Research - (2020) Advances in Dental Surgery
Study of Endometrial and Uterine Doppler Markers when Using Letrozole and Clomiphene Citrate in Subfertile Patients with PCOS or Unexplained Subfertilit
Median Atia Alkhalaf Almamou1* and Asim Kurjak2
*Correspondence: Median Atia Alkhalaf Almamou, Sarajevo Medical School, University of Sarajevo School of Science and Technology (SSST), Sarajevo, Bosnia and Herzegovina, IVF Unit, Obstetrics and gynaecology Department, Almoosa Specialist Hospital, Saudi Arabia, Email:
Abstract
Background: Clomiphene citrate has agonist and antagonist estrogen receptor properties with a discrepancy between pregnancy rate and ovulation rate. Letrozole works as a competitive, nonsteroidal aromatase inhibitor. Both stimulate ovarian follicles development. Methods: In this randomized prospective study, We recruit infertile Patients with PCOS or unexplained subfertility from the subfertility clinic at Al Moosa Specialist Hospital. We randomized patients to use either clomiphene or letrozole. On the day of the hCG trigger and the mid-luteal day, we recorded Endometrial thickness, pattern, blood flow, endometrial scoring system, uterine artery and sub endometrial arteries Doppler indices. Results: The mean of sub endometrial artery PI and RI on the day of the trigger was lower with the clomiphene treated patients than the letrozole treated patients with a p-value of .0003 and .0004, respectively. Endometrium pattern was more favourable in the letrozole treated patients than in the clomiphene treated patients with a p-value of 0.008 on the mid-luteal phase day. Letrozole treated patients had a better pregnancy rate. However, the evidence is not sufficient to suggest that there is a difference with a p-value of .728. Conclusions: When comparing letrozole with clomiphene citrate group, letrozole is associated with a more favourable (type A) pattern but less favourable endometrial vascularity on the mid-luteal day. While on the day of the trigger letrozole was less favourable in terms of sub endometrial impedance (higher sub endometrial PI. and RI). Letrozole treated patients has a better pregnancy rate, but the difference is not significant.
Keywords
Clomiphene citrate, Clomiphene citrate, Pregnancy, Subfertility, Letrozole
Introduction
Clomiphene has both agonist and antagonist estrogen receptors properties [1]. The agonist actions occur if there are low concentrations of endogenous estrogen; otherwise, the main action of clomiphene citrate is antagonist competition at the estrogen receptors [2]. At the level of the hypothalamus, clomiphene antagonizes the estrogen receptors. It depletes the receptors of estrogen by binding to them. This action will diminish the estradiol negative feedback effect, which will cause an increase in the pulse frequency of GnRH. This action will boost the pituitary production of FSH and LH [3].
The expected pregnancy rate with clomiphene citrate is approximately 15%, of those 50% conceive during a 6-month course of therapy. Cycle fecundability decreases dramatically after that [4]. There is a discrepancy between pregnancy rate and ovulation rate. The reason behind that is the detrimental effect on the endometrial thickness [5] and cervical mucus [6].
Letrozole is triazole derivatives (antifungal) that work as a competitive, nonsteroidal aromatase inhibitor. It limits the production of oestrogen, causing an elevation in pituitary FSH and LH which stimulates the development of ovarian follicles. Letrozole is superior to clomiphene citrate in polycystic ovary syndrome, concluded by Legro et al. multicenter randomized controlled trial (RCT) [7]. Aromatase inhibitors are used mainly as adjuvant therapy in postmenopausal breast cancer [8], but we are using them increasingly as a treatment for ovulation induction.
Although both clomiphene citrate and aromatase inhibitors stimulate FSH and LH by decreasing the oestrogen negative feedback, clomiphene citrate depletes central oestrogen receptors while letrozole directly decreases the production of oestrogen.
Normal endometrial maturation is required for implantation to occur. Higher pregnancy rate is associated with endometrium thickness equal or over 10 mm, as Kovacs et al. found in their study [9].
Objective
To assess the use of ultrasound markers to compare the impact of clomiphene and letrozole on pregnancy rate and receptivity of the endometrium.
Hypothesis
There is a distinction between clomiphene and letrozole in terms of ultrasound markers (Endometrial thickness, pattern, blood flow, endometrial receptivity scoring system and doppler indices of uterine and sub endometrial arteries) and pregnancy rate in patients with subfertility.
Materials and Methods
Studied cases selection
The study is a randomized prospective comparative research. We got ethical approval for the study from Al Moosa Specialist Hospital ethical committee. Informed consent (verbal and written) obtained from all couples. We recruited patients with PCOS or unexplained subfertility from the subfertility clinic at Al Moosa Specialist Hospital.
We diagnosed PCOS according to the Modified Rotterdam Consensus, 2003 [10]. We diagnosed PCOS with two out of these three conditions: ovulation dysfunction, hyperandrogenism (clinical or biochemical), and ultrasonic polycystic ovary picture. Polycystic ovary picture on ultrasound diagnosed if there are ≥ 12 follicles with a 2-9 diameter in any side, ovarian enlargement ≥ 10 ml or both [10].
Hirsutism is a terminal hair presence with a male's pattern in females. We can find hirsutism in 5%–15% of women. Modified Ferriman and Gallwey is a scoring system to quantify hirsutism, including nine androgen-dependent sites [11]. We diagnosed hirsutism when modified Ferriman–Gallwey (mFG) score is ≥ 6. We scored each body area from 0 (terminal hairs absence) to 4 (terminal hair growth is extensive), then we added each area score together to get the total score.
Inclusion criteria
Female gender.
Age should be from 18 to 38 years.
Subfertility period should be at least one year.
Hysterosalpingography or Saline Infusion Sonography is normal.
Normal semen analysis using modified WHO criteria 2010. Normal semen values as recognized by WHO are: ≥ 1.5 millilitre volume, ≥ 15 million per millilitre count, ≥ 39 million total count, ≥ 40% total motility, ≥ 32% forward motility, ≥ 58% viable, ≥ 4% morphologically normal, < 1 million/millilitre Leucocyte counting [12].
BMI ≤ 35 kg/m2.
Excluding criteria
Medical record of surgery in the pelvis, endometriosis or PID.
Intramural uterine myoma (> 4 cm) or sub endometrial myomas or congenital uterine anomaly.
Medical diseases such as adrenogenital syndrome, Cushing's syndrome, or androgenproducing neoplasms.
Laparoscopy findings of adhesions or abnormalities in the pelvis.
Primary amenorrhoea, premature ovarian failure, hypogonadism.
Male factor subfertility.
Untreated thyroid disorders or untreated hyperprolactinemia.
Cancelled cycles or Premature ovulation (before hCG injection).
Informed consent refusal.
The same investigator did the ultrasounds to avoid the interobserver variability. We used a transvaginal probe (5-9 MHz) Voluson P80 (GE Medical Systems). All patients instructed not to drink coffee or tea, not to smoke and to not use any medications or herbs within 8 hours before each exam. We performed all ultrasound examinations after the patient had emptied her bladder, with the use of the lithotomy position.
We divided the patients using a random number using an online randomization website (www. random.org) into two groups:
Group 1: Clomiphene citrate group.
Group 2: Letrozole group.
Letrozole 5 mg once per day for five days. Clomiphene citrate 100 mg once per day for five days. Both to be started from the third day of the period.
Evaluation of study subjects
At the second or third day of the cycle, a transvaginal ultrasound examination done to assess Antral Follicle Count (AFC) and to exclude any pelvic pathology before treatment.
An anovulatory cycle would be presumed if by day 16 we found no follicle ≥12 mm where we would cancel the cycle.
When we found at least one follicle with mean diameter ≥ 18 mm (mean of two perpendicular diameter measurements) [13], we would administer hCG 10000 units IM (Choriomon, IBSA, Institut Biochimique SA or Pregnyl, Organon) the same morning and timing of intercourse would be 24 and 36 hours from hCG injection.
We conducted an ultrasonographic assessment of the endometrial thickness, pattern, blood flow and endometrial scoring system, uterine artery and sub endometrial arteries doppler indices done on the day of hCG and nine days after the day of hCG triggering. Measurement of the stripe of the endometrium was at the broadest part at the median plane, from side to side from the outer echogenic border.
The pattern of the endometrium was described using a 3-grade system by Gonen and Casper in 1990 on the day of hCG [14]
Type A: The endometrium has uniform high echogenicity, with a non-visible median line.
Type B: It has iso-echogenic homogeneity, with a non-visible median line.
Type C: There is a multilayered endometrial pattern composed of a high echogenicity external and median lines with low echogenicity between the lines.
We recorded endometrial and sub endometrial vascularity on the day of hCG trigger and nine days post hCG administration. On the day of the hCG trigger, we recorded vascular penetration towards the endometrial canal in 4 types
Zone 1: Vascularity is distal to high echogenicity external lines.
Zone 2: Vascularity reaches high echogenicity external lines.
Zone 3: Vascularity reaches low echogenicity part between external and internal lines.
Zone 4: Vascularity reaches high echogenicity internal line.
On the mid-luteal phase day, we recorded vascular penetration towards the endometrial canal in 2 zones
Zone 1: vascularity is distal to high echogenicity external lines.
Zone 2: vascularity reaches at least the high echogenicity external lines up to the internal median line.
And finally, we evaluated the System of Applebaum Score.[15] This system includes this item
Thickness of the endometrium:
≤ 7 millimeter=0.
7-9 millimeter=2.
10-13 millimeter=3.
≥ 14 millimeter=1.
Endometrial layers
Absent of layers=0.
Blurry 5 lines=1.
Clear 5 lines=3.
Wave movments of the endometrium
<3 wave movments in 60 seconds=0.
≥ 3 wave movments in 60 seconds=3.
Ultrasound Homogeneity of the myometrium
Inhomogenic pattern=1.
Homogenic pattern=2.
PI of the uterine artery
≥ 3.0=0.
2.5-2.99=0.
2.2-2.49=1.
≤ 2.19=2.
Zone 3 vasculairty of the endometrium
No vascilarity=0.
Dispersed vascularity=2.
Multi-spots vascularity=5.
Vascularity of the myometrium (spiral arteries)
Absent=0.
Present=2.
So, the maximum score 20.
We analyzed the sub endometrial arteries PI and RI using doppler 1–3 mm under the outer line of the endometrium.
Using a 2D doppler assessment, we assessed the PI and RI of the uterine artery. On both lateral cervical edges, we set the cursor on the uterine artery main subdivisions. We configured the filter at lowest 1.3 PRF for uterine artery and 0.3 PRF for the sub endometrial arteries.
We positioned the doppler gate on the best colour signal vessel. When we obtained a good wave, we record PI and RI. We calculated the average of both PI and RI of the uterine artery.
In all patients, we gave dydrogesterone as luteal phase support (Duphaston 10 mg, BD, orally) for 13–15 days starting from day three after hCG injection. We requested a serum pregnancy test two weeks after timed intercourse. If the serum pregnancy test were positive, we would do a pelvic antenatal sonographic study at six gestational weeks to confirm successful pregnancy. If the pregnancy was negative, we could repeat the cycle twice after couple approval.
Data analysis
We used Microsoft Excel and IBM SPSS for statistical calculations, while we used G Power software for power and sample size prediction.
Results
For Fisher's exact test, We predicted the sample size using G Power. The applied assumption to calculate the sample size are control group proportion= .05, experimental group proportion= .4, two-sided α error=0.05, power level=80% and allocation ratio n1/n2=1. The predicted sample size was 48 cycles or more.
For the point biserial correlation, we calculated the sample size using the same software with a moderate effect size of .37 and an α error of 0.05 and a power of 80%. We concluded that the sample size should be 52 cycles or more.
We carry out a total of 54 cycles of ovulation induction cycles and randomized to 27 in each group. The evidence is not sufficient to suggest a difference in age or BMI with a p-value of .109 and .153, respectively (Table 1).
| Protocol | ||||||
|---|---|---|---|---|---|---|
| Clomiphene citrate group | Letrozole group | |||||
| Mean | N | Std. Deviation | Mean | N | Std. Deviation | |
| AGE | 26.4 | 27 | 4.9 | 28.3 | 27 | 4.5 |
| BMI | 25.6 | 27 | 5.1 | 27.9 | 27 | 6.2 |
Table 1: Age and BMI with letrozole and clomiphene citrate groups.
The comparison included the endometrial thickness, pattern and vascularity, sub endometrial arteries doppler markers, Uterine artery doppler markers and Applebaum Scoring System.
For the categorical data, we used Fisher's Exact Test. While we applied point-biserial Pearson correlation to calculate the correlation among the normally distributed continuous variables and the dichotomic variable (Clomiphene citrate or letrozole); otherwise, We used rank-biserial Spearman's correlation.
There was no difference between the groups in the mean mid-cycle endometrial thickness (8.6 ± 1.97 for letrozole vs 8.55 ± 2.4 for clomiphene, P= 0.61). Similarly, there was no difference in the mean mid-luteal endometrial thickness (11.47 ± 3.23 for letrozole vs 12.16 ± 2.89 for clomiphene, P=0.56).
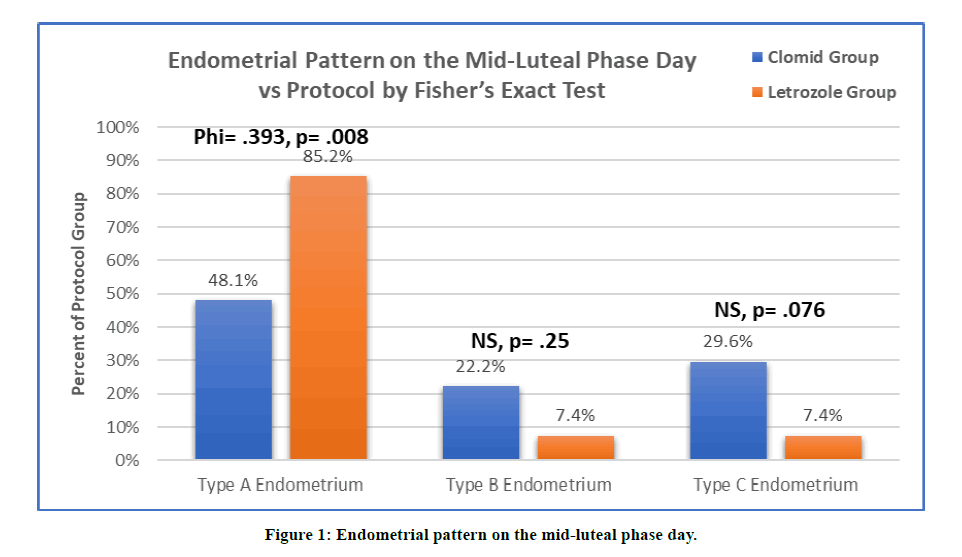
We performed Fisher's Exact Test to examine the relationship between the ovulation induction medication and endometrial pattern on the mid-luteal phase day. In clomiphene cycles, type A endometrium has been seen in 48.1% while in letrozole cycles it was seen in 85.2%. There was sufficient evidence to suggest that there is a moderate relationship between type A endometrium and oral ovulation induction medication with a p-value of 0.008 and a Phi of .393. Letrozole group caused more of type A endometrium on the mid-luteal phase day compared with clomiphene citrate group (Figure 1).
Figure 1: Endometrial pattern on the mid-luteal phase day.
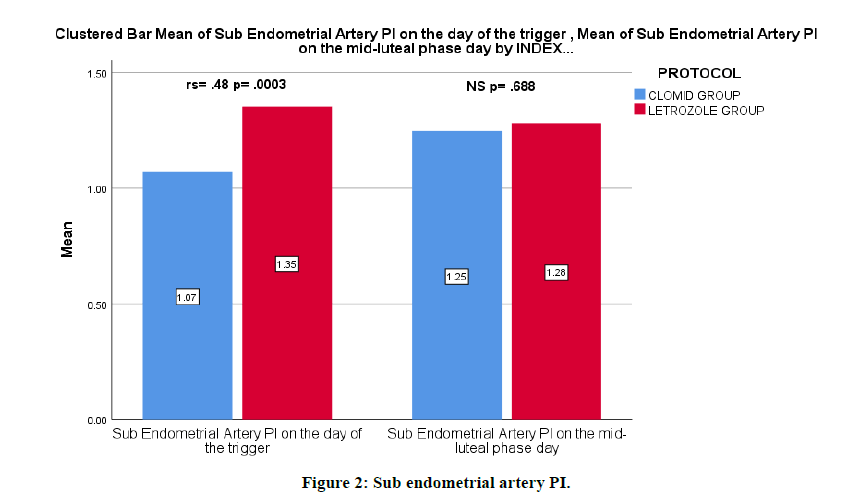
The mean of sub endometrial artery PI. on the day of the trigger was lower with the clomiphene citrate group (letrozole 1.35 ± 0.34 vs clomiphene citrate 1.07 ± 0.33). There was evidence to suggest that there is a correlation between the ovulation induction medication and sub endometrial artery PI. on the day of the trigger, rs=0.478, p=0.0003 (Figure 2).
Figure 2: Sub endometrial artery PI.
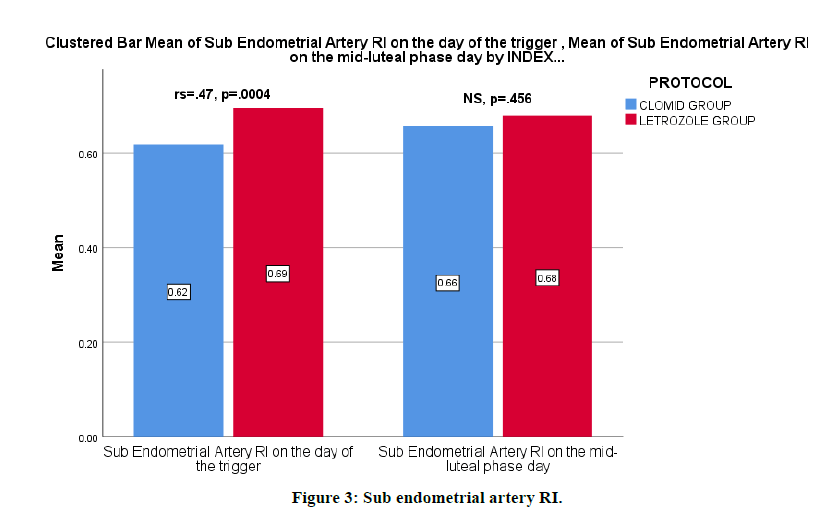
The mean of sub endometrial artery RI on the day of the trigger was lower with the clomiphene citrate group (letrozole .69 ± .06 vs clomiphene citrate 0.62 ± .1). There was evidence to suggest that there is a correlation between the ovulation induction medication and sub endometrial artery RI on the day of the trigger, rs=0.466, p=0.0004 (Figure 3).
Figure 3: Sub endometrial artery RI.
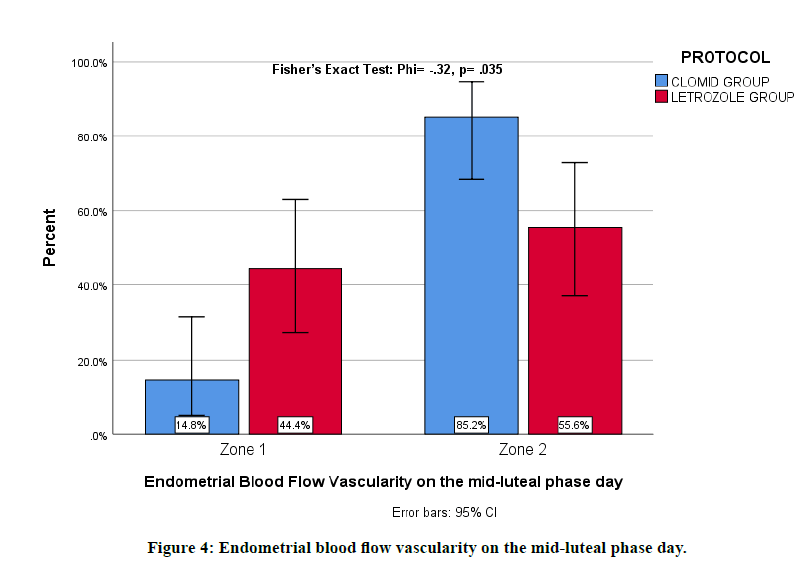
Endometrial blood flow vascularity on the midluteal phase day was studied using Fisher's Exact Test. We found Zone 2 in 85.19% in Clomiphene citrate group vs 55.56% in the Letrozole group. There was sufficient evidence to suggest that there is a moderate relationship with a p-value of 0.035 and a moderate phi test effect size of - .324. The endometrial vascularity on the midluteal phase day was more prominent with the Clomiphene citrate group (Figure 4).
Figure 4: Endometrial blood flow vascularity on the mid-luteal phase day.
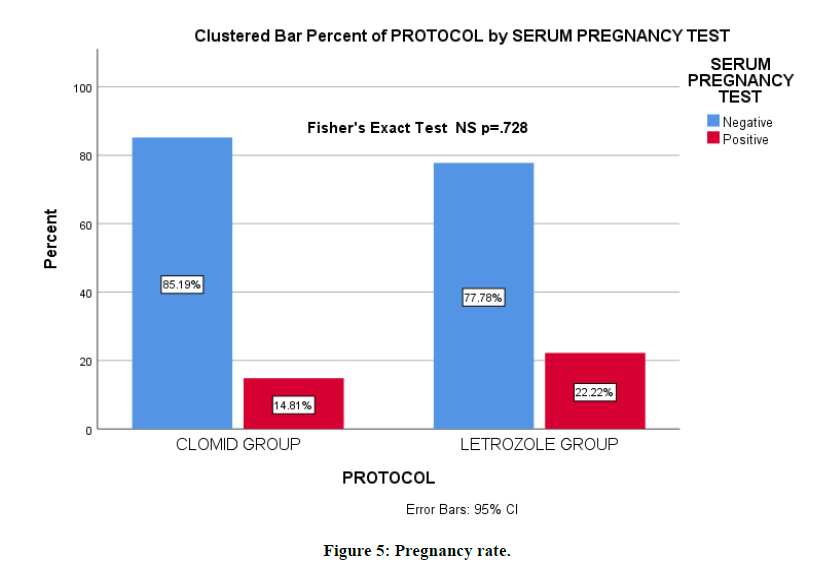
The pregnancy rate in the Clomiphene group was 14.8% vs 22.2% for letrozole group. The evidence was not sufficient to suggest that there is a relationship using Fisher's Exact Test with a p-value of 0.728 (Figure 5).
Figure 5: Pregnancy rate.
On the day of trigger, the evidence was not sufficient to suggest that there is a difference in terms of the endometrial pattern (type A p-value= .544, type B p-value=0.327, type C p-value=1), the sub endometrial blood flow vascularity (p=1), the Applebaum Uterine Scoring System (p=0.24), the PI of uterine artery (p=0.972) and the RI of uterine artery (p=0.785).
On the mid-luteal day, also the evidence was not sufficient to suggest that there is a difference between the groups in terms of the sub endometrial arteries PI (p=0.688), the sub endometrial arteries RI (p=0.456), the PI of the uterine Artery (p=0.602) and the RI of the uterine artery (p=0.614).
Discussion
Clomiphene citrate reduces endometrium thickness as reported by many studies, and thus the chances of conception [5], maybe because of oestrogen receptors reduction. Letrozole is proposed to be superior in terms of width or receptiveness of endometrium and pregnancy rate.
In our study, we found that width and pattern of endometrium on the day hCG trigger did not differ significantly between treatment groups. However, letrozole was more favourable in terms of the endometrial pattern at the midluteal phase than clomiphene citrate. Type A endometrium on the mid-luteal day was significantly more likely after treatment with letrozole.
The endometrial blood flow was similar in the treatment groups on the day of trigger, but on the mid-luteal day, there was evidence to suggest that there is a difference in the endometrial vascularity between the treatment groups. More mid-luteal zone 2 was associated with the clomiphene citrate group when compared with letrozole group.
The letrozole group was superior in Applebaum Uterine Score. However, the evidence is not sufficient to suggest that there is a difference.
In the Letrozole group, the reported sub endometrial arteries PI and RI were higher than those reported in the clomiphene group on the day of the trigger while the difference was not significant on the mid-luteal day.
Also, we examined the uterine artery PI and RI, but we found no difference between the treatment groups neither on the day of the hCG trigger nor on the mid-luteal day.
Our data indicate that pregnancy rates are higher with the letrozole group (22.2% vs 14.8%), but the difference was not statistically significant.
Conclusion
When comparing letrozole with clomiphene citrate group, letrozole is associated with more type A pattern on the mid-luteal day. While clomiphene is associated with more mid-luteal zone 2 when compared with letrozole group.
On mid cycle, letrozole has a higher sub endometrial PI and RI. Letrozole group is superior regarding the pregnancy rate, but the difference was not significant.
So, clomiphene citrate may be more favourable in terms of sub endometrial PI and RI on the mid-cycle day. In the mid-luteal phase, letrozole is more favourable in terms of endometrial pattern, while clomiphene is more favourable in terms of endometrial blood flow.
The net effect is a mild preference to the letrozole group when compared to clomiphene group.
Acknowledgement
The authors are grateful to the School of Science and Technology (SSST) university and the administration of Almoosa Specialist Hospital for their great assistance in achieving the goals of this research.
Ethical Considerations
A consent to participate was taken from all couples. Besides, the approval of the Ethical and Research Committee in Almoosa specialist hospital was obtained.
Fundings
The authors paid the whole expenses of this research, and no financial aid was taken from any agency.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Clark JH, Markaveric, BM. The agonistic-antagonistic properties of clomiphene: A review. Pharmacol Therapeu 1981; 15:467–519.
- Practice committee of the American society for reproductive medicine. Use of clomiphene citrate in women. Fertil Steril 2003; 80:1302–1308.
- Kettel LM, Roseff SJ, Berga SL, et al. Hypothalamic-pituitary-ovarian response to clomiphene citrate in women with polycystic ovary syndrome. Fertil Steril 1993; 59:532-538.
- Gorlitsky GA, Kase NG, Speroff L. Ovulation and pregnancy rates with clomiphene citrate. Obstet Gynecol 1978; 51:265–269.
- Gadalla MA, Huang S, Wang R, et al. Effect of clomiphene citrate on ovulation, endometrial thickness and live birth in anovulatory women: Systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017; 51:64–76.
- Thompson LA, Barratt CL, Thornton R, et al. The effects of cyclofenil and clomiphene citrate on cervical mucus volume and receptivity over the periovulatory period. Fertil Steril 1993; 59:125–129.
- Legro RS, Brzyski RG, Diamond MP, et al. Clomiphene versus letrozole for infertility in the polycystic ovary syndrome. New England J Med 2014; 371:119–129.
- Mitwally MF, Biljan MM, Casper RF. Pregnancy outcome with the use of aromatase inhibitors for ovarian stimulation. Am J Obstet Gynecol 2005; 192:381–386.
- Kovacs P, Matyas SZ, Boda K, et al. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod 2003; 18:2337–2341.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on polycystic ovary syndrome diagnostic criteria and long-term health risks related to it. Fertil Steril 2004; 81:19–25.
- Ferriman D, Gallwey JD. Body hair growth clinical assessment in women. J Clin Endocrinol Metabol 1961; 21:1440–1447.
- https://www.who.int/reproductivehealth/publications/infertility/9789241547789/en/
- Schild RL, Knobloch C, Dorn C, et al. Assessing receptivity of endometrium in an in vitro fertilization program by endometrial thickness, endometrial volume, spiral artery blood flow and uterine artery blood flow. Fertil Steril 2001; 75:361–366.
- Gonen Y, Casper RF. (1990). Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). In Vitro Fertilization Embryo Transfer J 1990; 7:146–152.
- Applebaum M. The uterine biophysical profile. Ultrasound Obstet Gynecol 1995; 5:67–68.
Author Info
Median Atia Alkhalaf Almamou1* and Asim Kurjak2
1Sarajevo Medical School, University of Sarajevo School of Science and Technology (SSST), Sarajevo, Bosnia and Herzegovina, IVF Unit, Obstetrics and gynaecology Department, Almoosa Specialist Hospital, Saudi Arabia2Medical School, Universities of Zagreb and Sarajevo, Zagreb, Croatia
Received: 26-Sep-2020 Accepted: 13-Oct-2020 Published: 20-Oct-2020