Research - (2021) Volume 9, Issue 1
The Effect of Adding Poloxamer Surfactant on the Penetration Depth of NaOCl and NaOH into Dentinal Tubules
Thaer A Mukhlif1* and Raghad A Al-Hashimi2
*Correspondence: Thaer A Mukhlif, Department of Esthetic and Restorative Dentistry, Ministry of Health, Al-Anbar Health Director, Iraq, Email:
Abstract
Objectives: The purpose of this in vitro study was to compare the effect of adding poloxamer surfactant to irrigant solutions on the penetration depth inside dentinal tubules.
Materials and method: 63 human single roots of permanent premolar (7 for each group) were used in this in vitro study. Roots embedded in container (filled with impression silicon) were instrumented with ProTaper Rotary instruments till size F4, each group (7 roots) were irrigated with one of the following solution: NaOH (5% (A1), 2.5/(A2), 0.5%(A3)), NaOH+Poloxamer (5%(B1), 2.5%(B2), 0.5%(B3)), NaOCl 5.25%(C1), NaOCl+Poloxamer (C2), and Normal Saline(D). Each group was irrigated with 3 ml of EDTA 17% for 1 Minute to remove smear layer then washing with normal saline. After that, the roots were filled with methylene blue that washed later by one of solutions used in this study. By longitudinal sectioning of the roots the specimens might be ready for imaging and examining with digital microscope, the differences in color for each root images were measured with Photoshop program. Then the result analyzed statistically with Anova test, Post hock, and T test.
Results: The study revealed that NaOH showed highest penetration than that of NaOCl, and the adding of Poloxamer surfactant significantly increase the penetration inside dentinal tubules for both solutions. There was also a significant difference in penetration depth between root thirds.
Conclusion: Adding poloxamer surfactant significantly increased penetration depth of irrigant solutions inside dentinal tubule, and there was a significant difference in penetration among solutions and between root thirds.
Keywords
Poloxamer, Penetration depth, Dentinal tubules, NaOCl, NaOH
Introduction
The primary goal of root canal treatment is the reduction of microorganisms by root canal cleaning and disinfection. Opportunistic dental pulp and root canals infections caused by oral commensal microorganisms can lead to pulpitis, necrosis of pulp and then periradicular lesions. With mechanical preparation alone, the remained untouched area might be more than one-third of root canal surface. Irrigation plays a major role in endodontic treatment, as it enables chemical dissolution for remnant pulp tissue, aid in removing debris and smear layer and in mechanical detachment of biofilm. The apical third of the canal system mostly has a complex morphology and therefore it is difficult to clean [1].
Dentinal tubules invasion by bacteria has been regarded as a potential source of infection persistent. Ando and Hoshino in 1990 found bacteria in infected teeth dentinal tubules at approximately half the distance between the cementodentinal junction and main root canal. Furthermore, Haapasalo, et al. found that Enterococcus faecalis could invaded dentinal tubules rapidly, and with cementum absence, the front of the infection after 3 weeks had reached 1000 μm. Therefore, the irrigants penetration depth inside dentinal tubules is an important factor that can potentially affect the irrigant effectiveness and contribute to the root canal treatment outcome. Sodium hypochlorite (NaOCl) is the most used root canal irrigant because of its tissue-dissolving and antimicrobial activity [2]
Neglia et al. in 2008 in an ex vivo study on NaOCl5.25% solution effectiveness against E. faecalis found that the drop in the bacterial load was rapid in most of the teeth irrigated with NaOCl, but this drop remains for up to 48 h after irrigation, because in the following 96 h the bacterial load had raised the infection again to its previous level because of recolonization of70% of the samples. For measuring NaOCl penetration inside dentinal tubules, Zou et al. in 2010 developed a stained dentin block model by using varying temperature and concentration of the solution, and various exposure time. The major NaOCl drawback is its high surface tension, which may affect its wettability that play an important role in the antimicrobial solutions penetration inside dentinal tubules and into root canal system irregularities [2].
Agitation techniques with devices such as sonic, ultrasonic, Endovac, and plastic rotary files can increase the final irrigant effect on the apical third of root canal walls. The penetration depth of irrigation solutions and therefore their disinfecting effect on dentinal tubules are limited. These limitations lead to incomplete bacterial removal from dentinal tubules after cleaning and shaping of the root canal system. Irrigation systems which increase the penetration depth of irrigants inside dentinal tubules and the root canal while encountering minimal apical extrusion of irrigants. As a result of that cytotoxic effects on periapical tissues could be eliminated and superior effect sexhibited [3].
Depending on time, concentration, and temperature, NaOCl as a main root canal irrigant can penetrate up to 300 mm through the dentinal tubules (Zou et al.,2010). However, bacterial penetration within dentinal tubules can reach up to a 1000-mm. Microorganisms in the dentinal tubules can act as a reservoir from the surrounding tissue and root canal infection; therefore, reinfection might occur, therefore for complete eradication of bacteria in infected dentinal tubule, intracanal medicaments should penetrate through the dentinal tubules [4].
Sodium hydroxide, also known as lye or caustic soda, is a highly corrosive compound. It is used to control pH and alkalinity in aqueous solutions and is effective in organic matter breaking down. It is a strong base, and very soluble in water, glycerin, and alcohol.
A recent publication from the virology field suggested a solution composed of a combination of NaOH, SDS and an alcohol may be with a fast, broad range disinfective properties on fungi, bacteria, and viral prion proteins. There has been no research data performed on NaOH containing solutions in the endodontic literature, because many opinions considered using NaOH endodontics is unsafe and unsuitable [5].
Mo in 2016 revealed that NaOCl groups resulted in more killed biofilm, however visually there were more remaining biofilm voluminous areas. In contrast, with Double and Triple solution of NaOH with surfactant groups were proportionately less biofilm microbes killing, however, the remaining non-detached biofilm areas were low in volume and sparse.
Surface tension modification of irrigant solutions might improve irrigation efficacy by permitting irrigants to flow into far areas because surface tension inhibits the liquid spreading over a surface and inhibit its capacity to permeate capillary tubes. The liquid spreading or concentration in contact with a surface depends on attraction forces between the liquid molecules and with those forces exerted by surface molecules when was in contact with liquid molecules. The addition of surface-active agents might increase the surface area, thus making surfaces easily wetted. The surface-active agents that used in cleaning processes are referred to as detergents. Reducing surface tension of root canal irrigants make the root canal dentine free of debris, more adapted to the dentinal walls, better tubule penetration and more rapid fresh solution exchange [6].
Poloxamers are a major non-ionic surfactant, which are widely used in wound care. They are triblock copolymers with a central hydrophobic chain of polypropylene oxide (PPO, polyoxypropylene) connected by two hydrophilic chains ofpolyethylene oxide (PEO, polyoxyethylene). Through the polymers chain length adjusting and the PPO: PEO ratio, many different poloxamers in industry have been produced which have slightly different characteristics [7].
poloxamer 407 is a non-ionic surfactant which above a particular concentration and temperature of polymer have reversible gelation properties. Due to its easy preparation, a sterile injectable formulations based on poloxamer 407 copolymer have a good candidate for drug delivery, especially when requiring a controlled release of the drug [8].
Poloxamers have gained more attention because it can repair the biological membranes damaged by diseases and trauma. They can stimulate the immune response, fat metabolism, cell proliferation, tissue microcirculation and collagen synthesis. At the same time, when used topically they provide the stability of several a water-soluble medicinal substance. The Poloxamer gel not only provides a non-toxic detergent cover to the wound but may also have a beneficial action in accelerating wounds healing, has immunomodulatory characteristics and neutrophils inactivation. Poloxamer is attractive due to it is transformed from a low-viscosity solution at low temperature to a semisolid gel at higher temperature. Previous in vitro study done by Babickaite et al. in 2016 showed that a chlorhexidine-poloxamer gel is effective against gram-negative and gram-positive [9].
As the PEO-PPO block copolymer concentration in solution reaches a certain critical micellization concentration (cmc) at a fixed temperature, the micellization process commences. Conversely, at a fixed block copolymer concentration, increasing the temperature to the critical micellization temperature (cmt) also induces micellization. The effect of temperature arises from the reduced PEO solubility, and especially the PPO, blocks in water upon heating [10].
Materials and Methods
Specimen selection and preparation
A total of 63 (each group 7 tooth) human single root premolars that were extracted, for many reasons not related to this study. Two digital radiograph in bucco-lingual and Mesiodistal direction were taken to select onlysingle canal permanent premolars with ≥16 mm root lengths were included. Teeth withopen apices, caries, calcified canals,root fractures, resorptive defects, previous root fillings were excluded [11].The specimens were embedded in an experimentalroot socketresembile of silicon impression material. Penetration of NaOCl inside dentinal tubules was measuredby stained dentine model according to Zou et al. (2010), but with some modifications to resemble in vivo conditions. With a slow-speed diamond disc (Taiwan) tooth were decoronated and the remaining root adjusted to 15 mm. The working length (WL) was measured by inserting a size 10 K-File (DENTSPLY, Switzerland) into the root canal until the tip was visible beyond the apex and 0.5 millimeter was then subtracted from this length [11].
To prepare Poloxamer, the amount of polymer required was dispersed in deionized water with continuous stirring at room temperature for one hour. The partially dissolved Poloxamer solutions were stored at 4ºC (in the refrigerator) until the whole polymer was completely dissolved (need approximately 24 h) [12]. The concentration of poloxamer used in this study depend on the Critical Micelle Concentration (CMC). Slight change in surface tension is detected at exceptionally low surfactant concentration. Surfactant addition drastically decreases the surface tension. Surface becomes saturated at CMC point, and any surfactant molecules addition do not effect on the surface tension. CMC of present test was between 0.1 to 0.25 (Figure 1).
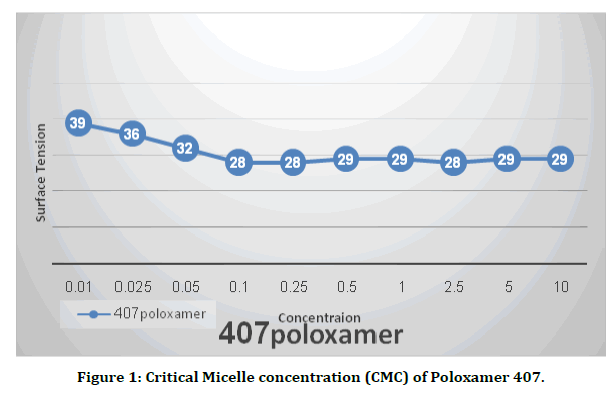
Figure 1. Critical Micelle concentration (CMC) of Poloxamer 407.
Root canals were prepared using Protaper Rotary Instruments to enlarge the canals till file size F4 according to manufacturers’ instructions. During instrumentation canals were irrigated with 5ml of each one of nine-solution used in study: NaOH in three concentration [(5%(A1), 2.5%(A2), and 0.5%(A3)], NaOH with Poloxamer in three concentration also [5%(B1), 2.5%(B2), 0.5%(B3)], NaOCl 5.25%(C1), NaOCl 5.25% with Poloxamer (C2), and Normal Saline (D) as a control group [13]. One ml of saline was delivered between each file using a syringe and 30G needle (Sinaledent, China) to flush the dentin debris created during instrumentation [14].
The root canals were then irrigated with three mL of 17% EDTA for one minute to remove the smear layer with manual activation with apically fitted gutta percha. All canals received a final rinse of 3 mL of sterile saline [15]. The canals then filled with 1% methylene blue which was activated for 30 s manually with apically fitted gutta percha in order to visualize the penetration depth of the last irrigant. Then the canal was dried with paper points and teeth were stored dry until further use [1]. Five ml from each irrigant solution groups were used to remove the methylene blue from root canal by bleaching method and then dried with paper point (Figure 2).
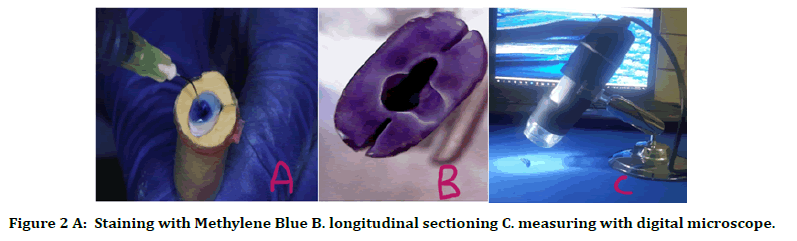
Figure 2. A: Staining with Methylene Blue B. longitudinal sectioning C. measuring with digital microscope.
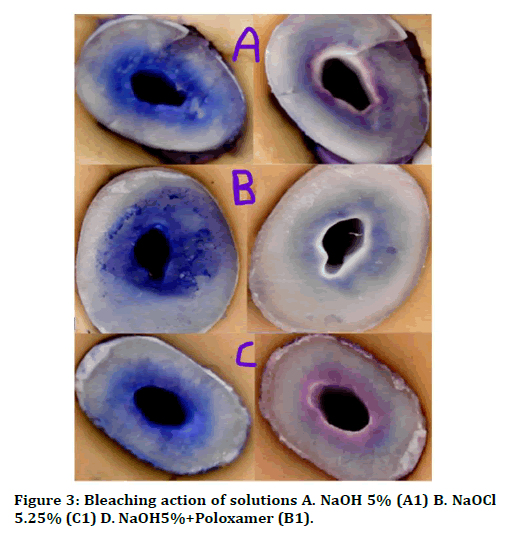
Figure 3. Bleaching action of solutions A. NaOH 5% (A1) B. NaOCl 5.25% (C1) D. NaOH5%+Poloxamer (B1).
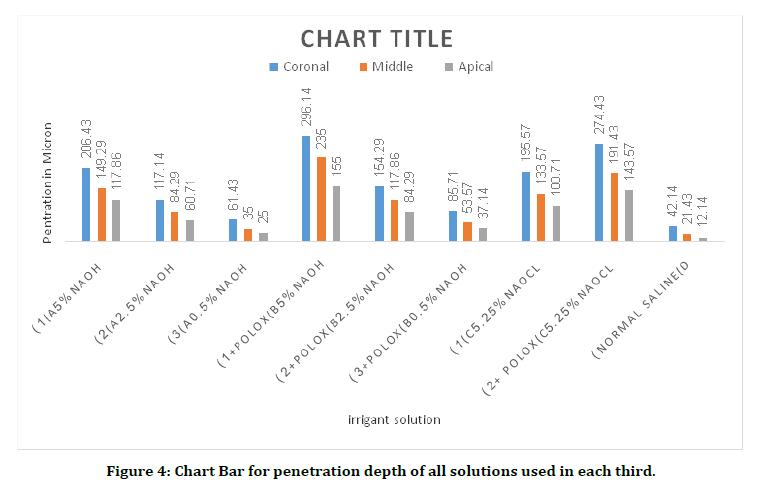
Figure 4. Chart Bar for penetration depth of all solutions used in each third.
Specimens for all groups grooved longitudinally with a diamond disc (Taiwan) and split into two halves with a chisel, and the halve which have more root canal borders was selected for evaluating penetration depth of dye inside dentinal tubules. All measurements were done by using the USB digital microscope (China). The measurements were done under X20 and 40X magnification in three zones of each specimen:
Coronal zone: 5mm coronal part.
Middle zone: 5mm middle part.
Apical zone: 5mm apical part.
four measurements for each zone were done and the mean of them was considered as penetration depth value at that site. Achieved data was evaluated by descriptive statistic methods via statistic software SPSS 25. For comparison among solutions in each third (Apical, middle & coronal) ANOVA test with post hock for multiple comparison, while independent T test was used to compare among thirds for the same specimen. For all statistic process P value<0.05 considered as significant [15].
The irrigant solution penetration depth was measured by using intensity profiles of horizontal line from the root canal toward the periphery, the irrigant penetration depth was defined as the region where methylene dye was removed “bleached” and a dentinal tubules white line was observed. This line corresponds the difference in color along the image horizontal axis, therefore “white” pixels detecting intensity peaks. Images from all specimens were evaluated by two blinded operators [16].
The Method of Calculating the Bleached Zone The captured images from microscopic with 20X magnifications, were opened with Photoshop software. Polygonal lasso tool was used to detect the limits of bleached zone, from Photoshop histogram menu number of selected pixels was read, while pixel in Info menu give the height of canal interior wall. The following formula based on pixel was used to calculate the penetration depth of irrigation solution:
b=Total pixels ̸ a
(b=Average of irrigation solution penetration depth, a= Canal interior wall height). For example, images with 96 dpi resolutions, every 100 pixels were equivalent to 25.4 mm according to a special formula, and by dividing that to the magnification, the real size was obtained and then converted to micron to get the average of penetration in the total penetrated surface. Statistical analysis was performed [17].
Results
Penetration test is so important to show irrigant entrance inside dentinal tubules. Descriptive statistic (Table 1) and Bar Chart (Figure) showed that the largest distance (Mean 296.14 mm) inside dentinal tubules was recorded by NaOH+Poloxamer (group B1) in coronal section, and the lowest distance (Mean 12.14mm) recorded by Normal Saline(D) in apical section.
| Groups | N | Mean Coronal | Std.Dev. | Mean Middle | Std.Dev. | Mean Apical | Std.Dev. |
|---|---|---|---|---|---|---|---|
| A1 | 7 | 206.43 | 5.563 | 149.29 | 6.726 | 117.86 | 4.88 |
| A2 | 7 | 117.14 | 4.88 | 84.29 | 4.499 | 60.71 | 4.499 |
| A3 | 7 | 61.43 | 5.563 | 35 | 4.082 | 25 | 2.887 |
| B1 | 7 | 296.14 | 5.984 | 235 | 7.071 | 155 | 6.455 |
| B2 | 7 | 154.29 | 6.726 | 117.86 | 4.88 | 84.29 | 4.499 |
| B3 | 7 | 85.71 | 5.345 | 53.57 | 3.78 | 37.14 | 5.669 |
| C1 | 7 | 195.57 | 7.138 | 133.57 | 3.78 | 100.71 | 3.45 |
| C2 | 7 | 274.43 | 8.96 | 191.43 | 9.88 | 143.57 | 7.48 |
| D | 7 | 42.14 | 2.673 | 21.43 | 4.756 | 12.14 | 2.673 |
| Total | 63 | 159.25 | 86.535 | 113.49 | 68.766 | 81.83 | 49.32 |
Table 1: Descriptive statistic of irrigant penetration.
An Anova test (Table 2) showed that there was a significant difference among groups within each root third (apical Middle, Coronal). The penetration of irrigant were decreased with concentration decrease (from A1 to A3, and from B1 to B3) and the differences was high significant as shown by post hock multiple comparison. Adding surfactant also showed significant difference for both solutions used (NaOH and NaOCl) as shown in Table 3. Comparison among thirds within same root specimen for each solution revealed that there was a high significant difference in penetration depth between thirds, that the penetration decreased from coronal to middle to apical thirds as showed by T test comparison in Table 4.
| Section | Sum of squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Coronal | 462273.651 | 8 | 57784.206 | 1558.393 | 0 |
| Middle | 360043.651 | 8 | 36419.147 | 1075.503 | 0 |
| Apical | 149486.508 | 8 | 18685.813 | 759.488 | 0 |
Table 2: Anova test for irrigant penetration.
| Solution | Groups | Coronal | Middle | Apical | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Diff. | Stand. Error | P-Value | Mean Diff. | Stand. Error | P-Value | Mean Diff. | Stand. Error | P-Value | ||
| NaOH 5% (A1) | A2 | 89.29 | 3.255 | 0 | 65 | 3.11 | 0 | 57.14 | 2.651 | 0 |
| A3 | 145 | 3.255 | 0 | 114.29 | 3.11 | 0 | 92.86 | 2.651 | 0 | |
| B1 | -89.71 | 3.255 | 0 | -85.71 | 3.11 | 0 | -37.14 | 2.651 | 0 | |
| B2 | 52.14 | 3.255 | 0 | 31.43 | 3.11 | 0 | 33.57 | 2.651 | 0 | |
| B3 | 120.71 | 3.255 | 0 | 95.71 | 3.11 | 0 | 80.71 | 2.651 | 0 | |
| C1 | 10.86 | 3.255 | 0 | 15.71 | 3.11 | 0.002 | 17.14 | 2.651 | 0 | |
| C2 | -68 | 3.255 | 0 | -42.14 | 3.11 | 0 | -25.71 | 2.651 | 0 | |
| D | 164.29 | 3.255 | 0 | 127.85 | 3.11 | 0 | 105.71 | 2.651 | 0 | |
| NaOH 2.5% (A2) | A3 | 49.29 | 3.255 | 0 | 49.29 | 3.11 | 0 | 35.71 | 2.651 | 0 |
| B1 | -186.4 | 3.255 | 0 | -150.7 | 3.11 | 0 | -94.29 | 2.651 | 0 | |
| B2 | -33.57 | 3.255 | 0 | -33.57 | 3.11 | 0 | -23.57 | 2.651 | 0 | |
| B3 | 30.71 | 3.255 | 0 | 30.71 | 3.11 | 0 | 23.57 | 2.651 | 0 | |
| C1 | -49.29 | 3.255 | 0 | -49.29 | 3.11 | 0 | -40 | 2.651 | 0 | |
| C2 | -107.1 | 3.255 | 0 | -107.1 | 3.11 | 0 | -82.86 | 2.651 | 0 | |
| D | 62.86 | 3.255 | 0 | 62.86 | 3.11 | 0 | 48.56 | 2.651 | 0 | |
| NaOH 0.5% (A3) | B1 | -235.7 | 3.255 | 0 | -200 | 3.11 | 0 | -130 | 2.651 | 0 |
| B2 | -82.86 | 3.255 | 0 | -82.86 | 3.11 | 0 | -59.29 | 2.651 | 0 | |
| B3 | -18.57 | 3.255 | 0 | -18.57 | 3.11 | 0 | -12.14 | 2.651 | 0 | |
| C1 | -98.57 | 3.255 | 0 | -98.57 | 3.11 | 0 | -75.71 | 2.651 | 0 | |
| C2 | -156.4 | 3.255 | 0 | -156.4 | 3.11 | 0 | -118.6 | 2.651 | 0 | |
| D | 13.57 | 3.255 | 0 | 13.57 | 3.11 | 0.005 | 12.86 | 2.651 | 0 | |
| NaOH 5% +Poloxamer (B1) | B2 | 152.86 | 3.255 | 0 | 117.14 | 3.11 | 0 | 70.71 | 2.651 | 0 |
| B3 | 217.14 | 3.255 | 0 | 181.42 | 3.11 | 0 | 117.85 | 2.651 | 0 | |
| C1 | 137.14 | 3.255 | 0 | 101.42 | 3.11 | 0 | 54.29 | 2.651 | 0 | |
| C2 | 79.29 | 3.255 | 0 | 43.57 | 3.11 | 0 | 11.43 | 2.651 | 0 | |
| D | 249.29 | 3.255 | 0 | 213.57 | 3.11 | 0 | 142.9 | 2.651 | 0 | |
| NaOH 2.5% +Poloxamer (B2) | B3 | 64.29 | 3.255 | 0 | 64.28 | 3.11 | 0 | 47.14 | 2.651 | 0 |
| C1 | -15.71 | 3.255 | 0 | -15.71 | 3.11 | 0 | -16.43 | 2.651 | 0 | |
| C2 | -73.57 | 3.255 | 0 | -73.57 | 3.11 | 0 | -59.29 | 2.651 | 0 | |
| D | 96.43 | 3.255 | 0 | 96.42 | 3.11 | 0 | 72.14 | 2.651 | 0 | |
| NaOH 0.5% +Poloxamer (B3) | C1 | -80 | 3.255 | 0 | -80 | 3.11 | 0 | -63.58 | 2.651 | 0 |
| C2 | -137.9 | 3.255 | 0 | -137.9 | 3.11 | 0 | -106.4 | 2.651 | 0 | |
| D | 32.14 | 3.255 | 0 | 32.14 | 3.11 | 0 | 25 | 2.651 | 0 | |
| NaOCl 5.25% (C1) | C2 | -57.86 | 3.255 | 0 | -57.86 | 3.11 | 0 | -42.86 | 2.651 | 0 |
| D | 112.14 | 3.255 | 0 | 112.14 | 3.11 | 0 | 88.57 | 2.651 | 0 | |
| NaOCl 5.25% +Polox (C2) | D | 170 | 3.255 | 0 | 170 | 3.11 | 0 | 131.4 | 2.651 | 0 |
Table 3: LSD multiple comparison of penetration in coronal section.
| Solutions | Third | Third | Mean Difference | Std. Error | Sig. |
|---|---|---|---|---|---|
| NaOH 5% (A1) | Coronal | Middle | 57.143 | 3.299 | 0 |
| Apical | 87.857 | 2.766 | 0 | ||
| Middle | Apical | 31.429 | 3.141 | 0 | |
| NaOH 2.5% (A2) | Coronal | Middle | 32.857 | 2.736 | 0 |
| Apical | 56.429 | 2.736 | 0 | ||
| Middle | Apical | 23.571 | 2.405 | 0 | |
| NaOH 0.5% (A3) | Coronal | Middle | 36.429 | 2.369 | 0 |
| Apical | 26.429 | 2.608 | 0 | ||
| Middle | Apical | 23.571 | 2.405 | 0 | |
| NaOH 5% +Polox (B1) | Coronal | Middle | 61.143 | 3.501 | 0 |
| Apical | 141.143 | 3.327 | 0 | ||
| Middle | Apical | 80 | 3.619 | 0 | |
| NaOH 2.5%+Polox (B2) | Coronal | Middle | 36.429 | 3.141 | 0 |
| Apical | 70 | 3.058 | 0 | ||
| Middle | Apical | 33.571 | 2.508 | 0 | |
| NaOH 0.5% +Polox(B3) | Coronal | Middle | 32.143 | 2.474 | 0 |
| Apical | 47.857 | 2.736 | 0 | ||
| Middle | Apical | 15.714 | 2.333 | 0 | |
| NAOCl 5.25%(C1) | Coronal | Middle | 62 | 3.053 | 0 |
| Apical | 94.857 | 2.997 | 0 | ||
| Middle | Apical | 32.857 | 1.934 | 0 | |
| NAOCl 5.25%+Polox(C2) | Coronal | Middle | 83 | 5.041 .000 | 0 |
| Apical | 130.857 | 4.412 .000 | 0 | ||
| Middle | Apical | 47.857 | 4.684 .000 | 0 | |
| Normal Saline(D) | Coronal | Middle | 20.714 | 2.062.000 | 0 |
| Apical | 30 | 1.429.000 | 0 | ||
| Middle | Apical | 9.286 | 2.062 .001 | 0.001 |
Table 4: T test comparison among thirds within same root for all solutions.
Discussion
Several studies showed that there is no endodontic irrigant solution with all required properties, therefore it is still important to search for a new irrigants. NaOCl is the most common root canal irrigant with an antimicrobial efficacy against microorganisms together with its potential to remove organic components. Previous studies revealed that dentin wett ability can be increased with NaOCl. However, due to its high surface tension, it may fail to reach microorganisms in the depth of tubules [18].
Root canal morphology is so complex with irregularities areas that contain microorganisms and pulpal tissues that cannot be reached by instruments. Therefore, proteolytic and an antiseptic fluid is necessary to clean and disinfect these areas. The way by which an irrigant spreads onto the canal and approach the non-instrumented areas is so important in root canal treatment. Many research has been done to reveal the mechanisms underlying irrigant penetration to draw clinical conclusions. For example, poor penetration depth of the irrigant beyond the needle tip, limiting the disinfection of the apical part of the root canal, has been reported [19].
The use of sodium hydroxide in this study was due to it is effectivity in removing proteins and nucleic acids. It is effective also for inactivating most viruses, yeasts, bacteria, and endotoxins. The in activation of bacteria by NaOH inactivation is dependent upon concentration, contact time, and temperature. To save time, it is common practice in industrial manufacturing a salt, such as sodium chloride, was added to the sodium hydroxide solution to combine cleaning with sanitization. As a cleaning agent, NaOH can dissolves proteins saponifies fats and. In general, it can solubilize precipitated proteins. In the presence of chlorine Its hydrolyzing power could be enhanced [20].
The type of a surfactant selected for this study was nonionic than other types of surfactant because non-ionic surfactants could provide a better wetting ability than ionic surfactants because they have a hydrogen bonding ability on its polar hydrophilic portion, which can provide strong connections with water molecules [12].
Non-ionic surfactants are the second surfactant used with about 45% of the overall industrial production. Because their hydrophilic group is of a non-dissociable type, they do not ionize in aqueous solution, such as alcohol, phenol, ether, ester, or amide [21].
The use of Poloxamer other than other types of nonionic surfactant was due to it is previous use with drugs as solubilizer, emulsifier and stabilizer, and due to its being non-toxic and non-irritant and used as wetting agents in ointments [22]. The type of Poloxamer selected for this study was poloxamer 407 because among several natural and synthetic polymers used to form polymeric micelles, Poloxamer407, is the most attractive due to its biocompatibility, biodegradability, and low toxicity [13]. Also due to the ability of their molecules to form entanglements or noncovalent bonds with mucus, thus favoring a great degree of interaction with various biological tissues, prolonging the residence time of the formulation at the application site [23].
For irrigant solutions the penetration test is so important due to its relation to microorganism disinfection inside dentinal tubulesin order to overcome the limited irrigant penetration inside it. These limitations lead to incomplete removal of bacteria from dentinal tubules after root canal cleaning and shaping. The best irrigation system is that which increase the irrigants penetration inside dentinal tubules and root canal while causing minimal extrusion of irrigants apically, thus eliminating cytotoxic effects on periapical tissues, exhibit superior effects [3].
The selection of reverse bleaching method in this study to evaluate the penetration of irrigant was due to its easy to use, precise results and availability of equipment. Penetration of NaOCl in human dentin is difficult to be measured experimentally. It is a strong oxidant, so NaOCl with added dyes are often altered in their molecular structure. However, dyes can be used to stain dentin, while NaOCl penetration can be evaluated by its bleaching ability [24].
From the result of this study NaOH 5% (group A1) was significantly had a greater penetration than NaOCl 5.25% (group C1). This may be attributed to the lower surface tension and viscosity of NaOH due to lower molecular weight compared with NaOCl. [25] revealed that viscosity and surface tension are the two main factors which influence the flow and penetration depth of the irrigants dentin, and the wettability of the irrigating solution on dentine is related to its viscosity and surface tension. The surface tension is the force between molecules that produces a tendency to decrease liquid surface area. This force tends to limit the liquids ability to penetrate in a capillary tube. The solution wettability governs the capability of its penetration both into the main and lateral canals, and into the dentinal tubules [26-29].
The finding of this study showed that a decreasing in penetration depth when the concentration of irrigant decreased. This agreed with [30] who concluded that temperature, concentration, and time all play a role in determining NaOCl penetration depth inside dentinal tubules. When these factors were present together, deepest penetration was obtained and the increasing in concentration from 1% to 6% results in 30%– 50% increase in penetration. In coincidence with this study [29] revealed that an increasing NaOCl concentration or contact time could improve tubular penetration for each of technique used in their study.
The result of this study revealed that there was an increase in irrigant penetration when surfactant was added to both solutions used (NAOH and NaOCl), and this agreed with [20] who concluded that surface-active agents improve NaOCl penetration in the main canal but didn’t have effect on its dissolution ability to the pulp tissue. When ethanol was added to NaOCl compared with NaOCl alone,it was found that this combination inside capillary tubes moved further depending on the ethanol amount in the mixtures (Cunningham et al. 1982) in agreement with present study clarified that ChlorCid with surfactant (ChlorCid™ Surf) showed higher penetration in cervical and middle sections, compared with ChloCid without surfactant (ChlorCid™), indicating that the presence of surfactant in composition led to higher penetration inside dentinal tubules. Thus, they concluded that ChlorCid™ Surf lower contact angle on root canal dentin, lower surface tension, and higher penetration depth inside dentinal tubules.
This finding contrasted with the study previously done by Faria et al. in 2019 [9] who concluded that the addition of surfactants to NaOCl solutions did not increase penetration into dentinal tubules. This contrast in results may be related to the differences in methods that they used dentine blocks in their study, while intact roots (resemble clinical work) with longitudinal sectioning were used in present study. They also used different surfactant (cetrimide) and different dye (crystal violet) in their study instead of Poloxamer surfactant and methylene blue dye used in present study.
The result of present study clarified that for all groups the penetration depth of an irrigant decreased from coronal to middle and to apical third with high significant differences among thirds. This was in coincidence with Kucuk et al. [16] who concluded that the penetration of irrigant penetration into dentinal tubules was significantly greater in the middle section than in the apical section of the root canal. Vadhana et al. in 2015 [25] In agreement with this study showed that the penetration depth of 2% CHX into root dentinal tubules is deeper in coronal third when compared to middle and apical third. The present study result was disagreeing with Generali et ai in 2018 [11] who showed that an increase of bleaching towards the coronal direction and no differences between groups, this differences from present study may be related to their staining procedure by using of 10% copper sulphate solution containing 5% ammonium hydroxide, then in a1% rubeanic acid alcohol solution., while in present study Methylene blue was used.
Limitation
The limited parts of this study were related to the inability to use it in vivo but replaced with in vitro study by extracted teeth which give comparable results.
Conclusion
From the result of present study, we concluded that NaOH had higher penetration inside dentinal tubules than that of NaOCl and adding poloxamer surfactant for both solutions used lead to higher penetration inside dentinal tubules. There was also a decrease in surface tension of NaOH when the concentration decreased.
References
- Galler KM, Grubmuller V, Schlichting R, et al. Penetration depth of irrigants into root dentine after sonic, ultrasonic, and photoacoustic activation Inter Endodo J 2019; 52:1210–1217.
- Palazzi F, Blasi A, Mohammadi Z, et al. Penetration of Sodium Hypochlorite modified with surfactants into root canal dentin. Braz Dent J 2016; 7:208-216.
- Ghorbanzadeh A, Aminsobhani M, Sohrabi K, et al. Penetration depth of sodium hypochlorite in dentinal tubules after conventional irrigation, passive ultrasonic agitation and Nd:YAG laser activated irrigation. J Lasers Med Sci 2016; 7:105-111.
- Sungur DD, Aksel H, Purali N, et al. Effect of a low surface tension vehicle on the dentinal tubule penetration of calcium hydroxide and triple antibiotic paste. J Endod 2017; 43:452-455.
- Mo AJ. Effect of NaOH Solutions on planktonic bacteria, biofilms, and lipopolysaccharide thesis (Master). The University of British Columbia (Vancouver) 2016; 1-59.
- Rossi-Fedele G, Prichard JW, Steier L, et al. The effect of surface tension reduction on the clinical performance of sodium hypochlorite in endodontics. Inter Endod J 2013; 46:492–498.
- Percival SL, Chen R, Mayer D, et al. Mode of action of poloxamer-based surfactants in wound care and efficacy on biofilms. Int Wound J 2018; 15:1–7.
- Fakhari A, Corcoran M, Schwarz A, et al. Thermogelling properties of purified poloxamer 407. Heliyon 2017; 3:1-26.
- Babickaite L, Grigonis A, Ramanauskiene K, et al. Therapeutic activity of chlorhexidine-poloxamer antiseptic gel on wound healing in rats: a preclinical study. Polish J Veter Sci 2018; 21:175–183.
- Bodratti AM, Alexandridis P. Formulation of poloxamers for drug delivery. J Functional Biomaterial 2018; 9:1-24.
- Varshosaz J, Tabbakhian M, Salmani Z, et al. Designing of a thermosensitive chitosan/poloxamerin situ gel for ocular delivery of ciprofloxacin. Open Drug Delivery J 2008; 2:61-70.
- Varela P, Souza E, de Deus G, et al. Effectiveness of complementary irrigation routines in debriding pulp tissue from root canals instrumented with a single reciprocating file. Intern Endod J 2018; 52:475-483.
- Saxena V, Hussain MD. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Inter J Nano-med 2012; 7:713–721.
- Vandrangi P. Evaluating penetration depth of treatment fluids into dentinal tubules using the gentle wave system. Dentistry 2016; 6:3:1-5.
- Iandolo A, Abdellatif D, Amato M, et al. Dentinal tubule penetration and root canal cleanliness following ultrasonic activation of intracanal-heated sodium hypochlorite. Austr Endod J 2019; 46:1-6.
- Coaguila-Llerena H, Barbieri I, Tanomaru-Filho M, et al. Physicochemical properties, cytotoxicity, and penetration into dentinal tubules of sodium hypochlorite with and without surfactants. Restorative Dent Endodont 2020; 45:e47.
- Hülsmann M, Rümmelin C, Schäfers F. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endodont 1997; 23:301-306.
- Abbaszadegana A, Ghahramani Y, Farshada M, et al. In Vitro evaluation of dynamic viscosity, surface tension and dentin wettability of silver nanoparticles as an irrigation solution. Iranian Endod J 2019; 14:23-27.
- Bukiet F, Soler T, Guivarch M, et al. Factors affecting the viscosity of sodium hypochlorite and their effect on irrigant flow. Inter Endod J 2013; 46:954-961.
- Amersham Biosciences. Use of sodium hydroxide for cleaning and sanitizing chromatography media and systems (Code No. 18-1124-57 AD, 2001-06.)
- Kosarieh E, Khavas SS, Rahimi A, et al. The comparison of penetration depth of two different photosensitizers in root canals with and without smear layer: An in vitro study. Photodiagnosis Photodynamic Therapy 2016; 13:10-14.
- Devi RD, Sandhya P, Hari BNV. Poloxamer: A novel functional molecule for drug delivery and gene therapy. J Pharm Sci Res 2013; 5:159–165.
- Kamath P, Kundabala M, Shenoy S, et al. An evaluation of horizontal depth of penetration of various irrigants into the dentinal tubules when used alone and in combination with diode laser: An in vitro study. J Interdisciplinary Dent 2014; 4:130.
- Kucuk M, Kermeoglu F. Efficacy of different irrigation methods on dentinal tubule penetration of Chlorhexidine, QMix and Irritrol: A confocal laser scanning microscopy study. Austr Endod J 2018; 45:202-208.
- Vadhana S, Latha J, Velmurugan N, et al. Evaluation of penetration depth of 2% chlorhexidine digluconate into root dentinal tubules using confocal laser scanning microscope. Restor Dent Endo 2015; 40:149–154.
- Virdee SS, Farnell JJ, Silva MA, et al. The influence of irrigant activation, concentration and contact time on sodium hypochlorite penetration into root dentine: An ex vivo experiments. Inter Endod J 2020; 53:986–997.
- Zou L, Shen Y, Li W, et al. Penetration of sodium hypochlorite into dentin. J Endodo 2010; 36:793-796.
- Faria G, Viola KS, Llerena HG, et al. Penetration of sodium hypochlorite into root canal dentine: effect of surfactants, gel form and passive ultrasonic irrigation. Intern Endod J 2019; 52:385-392.
- Generali L, Campolongo E, Consolo U, et al. Sodium hypochlorite penetration into dentinal tubules after manual dynamic agitation and ultrasonic activation: A histochemical evaluation. Odontology 2018; 106:454-459.
- Khaord P, Amin A, Shah MB, et al. Effectiveness of different irrigation techniques on smear layer removal in apical thirds of mesial root canals of permanent mandibular first molar: A scanning electron microscopic study. J Conservative Dent 2015; 18:321.
Author Info
Thaer A Mukhlif1* and Raghad A Al-Hashimi2
1Department of Esthetic and Restorative Dentistry, Ministry of Health, Al-Anbar Health Director, Iraq2Department of Endodontics, Dean of College of Dentistry, University of Baghdad, Iraq
Citation: Thaer A Mukhlif, Raghad A Al-Hashimi, The Effect of Adding Poloxamer Surfactant on the Penetration Depth of NaOCl and NaOH into Dentinal Tubules, J Res Med Dent Sci, 2021, 9 (1): 200-209.
Received: 01-Dec-2020 Accepted: 23-Dec-2020
