISA).Calcium and alkaline phosphatase was assayed in serum according to the industrial company BIOLABO (France).
Statistical analysis
Analysis of data was made by using Statistical Package for Social Science (SPSS) system/ version 20 Results expressed as mean ± Standard Error S.E.
Results
The levels of biochemical parameters in osteoporosis patients and healthy groups according to gender (mean ± S.E)
In the Table 1 which describe levels of biochemical parameters showing that in osteoporosis patients markedly rise in level biochemical parameters that measured in the study, where the significant increase (p≤ 0.05) in CX3C in female, and significant increase in bone sialoprotein concentration for both genders when compared with healthy control group .and insignificant increase (p ≤ 0.05) in gelsolin and cathepsin K level in female osteoporosis and no significant difference in male.
| Groups | Gender | Male | Female |
|---|---|---|---|
| Parameters | Mean ± S.E | ||
| CX3C(ng/ml) | Patient | 10.16 ± 1.3 | 11.53 ± 2.6 |
| Healthy | 9.76 ± 1.5 | 3.46 ± 0.6 | |
| p-value | 0.121 | 0.02 ⃰ | |
| Bone sialoprotein (ng/ml) | Patient | 47.53 ± 6.7 | 45.36 ± 12.4 |
| Healthy | 31.45 ± 5.1 | 19.32 ± 9.8 | |
| p-value | 0.005 ⃰ | 0.008 ⃰ | |
| Gelsolin (ng/MLS) | Patient | 50.06 ± 10.2 | 75.61 ± 12.5 |
| Healthy | 48.13 ± 8.1 | 42.46 ± 10.5 | |
| p-value | 0.09 | 0.02 ⃰ | |
| Cathepsin K (ng/ml) | Patient | 3.07 ± 0.2 | 4.77 ± 0.6 |
| Healthy | 2.99 ± 0.1 | 1.25 ± 0.8 | |
| p-value | 0.41 | 0.04 ⃰ | |
| Vit. D3(ng/ml) | Patient | 18.44 ± 4.3 | 15.26 ± 2.2 |
| Healthy | 20.48 ± 4.4 | 20.66 ± 3.4 | |
| p-value | 0.215 | 0.06 | |
| Ca (mg/dL) | Patient | 8.46 ± 1.3 | 8.22 ± 0.8 |
| Healthy | 8.43 ± 1.4 | 9.38 ± 2.2 | |
| p-value | 0.687 | 0.311 | |
| PO4 (mg/dL) | Patient | 4.74 ± 0.8 | 4.70 ± 0.9 |
| Healthy | 3.77 ± 0.7 | 4.18 ± 1.1 | |
| p-value | 0.567 | 0.244 | |
| ALP (Iu/L) | Patient | 93.07 ± 15.2 | 114.5 ± 17.8 |
| Healthy | 79.00 ± 11.7 | 69.85 ± 12.1 | |
| p-value | 0.06 ⃰ | 0.002 ⃰ | |
| *Significant difference at (p≤0.05) | |||
Table 1: Levels some of biochemical parameters in osteoporosis patients and healthy according to gender.
This study show no significant difference level of vitamin D , calcium and phosphorous in both gender while there is significant increase at (p≤0.05) in alkaline phosphatase in both gender (Figures 1A to Figure 1D).
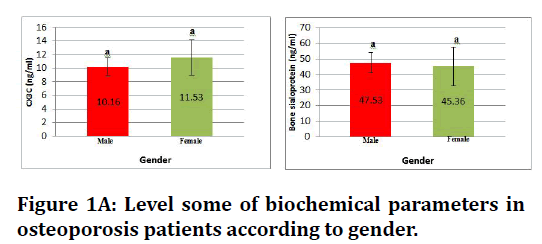
Figure 1A. Level some of biochemical parameters in osteoporosis patients according to gender.
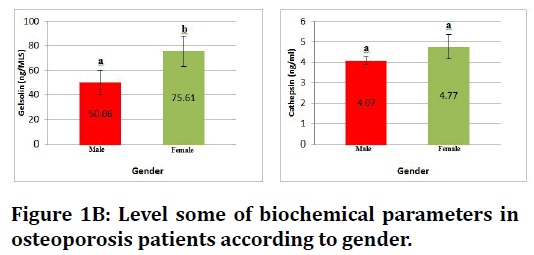
Figure 1B. Level some of biochemical parameters in osteoporosis patients according to gender.
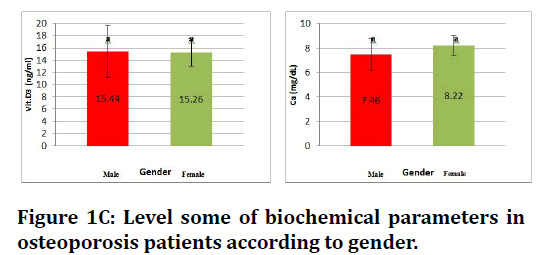
Figure 1C. Level some of biochemical parameters in osteoporosis patients according to gender.
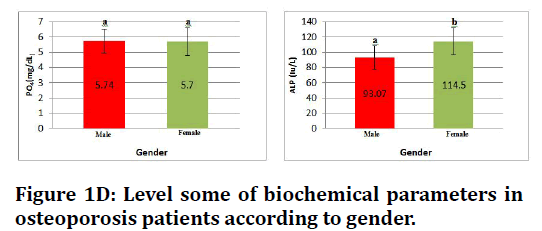
Figure 1D. Level some of biochemical parameters in osteoporosis patients according to gender.
Discussion
Sex is a well-established nonmodifiable factor for osteoporosis [9]. The risk of osteoporosis is greater in women than in men [1].
For instance, using data from the National Nutrition and Health Survey in Taiwan (2005-2008), the estimated prevalence of osteoporosis was 23.9% and 38.3% in men and women, respectively [10]. One major reason for sex differences in osteoporosis prevalence is menopause [9].
Menopausal women are estrogen deficient and more likely to have bone loss, osteoporosis, and fractures than premenopausal women. Results in this study recorded that women are more affected than males Table 1.
Women suffer from osteoporosis mainly due to the effects of menopause. CX3CL1 (also called fractalkine, FKN) is the only known member of the CX3C subfamily [11]. Unlike all other chemokines, CX3CL1/FKN exists as both a transmembrane protein and a soluble chemoattractant and is widely expressed on natural killer (NK) cells, monocytes and T cells [12]. So far the potential involvement of CX3CL1/ FKN in bone remodelling processes has been explored in only a few studies. Han et al. [13] found that the CX3CL1– CX3CR1 axis plays a pivotal role in osteoclast recruitment and subsequent bone resorption [13]. Also, inhibition of the CX3CL1- CX3CR1 axis through using anti-CX3CL1 mAb could inhibit osteoblast-guided differentiation of osteoclasts in vitro [14]. In addition, it has been identified that the CX3CR1–CX3CL1 axis plays an important role in the maintenance of osteoclastic precursors [15]. The result study showed signifigant increase in the serum CXC3 concentration in the female osteoporosis compared to control this finding, agreeing with its mention Yi-Ding Chen et al. [16] that FKN concentration in serum was elevated in postmenopausal osteoporotic patients compared with postmenopausal non-osteoporotic females and healthy controls.
Elevated serum FKN levels in osteoporosis patients were correlated with attenuated BMD, increased bone turnover markers, inflammation markers The CX3CL1/CX3CR1 axis plays a principal role in osteoclast maturation and binding them with immune cells to the surface of the bone tissue. It promotes the development of inflammation and production of many inflammatory cytokines near the bone surface (i.e., TNF-α, IL-1β, and IL-6). High concentrations of CX3CL1 in serum are directly proportional to increased concentrations of bone turnover and inflammatory factors in human blood serum (TRACP-5b, NTx, IL-1β, and IL-6) [17]. Bone sialoprotein (BSP) is a novel parameter of bone metabolism, BSP is a non collagenous bone matrix protein produced by osteoblasts [18].
However, presently it is not clear whether serum levels of bone sialoprotein reflect bone formation or bone resorption, respectively. Although it has been suggested that BSP levels reflect processes preferentially related to bone resorption [19,20]. Our study showed higher significant in male and female osteoporosis patients compared with the control group. Serum BSP was signi cantly elevated in postmenopausal osteoporosis compared to that of healthy perimen- opausal controls. Serum BSP decreased after diVerent antiresorptive treatments and this decrease paralleled the decrease of bone resorption markers , circulating BSP appears to be a valuable marker of bone resorption and monitoring therapy with antiresorptive drugs in postmenopausal osteoporosis. Pietschmann et al. [21] showed significantly decrease in the level of BSP in the men with idiopathic osteoporosis .BSP is expressed during early phases of bone deposition therefore it is tempting to speculate that in male idiopathic osteoporosis most aspects of bone formation are normal while there appears to be a defect at distinct stages of osteoblastic differentiation. The possibility of a different metabolism of BSP in males and females subjects also should be considered. GSN plays an important role in severing and capping actin filament in actin cytoskeleton organization [22]. It was critical for podosome assembly, rapid cell movements and signal transduction [23]. Podosomes are cytoskeletal structures where a rapid polymerization/depolymerization of actin occurs [24]. Which are of great importance for cell adhesion, mechanical perception and matrix remodeling in osteoclasts, activated endothelial cells, macrophages and dendritic cells [25]. Osteoclast can degrade bone matrix including extracellular matrix and some mineral composition, which are critical for bone remodeling and maintenance of calcium balance [26]. The activation of osteoclast for bone resorption process relies on the integrity of the actin cytoskeleton and adhesion and motility function of podosomes [27]. Previous study showed that GSN deficiency would lead to the inability to produce podosomes, the abnormal actin cytoskeletal architecture; and the reduced rates of osteoclast motility; and blocked podosome-related signal transduction; and eventually cause decreased bone resorption; and increased bone mass and strength [28]. Plasma GSN level presented no significant difference between two genders. However, significant differences in plasma GSN level were observed between high and low BMD subjects in the females specifically. The results of the current study showed a clear increase in the GSN concentration in female osteoporosis patients compared with the healthy group, this finding, agreeing with its mention Wen et al. [29], that plasma GSN levels were significantly higher in extremely low BMD subjects than high BMD in Chinese postmenopausal women and there are significant differences in plasma GSN level were observed between high and low BMD subjects in male and the females specifically .The findings imply that the function of GSN is probably influenced by gender-specific factors, e.g., sex hormones. Previous study suggested that GSN serves as a regulator in regulating androgen-mediated effects on osteoclastogenesis and bone resorption [27]. Whether GSN interact with estrogen to influence bone metabolism and BMD is unknown and has yet to be investigated in the future. Cathepsin K is one of the main catalytic enzymes expressed and secreted by the osteoclasts. It plays a predominant role in the degradation of bone type I collagen, and consequently is a drug target in osteoporosis [30]. It is a lysosomal enzyme synthesized as a procathepsin K, which is activated in the lysosomes through a process involving autocatalytic cleavage at low pH, and then released into the resorption lacunae [31]. It may be possible that some of cathepsin K, is further released in the circulation and could be a specific biological marker of osteoclast activity. Several assays have been developed, although it remains unclear whether they detect the proenzyme, the active form or both. The results of the current study showed a clear increase in the cathepcin Kconcentration in male and female osteoporosis patients compared with the healthy group, this finding, agreeing with its mention Meier et al. [32], shown that serum cathepsin K is increased in patients with osteoporosis and Paget's disease. The data of this study showed no significant difference in the level of serum Calcium between osteoporosis patients and control group Our work agrees with Najlaa et al. [33] who found no significance difference in calcium level in osteoporotic women and in control group and agree with Muhammad et al. [34] who found no significance difference in calcium level in osteoporotic men and women and in control group. In the current study serum phosphorus showed no significantly difference between women osteoporosis and control group this agree with Selvapandian et al. [35] and this may be serum Ca and P was regulated and homeostasis is maintained in serum regardless of their store in bone relation of phosphorus with BMD [36] and agree with Muhammad et al. [34] who found no significance difference in level serum phosphorus in osteoporotic men and women and in control group. Serum alkaline phosphatase showed higher in osteoporotic groups compared with control group this agree with Ramesh et al. [37]. This may be due to ALP can be drain from osteoblast which is rich with ALP also it found in plasma membrane of the cell in the liver, intestine, and placenta, all of which is may contribute to the total amount of alkaline phosphatase and agree with Muhammad et al. [34] who found significance difference in ALP level in osteoporotic men and women and in control group. The result of this study showed difference in vitamin D between osteoporosis patients and control group this agree with Ramesh et al. [37]. The decrease of serum vitamin D levels may be due to less outdoor activities of the women also with decrease exposure to sun light and due to our habits in wearing long dress this will prevent the vitamin D in the skin [38] to induce and convert to the active form which is the major storage form of vitamin D consecutively it is important for calcium and phosphorus absorption, when it is level decrease lead to increase parathyroid hormone which lead to decrease calcium absorption then effect on bone health.
Conclusion
The results showed a rise serum level of Bone sialoprotein and Alkalin phosphatase in male and female osteoporosis compared to control group. The results also showed no significant difference in serum level of (vitamin D, Calcium and Phsphorouse) in male and female osteoporosis patients compared to control group. In conclusion the Cathepcin K, Gelsolin and Bone sialoprotein play an important role in bone metabolism and increase level in osteoporosis patients.
References
- Ivanova S, Vasileva L, Ivanova S, et al. Osteoporosis: Therapeutic options. Folia Med 2015; 57:181.
- Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol 2017; 4:46.
- Cauley JA. Public health impact of osteoporosis. J Gerontol 2013; 68:1243-1251.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17:1726-1733.
- Chang HC, Hsieh CF, Lin YC, et al. Does coffee drinking have beneficial effects on bone health of Taiwanese adults? A longitudinal study. BMC Public Health 2018; 18:1-0.
- Mo D, Hsieh P, Yu H, et al. The relationship between osteoporosis and body composition in pre-and postmenopausal women from different ethnic groups in China. Ethn Health 2017; 22:295-310.
- Pagotto V, Silveira EA. Bone mineral density in the noninstitutionalized elderly: Influence of sociodemographic and anthropometric factors. Current Gerontol Geriatr Res 2016; 2016.
- Akhlaque U, Ayaz SB, Akhtar N, et al. Association of bone mineral density and body mass index in a cohort of Pakistanis: Relation to gender, menopause and ethnicity. Egyptian Rheumatol 2017; 39:39-43.
- Vijayakumar R, Büsselberg D. Osteoporosis: An under-recognized public health problem: Local and global risk factors and its regional and worldwide prevalence. J Local Global Health Sci 2016; 2016:2.
- Lin YC, Pan WH. Bone mineral density in adults in Taiwan: results of the Nutrition and Health Survey in Taiwan 2005-2008 (NAHSIT 2005-2008). Asia Pac J Clin Nutr 2011; 20:283-91.
- Chapman GA, Moores KE, Gohil J, et al. The role of fractalkine in the recruitment of monocytes to the endothelium. Eur J Pharmacol 2000; 392:189-95.
- Julia V. CX 3 CL 1 in allergic diseases: Not just a chemotactic molecule. Allergy 2012; 67:1106-10.
- Han KH, Ryu JW, Lim KE, et al. Vascular expression of the chemokine CX3CL1 promotes osteoclast recruitment and exacerbates bone resorption in an irradiated murine model. Bone 2014; 61:91-101.
- Koizumi K, Saitoh Y, Minami T, et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J Immunol 2009; 183:7825-31.
- Hoshino A, Ueha S, Hanada S, et al. Roles of chemokine receptor CX3CR1 in maintaining murine bone homeostasis through the regulation of both osteoblasts and osteoclasts. J Cell Sci 2013; 126:1032-1045.
- Chen YD, Huang CY, Liu HY, et al. Serum CX3CL1/fractalkine concentrations are positively associated with disease severity in postmenopausal osteoporotic patients. British J Biomed Sci 2016; 73:121-128.
- Wojdasiewicz P, Turczyn P, Dobies-Krzesniak B, et al. Role of CX3CL1/CX3CR1 signaling axis activity in osteoporosis. Mediators Inflam 2019; 2019.
- Bilezikian JP, Raisz LG, Martin TJ. Principles of bone biology. Academic Press 2008.
- Woitge HW, Pecherstorfer M, Li Y, et al. Novel serum markers of bone resorption: Clinical assessment and comparison with established urinary indices. J Bone Mineral Res 1999; 14:792-801.
- Hasan MS. Serum bone sialoprotein: A marker of bone resorption in postmenopausal osteoporosis. Scand J Clin Laboratory Investigation 2001; 61:513-21.
- Pietschmann P, Kudlacek S, Grisar J, et al. Bone turnover markers and sex hormones in men with idiopathic osteoporosis. Eur J Clin Investigat 2001; 31:444-51.
- Zhang L, Han C, Ye F, et al. Plasma gelsolin induced glomerular fibrosis via the TGF-β1/Smads signal transduction pathway in IgA nephropathy. Int J Mol Sci 2017; 18:390.
- Chellaiah M, Kizer N, Silva M, et al. Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J Cell Biol 2000; 148:665-78.
- Akisaka T, Yoshida H, Inoue S, et al. Organization of cytoskeletal F‐actin, G‐actin, and gelsolin in the adhesion structures in cultured osteoclast. J Bone Min Res 2001; 16:1248-55.
- Meddens M, Pandzic E, Slotman JA, et al. Actomyosin-dependent dynamic spatial patterns of cytoskeletal components drive mesoscale podosome organization. Nature Commun 2016; 7:1-7.
- Qin Y, Peng Y, Zhao W, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J Biol Chem 2017; 292:11021-33.
- Saltel F, Chabadel A, Bonnelye E, et al. Actin cytoskeletal organisation in osteoclasts: A model to decipher transmigration and matrix degradation. Eur J Cell boil 2008; 87:459-68.
- Wang WY, Ge B, Shi J, et al. Plasma gelsolin is associated with hip BMD in Chinese postmenopausal women. Plos One 2018; 13:e0197732.
- Mazid Helali A, Matin Iti F, Naina Mohamed I. Cathepsin K inhibitors: A novel target but promising approach in the treatment of osteoporosis. Curr Drug Targets 2013; 14:1591-600.
- Dodds RA, James IE, Rieman D, et al. Human osteoclast cathepsin K is processed intracellularly prior to attachment and bone resorption. J Bone Min Res 2001; 16:478-86.
- Meier C, Meinhardt U, Greenfield JR, et al. Serum cathepsin K concentrations reflect osteoclastic activity in women with postmenopausal osteoporosis and patients with Paget's disease. Clin Laborator 2006; 52:1-0.
- Ali NK. Estimation of some mineral (calcium, phosphorous, vitamin 25 (OH) D and alkaline phosphatase) in osteoporosis patients in Kirkuk city. J Osteopor Phys Act 2018; 6.
- Saqib MA, Rafique I, Hayder I, et al. Comparison of vitamin D levels with bone density, calcium, phosphate and alkaline phosphatase-An insight from major cities of Pakistan. J Pak Med Assoc 2018; 68:543-547.
- Selvapandian K, Arshiya B, Priya A, et al. Study of bone mineral density and serum vitamin D levels in health postmenopausal women. J Evid Based Med Healthc 2016; 3:3515-9.
- Jayaram N, Bijoor AR, Rajagopalan N, et al. The value of serum and urinary n-telopeptide in the diagnosis of osteoporosis. Indian J Orthop 2002; 36:9-13.
- Narula R, Tauseef M, Ahmad IA, et al. Vitamin D deficiency among postmenopausal women with osteoporosis. J Clin Diag Res 2013; 7:336.
- Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacotherap 2012; 3:118.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Shaimaa Hussein Ali1*, Hussein Jasim Obaid Al-Harbi2 and Hussein Jasim Obaid Al-Harbi2
1College of Science, Al-Qasim Green University, Iraq2College of Science, University of Babylon, Iraq
Received: 29-Jan-2022, Manuscript No. JRMDS-22-49425; , Pre QC No. JRMDS-22-49425 (PQ); Editor assigned: 31-Jan-2022, Pre QC No. JRMDS-22-49425 (PQ); Reviewed: 14-Feb-2022, QC No. JRMDS-22-49425; Revised: 18-Feb-2022, Manuscript No. JRMDS-22-49425 (R); Published: 25-Feb-2022
