Research - (2022) Volume 10, Issue 8
The Role of Agomelatine against Testicular Tissue Damage Due To Lipopolysaccharide-Induced Sepsis in Rats
Arzu Yalcin1*, Oğuzhan Kavrık1, Ulker Tunca1, Mustafa Saygin1 and Ozlem Ozmen2
*Correspondence: Arzu Yalcin, Suleyman Demirel University, Faculty of Medicine, Department of Physiology, Isparta, Turkey, Email:
Abstract
Background: We investigated role of Agomelatine (AGO) in the sepsis model. Materials and Methods: 24 male Wistar rats (12-month-old, 250-300 g) were used in the study. The study consists of 3 groups. Control group: 0.1 ml normal saline by gavage (single dose) ± 0.1 ml normal saline intraperitoneal (i.p, single dose) to right inguinal region of the rats. LPS group: 0.1 ml normal saline by gavage (single dose) + 5 mg / kg LPS (single dose) i.p to right inguinal region. LPS+AGO group: 5 mg / kg LPS (single dose) i.p to right inguinal region+20 mg/kg AGO (by gavage, single dose, 30 min before the LPS administration). Results: In the testis tissue in LPS+AGO, catalase expression was significantly increased as opposed to that of the control and LPS groups (p<0.05). When MDA activity was analyzed, LPS+AGO group showed a statistically significant decrease compared to LPS and control groups (p<0.05). Diffuse hyperemia, expressions of Caspase-8, Haptoglobin, IL-4, IL-10 were increased in LPS group according to control group (p<0.001). Expressions of Caspase-8, Haptoglobin, IL-4, IL-10 were decreased in AGO-treated group compared to LPS group (p<0.001). Sirtuin-1 expression was increased in AGO-treated group according to LPS group (p<0.001). Conclusion: AGO may reduce sepsis-induced inflammation.
Keywords
Sepsis, Oxidative stress, Agomelatine, Testis, Epididymis
Introduction
Although there has been a number of new treatment methods developed over recent years, sepsis continues as a high-mortality disease. Although the pathophysiology of sepsis has been significantly clarified, there are still many unexplained conditions. Inflammatory diseases are seen as risks to male reproductive function and fertility [1]. However, although such diseases are associated with inimical results on the male reproductive system, the exact mechanisms are still not completely known [2]. Sepsis is described as the systemic inflammatory response of the host against infection. The pathophysiology of sepsis consists of 3 parts: systemic inflammation, coagulation, and fibrinolysis. Coagulopathy in sepsis increases the severity of multiple organ failure and the development of septic shock. Organ perfusion deteriorates, which leads to the advancement of organ insufficiency. Apoptosis and necrosis signals happen during the septic response. The body responds with an incorrectly arranged response as a result of the death of the wrong cells at the wrong moment [3,4]. Lipopolysaccharide (LPS), as an endotoxin obtained from the cell wall of Gram-negative bacteria, results in the suppression of gonadal steroid genesis [1,5]. Many studies have shown that LPS induces the production of inflammatory cytokines through the activation of several mediators [3,6]. In the inflammatory process, high level of ROS production disrupts oxidant-antioxidant balance and leukocytes may be involved. In addition to phagocytosis activity, proinflammatory cytokines are found to support oxidative stress [7,8]. When ROS products exceed the antioxidant enzyme defense line, the damage rate of spermatozoa is increased [9]. Increased cytokines can cause permanent damage to spermatogenesis through germ cell apoptosis. However, its causes and molecular mechanisms are still not well understood. Agomelatin acts as an antidepressant type melatonin receptor agonist and 5-HT receptor antagonist [10]. AGO was developed in the mid-1990s at the Servicer research institute in France to create more effective and safer antidepressant drugs. It is a synthetic analogue of Melatonin, which is synthesized and released in the pineal gland [11]. There are few studies on the protective impact of AGO upon LPS damage. In addition to its antidepressant effect, it is thought to have an ability to change specific elements of the immune response during an inflammatory process which was shown in studies on the rat brain [12,13]. In a study of diabetic rats, it was stated that AGO could be used as a potential ant fibrotic and anti-inflammatory agent. The mechanism herein is thought to be capable of inhibiting the production of pro-inflammatory cytokines by blocking TNF-alpha [14]. However, the mechanisms of LPS-induced damage to the genitals and the efficacy of AGO are still awaiting to be clarified.
In this study, the effect of sepsis on the testicular tissue and the role of AGO in the experimental model of sepsis induced by LPS were investigated.
Material and Methods
Animals
The Animal Experiments Local Ethics Committee of Mehmet Akif Ersoy University, Burdur, Turkey approved the procedures performed on rats in the current study (02.08.2017/307). The rooms in the Experimental Animal Production and Experimental Research Laboratory were used to monitor the housing, feeding, and care of the rats during the study.
Study procedure
A total of 24 male Wistar albino rats, weighing 250–300 g, were kept in cages under regular lighting conditions (12/12 h light cycle with light period from 7:00 to 19:00), temperature 24-26°C, and given ad libitum water and standard rat pellet food. Rats were randomly distributed in three groups (n=8) as below:
Control group: 0.1 ml normal saline by gavage (single dose) + 0.1 ml normal saline intraperitoneal (i.p, single dose) to right inguinal region of the rats
LPS group: 0.1 ml normal saline by gavage (single dose) + 5 mg / kg LPS (single dose, 048 K4126, Sigma Aldrich, Sweden) i.p to right inguinal region [15].
LPS+AGO group: 5 mg / kg LPS (single dose) i.p to right inguinal region + 20 mg / kg AGO (Valdoxan, Servier, Turkey) (by gavage, single dose, 30 min before the LPS administration). AGO dissolved in normal saline [11].
Animals were sacrificed 6 hours after LPS administration under xylazine (10 mg/kg) and ketamine (90 mg/kg) anaesthesia. One of the testicular and epididymis tissues were removed and stored -80 0C for the analyses of malondialdehit (MDA), superoxide dismutase (SOD) and catalase (CAT). Other the testicular and epididymis tissues were fixed in 10% buffered formalin for histopathological and immunohistochemical analyses to evaluate the expression of Cas-8, Haptoglobulin, IL-4, IL- 10 and SIRT-1.
Biochemical analyses
Testicular and epididymal tissues were placed in phosphate buffer (pH 7.4) and homogenized by a tissue homogenizer (IKA Ultra‐Turrax T25 Basic; Labortechnik). Afterward, the homogenate was centrifuged at 10,000 rpm for 10 min at +4°C. MDA levels were estimated by the double‐heating way of Draper and Hadley. 16 Because it seems to offer an easy means of estimating lipid peroxidation in biological substances. Tissue samples trichloroacetic acid (TCA) was then mixed with thiobarbituric acid (TBA) as supernatant. The absorbance of the samples was determined at 532 nm by a spectrophotometer (Shimadzu UV‐1601). The results were presented as μmol/mg protein in the testicles and epididymides. SOD was determined by a kinetic assay. The assay depends on the competitive inhibition by superoxide dismutase of the reaction between superoxide, produced in the aerobic oxidation of xanthine by xanthine oxidase and a tetrazolium reagent, 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyltetrazolium chloride. It converts the superoxide radical of superoxide dismutase enzyme in aerobic cells to hydrogen peroxide and molecular oxygen. It is used as an antioxidant enzyme which is one of the oxidative stress markers. The SOD activity was determined on a spectrophotometer (Shimadzu UV - 1601) at 560 nm and enzyme activity results were presented as U / mg protein [16,17]. CAT enzyme catalyzes the detoxification of hydrogen peroxide to water and oxygen. Therefore, hydrogen peroxide produced in the cell was removed to hinder the production of the hydroxyl free radical. The decomposition rate of substrate H2O2 is monitored spectrophotometrically at 240 nm for 30 seconds [18]. The results were presented as μ/mg protein.
Histopathological examinations
Testis and epididymis samples were placed in a fixative which stabilizes the tissues to prevent decay (10% neutral buffered formalin). Then, samples were routinely prepared by an automatic tissue processor (Leica ASP300S, Wetzlar, Germany), were fixed in paraffin, and were sectioned to 5-μm thickness (Leica Microsystems, Wetzlar, Germany). After staining with hematoxylineosin (HE), sections were observed under a light microscope. Histopathological changes were graded by a pathologist. Quantitative changes were detected by counting totally visible heads of the mature spermatozoa in randomly selected 10 seminiferous tubules from each rat under a 40X objective.
Immunohistochemical examinations
Next histopathological examination, serial portions of testis samples were immunostained with active caspase-8 [Anti Caspase-8 (ab25901)], haptoglobin [anti-haptoglobin antibody (ab135835)], interleukin-4 [Anti-IL4 antibody, (ab6922)], interleukin-10 [Anti- IL10 antibody, (ab34843)], and sirtuin-1 [Anti-SIRT1 antibody [E104] ab32441)] by the streptavidin-biotin technique. Every primary and secondary antibody used in this study were bought from Abcam (Cambridge, UK). To immunohistochemical procedures, following deparaffinization and rehydration, a hydrogen peroxide solution was implemented to the tissues. Subsequently, the sections were incubated with the primary antibodies for 20 min, followed by the protein block for 60 min, respectively. Immunohistochemistry was performed with a 20-minute biotinylated secondary antibody and streptavidin alkaline phosphatase conjugate. EXPOSE Mouse and Rabbit Specific HRP/DAB Detection IHC kit (ab80436) was utilized as a secondary antibody. To negative controls, the primary antiserum step was eliminated. All tests were performed by a pathologist. To assess the percentage of positively immnuostained cells for each marker, the cells from each group were counted in 10 different areas at a 40X magnification. The results were obtained from the image analyzer and statistically analyzed. Morphometric reports were performed utilizing the Database Manual CellSens Life Science Imaging Software (Olympus Co. Tokyo, Japan). SPSS software (version 15.0, SPSS Inc., Chicago, IL, USA) was applied to analyze the immunopositive cell numbers. The variables were evaluated by the Bonferroni test, and the ANOVA test was used to compare groups. The value of p<0.05 was considered statistically significant.
Statistical analyses
Statistical analyses were conducted using SPSS 22.0 (Chicago IL, USA) for Windows. Descriptive statistics of the groups are shown as mean ± standard deviation (X ± SD). The Kolmogorov-Simirnov test was used previous to the evaluation to determine whether the data showed normal distribution. Parametric tests (ANOVA) were performed on the groups in which the characteristics of the investigated properties were normal. A p value <0.05 was considered statistical significant within 95% confidence interval.
Results
Biochemical results
In the testes tissue, CAT activity was statistically significant in LPS+AGO group compared to control and LPS groups (respectively, p=0.005, p=0.016). SOD activity was statistically significant in LPS+AGO group compared to LPS groups (Table 1).
| Groups | MDA (µmol/mg protein) | CAT (ku/mg protein) | SOD (U/mg protein) |
|---|---|---|---|
| Control | 3.40 ± 1.18 | 4.00 ± 1.61 | 19.98 ± 1.83 |
| LPS | 4.53 ± 1.80 | 3.18 ± 0.73 | 15.03 ± 2.36 |
| LPS+AGO | 4.25 ± 1.78 | 7.26 ± 1.65ab | 24.94 ± 8.96b |
| Values are presented as means ± SD. The relationships between groups and results of biochemical markers are assessed by one-way ANOVA (LPS: Lippolysaccaride, Ago: Agomelatine, MDA: Malondaldehyde SOD: Super Oxite Dismutase; CAT: Catalase). | |||
| aStatistically significant compared the control group (p<0.05) | |||
| bStatistically significant compared to the LPS group (p<0.05) | |||
Table 1: Oxidative stress parameters of testes tissue.
The increase in MDA level in the LPS group was not found to be significant compared to the control. In the epididymal tissue, MDA level increased significantly in the LPS group compared to the control group (p=0.03). MDA level was significantly decreased in LPS+AGO group compared to LPS group and control group (respectively, p=0.001, p=0.005). CAT and SOD enzyme activities were not statistically significant (p>0.05) (Table 2).
| Groups | MDA (µmol/mg protein) | CAT (ku/mg protein) | SOD (U/mg protein) |
|---|---|---|---|
| Control | 8.27 ± 0.45 | 1.11 ± 0.54 | 15.46 ± 2.84 |
| LPS | 11.53 ± 1.66a | 0.98 ± 0.37 | 15.97 ± 1.39 |
| LPS+AGO | 4.05 ± 1.75ab | 1.57 ± 0.51 | 19.58 ± 8.09 |
| Values are presented as the mean ± SD, n=8. The relationships between groups and results of biochemical markers are assessed by one-way ANOVA. (LPS: Lippolysaccaride, Ago: Agomelatine, MDA: Malondaldehyde SOD: Super Oxite Dismutase; CAT: Catalase). | |||
| aStatistically significant compared the control group (p <0.05) | |||
| bStatistically significant compared to the LPS group (p <0.05) | |||
Table 2: Oxidative stress parameters of epididymis tissue.
Histopathological results
Necropsy revealed marked hyperemia in testes and epididymides of rats belonging to LPS-treated group, while in other groups, testes and epididymides were normal (Figure 1).
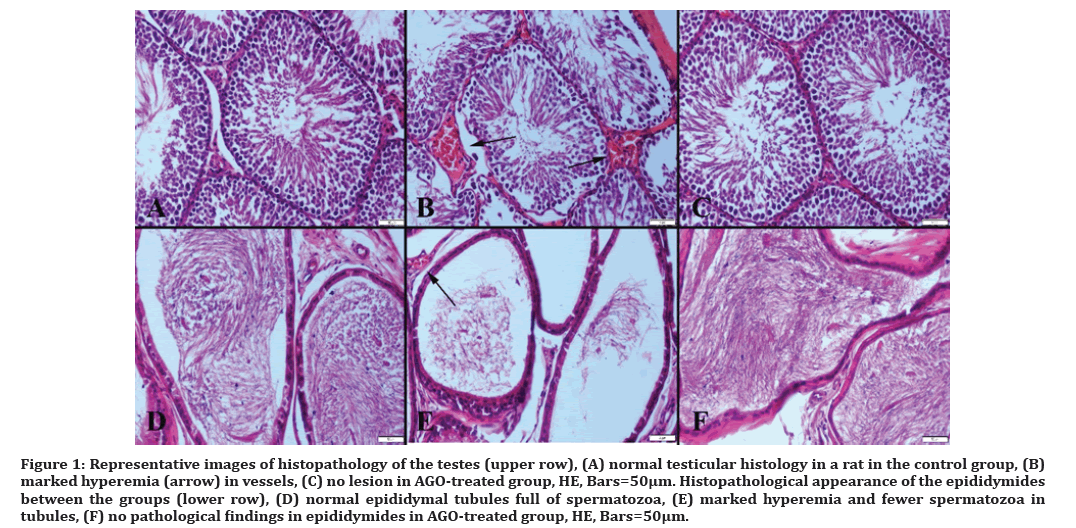
Figure 1: Representative images of histopathology of the testes (upper row), (A) normal testicular histology in a rat in the control group, (B) marked hyperemia (arrow) in vessels, (C) no lesion in AGO-treated group, HE, Bars=50µm. Histopathological appearance of the epididymides between the groups (lower row), (D) normal epididymal tubules full of spermatozoa, (E) marked hyperemia and fewer spermatozoa in tubules, (F) no pathological findings in epididymides in AGO-treated group, HE, Bars=50μm.
Degenerative and, in some rats, necrotic Sertoli and Leydig cells were observed in testes, a notable reduction in sperm concentration was shown in cauda epididymis, and most of the tubules contained a little number of spermatozoa in LPS group compared to the control group. Spermatid density in seminiferous tubules of testes showed a more signed decrease compared to the other groups. In AGO group, the treatment clearly ameliorated histopathology in testes induced by LPS.
Immunohistochemical results
The results of immunohistochemical examination in all groups are shown in Table 3.
| Control | LPS | AGO | p value | |
|---|---|---|---|---|
| Caspase-8 | 2.14 ± 1.34 | 11.57 ± 1.71a | 0.60 ± 0.26b | CON-LPS<0.001 |
| CON-AGO>0.05 | ||||
| LPS-AGO<0.001 | ||||
| Haptoglobin | 0.42 ± 0.29 | 10.57 ± 1.71a | 0.30 ± 0.21b | CON-LPS<0.001 |
| CON-AGO>0.05 | ||||
| LPS-AGO<0.001 | ||||
| IL-4 | 0.28 ± 0.18 | 6.14 ± 1.95a | 0.20 ± 0.13b | CON-LPS<0.001 |
| CON-AGO>0.05 | ||||
| LPS-AGO<0.001 | ||||
| IL-10 | 0.71 ± 0.35 | 15.71 ± 4.46a | 0.60 ± 0.26b | CON-LPS<0.001 |
| CON-AGO>0.05 | ||||
| LPS-AGO<0.001 | ||||
| SIRT-1 | 3.28 ± 2.62 | 0.14 ± 0.14a | 5.20 ± 2.14b | CON-LPS<0.05 |
| CON-AGO>0.05 | ||||
| LPS-AGO<0.001 |
Table 3: Statistical analysis results of immunohistochemistry scores.
Values presented as mean ± SD. The relationship between groups and results are assessed by One-way ANOVA Bonnferroni test. ap <0.05 when compared to the control group. bp <0.05 when compared to the LPS group.
Immunohistochemical analysis showed that LPS administration caused a marked increase in caspase-8, haptoglobin, IL-4, and IL-10 expressions along with a decrease in SIRT-1 immunoreaction in testes. AGO treatment improved all immunohistochemical findings (Figures 2-6). The sperm count in all groups is presented in (Figure 7).
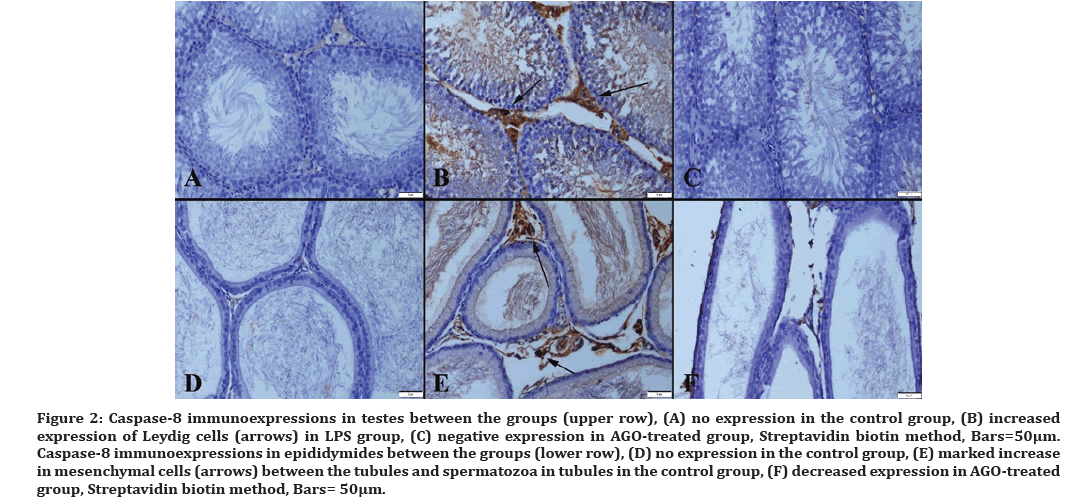
Figure 2: Caspase-8 immunoexpressions in testes between the groups (upper row), (A) no expression in the control group, (B) increased expression of Leydig cells (arrows) in LPS group, (C) negative expression in AGO-treated group, Streptavidin biotin method, Bars=50µm. Caspase-8 immunoexpressions in epididymides between the groups (lower row), (D) no expression in the control group, (E) marked increase in mesenchymal cells (arrows) between the tubules and spermatozoa in tubules in the control group, (F) decreased expression in AGO-treated group, Streptavidin biotin method, Bars= 50μm.
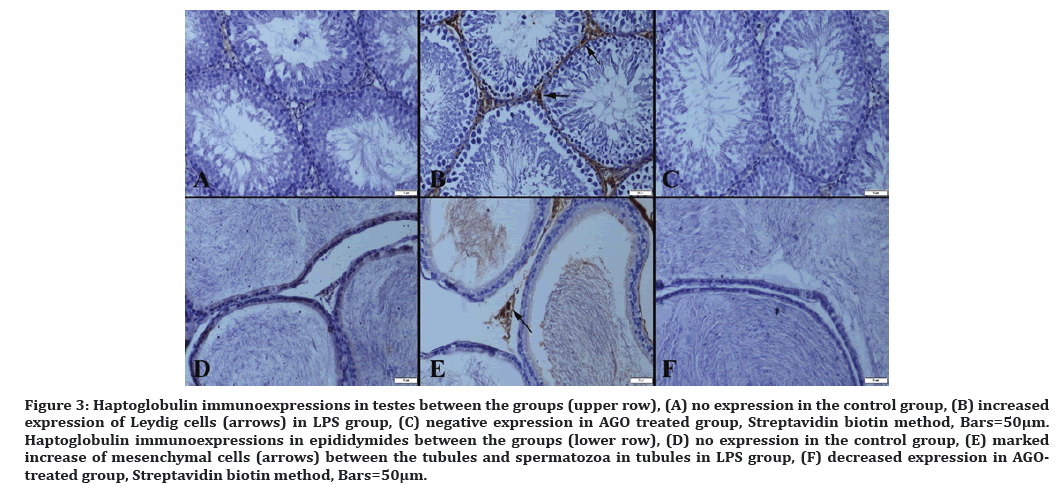
Figure 3: Haptoglobulin immunoexpressions in testes between the groups (upper row), (A) no expression in the control group, (B) increased expression of Leydig cells (arrows) in LPS group, (C) negative expression in AGO treated group, Streptavidin biotin method, Bars=50µm. Haptoglobulin immunoexpressions in epididymides between the groups (lower row), (D) no expression in the control group, (E) marked increase of mesenchymal cells (arrows) between the tubules and spermatozoa in tubules in LPS group, (F) decreased expression in AGOtreated group, Streptavidin biotin method, Bars=50μm.
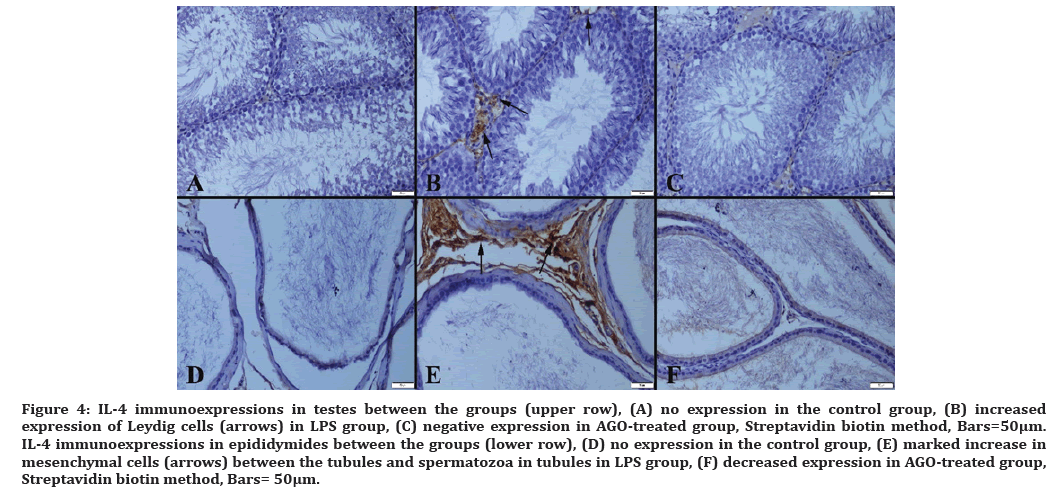
Figure 4: IL-4 immunoexpressions in testes between the groups (upper row), (A) no expression in the control group, (B) increased expression of Leydig cells (arrows) in LPS group, (C) negative expression in AGO-treated group, Streptavidin biotin method, Bars=50µm. IL-4 immunoexpressions in epididymides between the groups (lower row), (D) no expression in the control group, (E) marked increase in mesenchymal cells (arrows) between the tubules and spermatozoa in tubules in LPS group, (F) decreased expression in AGO-treated group, Streptavidin biotin method, Bars= 50μm.
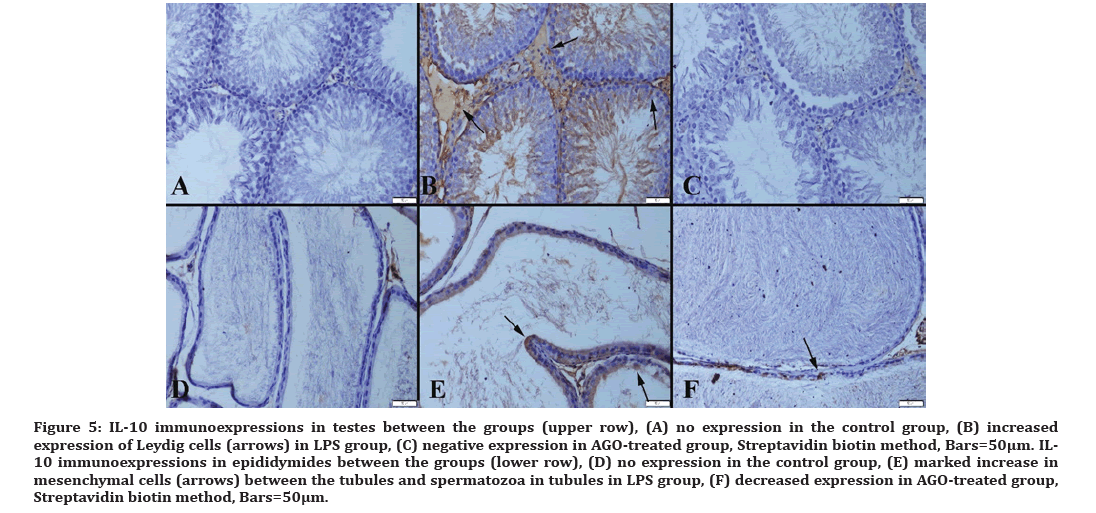
Figure 5:IL-10 immunoexpressions in testes between the groups (upper row), (A) no expression in the control group, (B) increased expression of Leydig cells (arrows) in LPS group, (C) negative expression in AGO-treated group, Streptavidin biotin method, Bars=50µm. IL- 10 immunoexpressions in epididymides between the groups (lower row), (D) no expression in the control group, (E) marked increase in mesenchymal cells (arrows) between the tubules and spermatozoa in tubules in LPS group, (F) decreased expression in AGO-treated group, Streptavidin biotin method, Bars=50μm.
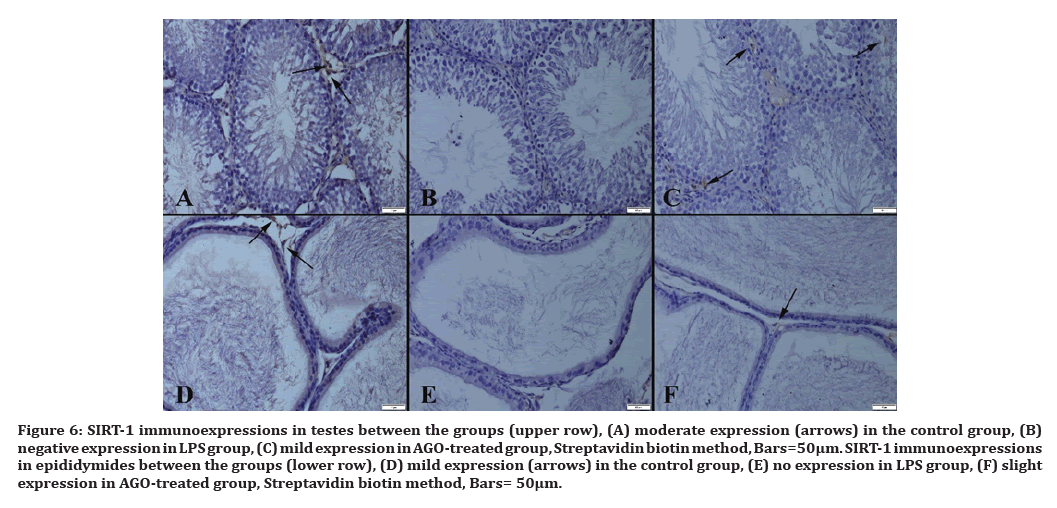
Figure 6:SIRT-1 immunoexpressions in testes between the groups (upper row), (A) moderate expression (arrows) in the control group, (B) negative expression in LPS group, (C) mild expression in AGO-treated group, Streptavidin biotin method, Bars=50µm. SIRT-1 immunoexpressions in epididymides between the groups (lower row), (D) mild expression (arrows) in the control group, (E) no expression in LPS group, (F) slight expression in AGO-treated group, Streptavidin biotin method, Bars= 50μm.
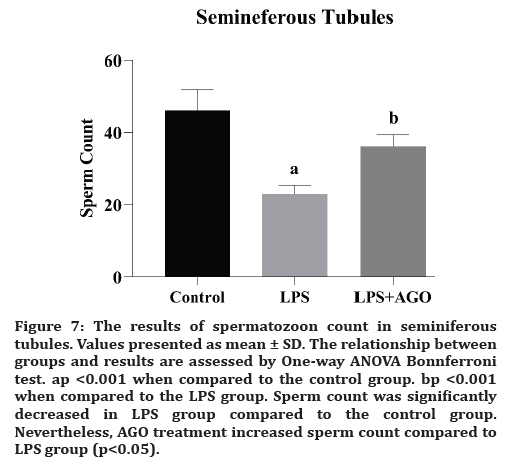
Figure 7:The results of spermatozoon count in seminiferous tubules. Values presented as mean ± SD. The relationship between groups and results are assessed by One-way ANOVA Bonnferroni test. ap <0.001 when compared to the control group. bp <0.001 when compared to the LPS group. Sperm count was significantly decreased in LPS group compared to the control group. Nevertheless, AGO treatment increased sperm count compared to LPS group (p<0.05).
Discussion
In our study, we demonstrated the protecting effects of AGO application against testicle and epididymal damage caused by LPS using immunohistochemical parameters. The changes caused by oxidative stress made us think that the damage caused by LPS can be reduced with AGO application. Histopathological findings and sperm count supported the results.
Adel RA Abd-Allah et al., presented the evidence that LPS-induced testicular injury may affect sperm motility, vital signs of oxidative stress, and inflammatory markers. The deterioration of the functions after oxidative stress activates the immune system [7] The studies aimed at investigating the blood-testis barrier suggested its protective effects against serious infections [7,19]. In our study, sperm count was significantly decreased in LPS group compared to the control group. But, AGO application increased sperm count compared to LPS group. We can say that there is a connection between inflammation and sperm count.
The relationship between reactive oxygen derivatives and sperm damage following endotoxin exposure was reported [20]. In their study, the inflammation pathway in the testis tissue was investigated in LPSinduced sepsis model. It clarified the relationship of TLR3 (Toll-like receptor 3) with the innate immune system [21]. According to the information obtained on the outcomes of apoptosis as an indicator of acute phase of endotoxemia, caspase-3 activity was found to be important [22]. Inflammation caused by bacterial lipopolysaccharide (LPS) leads to testicular tissue damage due to infection. Caspase-3, one of the stress response proteins, has been reported to induce acute stress following the deterioration of oxidant-antioxidant balance due to inflammation [23]. In in vitro sepsis study, which was aimed to reduce the effects of acute pro-inflammatory cytokine and caspase 3 and 8, the authors mentioned that caspase 3 and 8 are important in the acute phase of sepsis [24] In our study, caspase 8 activity was examined to show the formation of damage disrupting the blood-testis barrier. Immunohistological findings suggest that caspase-8 activity in sepsis can be reduced with AGO.
Proinflammatory cytokines lead to endothelial damage and sperm dysfunction [25]. Liu et al. in their study of LPS-induced changes in the expression of sepsis-induced inflammatory genes in the testicular tissue, examined IL-10 and IL-6 proinflammatory cytokine changes and showed that beta-cryptoxantine had a therapeutic effect [26]. In a study by Zhou et al. which used lipoxin A4 as a prophylaxis against testicular injury, IL-4 and IL-10 were used to monitor changes in cytokine modulation. Its additional supplementation against oxidative stress has been announced to improve the expressions of IL-4 and IL-10, reducing apoptosis and preventing damage [27]. Infection and autoimmune-induced testicular inflammation were studied in the research that provides information on T-cells as a marker of inflammation. When various cytokines were examined, IL-4 and IL-10 were found important, moreover, IL-10 was found to have important functions in certain pathways [28]. Overall, IL-4 and IL-10 have been studied extensively as the indicators of infection, and additional supplementation group given in the studies [29-31]. T cells do not produce cytokines such as interleukin-2, 4 and 7 when they are inactive. IL-4, which is considered to be one of the innate immunity mediators, is taking an important position among IL-4 proinflammatory cytokines released from activated T cells. In our study, we demonstrated the reduction of cytokines expressions by AGO which proved that AGO reduces testicular tissue damage. Our findings correspond to those of the studies mentioned above.
Haptoglobin is produced in response to IL-1, IL-6 and TNF-alpha cytokines as the acute phase protein. Inflammation is said to trigger hepatocytes with an acute phase response. As a result, haptoglobin is transported through the bloodstream to the site of infection for a natural immune response and tissue remodeling. Pain may be a symptom of an inflammatory response. In addition, haptoglobin is a specific indicator of stress induced by an inflammatory response to the tissue injury and castration [32]. In one study, castration did not affect serum cortisol and haptoglobin, there was no inflammation or infection [33]. There is no data on the association of sepsis with haptoglobin. But sepsis is caused by inflammation, inflammation environment. In our study, we first used haptoglobin as a marker of testicular tissue damage and demonstrated its increase in LPS group.
Studies on resveratrol against testicular damage, show that SIRT1 is important in reducing apoptosis. It is a known fact that SIRT1 activity decreases with age. It has been reported that stimulating p53 pathway by resveratrol may protect against reproductive toxicity by suppressing p53 and Ku70 acetylation [34]. In another study, germ cell-specific SIRT1 abolished the protective effects of CTRP3 in vivo and in vitro. The researchers reported that CTRP3, which has anti-apoptotic effects, increases sperm count, sperm viability and sperm motility. In addition, CTRP3 infusion has been reported to be associated with decreased endoplasmic reticulum stress and activation of SIRT1 [35]. It was found that red ginseng extract improves SIRT1 activity in testes of aging rats. SIRT1 is one of spermatogenesis-related key biomolecules that decreases its activity with aging, but it is possible to attenuate this change [36]. Histological results in our study showed that SIRT1 activity was reduced by inflammation in LPS group. AGO reversed this LPS-induced reduction. In this study, which correlates with the medical literature, we first immuno histochemically demonstrated that SIRT1 protein, which is associated with many functions, increases with AGO supplementation. In addition, a decrease in sperm count in LPS group changed to an increasing tendency with AGO.
Aly et al. investigated the potential toxicity of LPS and the protective effect of lycopene. They showed that lycopene may inhibit testicular degeneration, increase sperm motility and morphology, and support the antioxidant characterization of the testicular tissue [37]. In the study of AGO and gallic acid supplementation against testicular injury in diabetic rat testes, Yigitturk et al. found that AGO is a potentially useful agent against testicular damage due to its ability to reduce oxidative stress. Nevertheless, AGO has a poor therapeutic effect [14]. Studies of the harmful effect of LPS and its amelioration with AGO are so few that there is no specific study on the testicular tissue. In several studies of antidepressant-like effect of AGO, it was used as a preventive treatment against LPSinduced inflammation. Related studies have generally focused on the brain and behavior [12,13,38,39]. When we look at the findings of oxidative stress markers in our study, they do not provide sufficient information. While an increase in CAT expression in the testicular tissue was significant after AGO treatment, no significant expressions were seen for other markers. In the epididymal tissue, MDA expression was statistically significant, while other markers were not significant. We suggest that AGO supplementation cannot be a sufficient therapeutic agent for the testicular tissue. Histological findings revealed that sepsis occurred, but the provided AGO supplement was either insufficient or not strong in terms of efficacy.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Financial Disclosure
No funding sources.
References
- Hales DB, Diemer T, Hales KH. Role of cytokines in testicular function. Endocrine 1999; 10:201-217.
- Pacey AA, Eley A. Chlamydia trachomatis and male fertility. Hum Fertil 2004; 7:271-276.
- Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, et al. The pathogenesis of sepsis. Annu Rev Pathol 2011; 6:19-48.
- Cunneen J, Cartwright M. The puzzle of sepsis: Fitting the pieces of the inflammatory response with treatment. AACN Clin Issues 2004; 15:18-44.
- Nishio K, Horie M, Akazawa Y, et al. Attenuation of lipopolysaccharide (LPS)-induced cytotoxicity by tocopherols and tocotrienols. Redox Biol 2013; 1:97-103.
- Rossol M, Heine H, Meusch U, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol 2011; 31:379-446.
- Abd-Allah AR, Hela GK, Al-Yahya A., et al. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxidative Med Cellular Longevity 2009; 2:73-81.
- Sanocka D, Fraczek M, Jedrzejczak P, et al. Male genital tract infection: an influence of leukocytes and bacteria on semen. J Reprod Immunol 2004; 62:111-124.
- Fraczek M, Kurpisz M. The redox system in human semen and peroxidative damage of spermatozoa. Adv Hygiene Exp Med 2005; 59:523-534.
- Demir Ozkay U, Soztutar E, Can OD, et al. Effects of long-term agomelatine treatment on the cognitive performance and hippocampal plasticity of adult rats. Behav Pharmacol 2015; 26:469-480.
- Karakus E, Halici Z, Albayrak A, et al. Agomelatine: An antidepressant with new potent hepatoprotective effects on paracetamol-induced liver damage in rats. Human Exp Toxicol 2013; 32:846-857.
- Molteni R, Macchi F, Zecchillo C, et al. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur Neuropsychopharmacol 2013; 23:1645-1655.
- Inanir S, Copoglu US, Kokacya H, et al. Agomelatine protection in an LPS-induced psychosis-relevant behavior model. Med Sci Monit 2015; 21:3834-3839.
- Yigitturk G, Acara AC, Erbas O, et al. The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomed Pharma 2017; 87:240-246.
- Samuvel D, Shunmugavel A, Singh A, et al. S-Nitrosoglutathione ameliorates acute renal dysfunction in a rat model of lipopolysaccharide-induced sepsis. J Pharm Pharmacol 2016; 68:1310–1319.
- Drapper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990; 186:421–431.
- Woolliams JA, Wiener G, Anderson PH, et al. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood various breed crosses of sheep. Res Veterinary Sci 1983; 34:69–77.
- Aebi H. Catalase in Vitro. Methods Enzymology 1984;105, 121–126.
- Klein B, Haggeney T, Fietz D, et al. Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum Reprod 2016; 31:2192-2202.
- Urata K, Narahara H, Tanaka Y, et al. Effect of endotoxin-induced reactive oxygen species on sperm motility. Fertil Steril 2001; 76:163-166.
- Nejsum LN, Piec A, Fijak M, et al. Systemic LPS induces toll-like receptor 3 (TLR3) expression and apoptosis in testicular mouse tissue. Cell Tissue Res 2019; 378:143-154.
- Inoue T, Aoyama-Ishikawa M, Kamoshida S, et al. Endogenous interleukin 18 regulates testicular germ cell apoptosis during endotoxemia. Reproduction 2015; 150:105-114.
- Metukuri MR, Reddy CM, Reddy PR, et al. Bacterial LPS-mediated acute inflammation-induced spermatogenic failure in rats: Role of stress response proteins and mitochondrial dysfunction. Inflammation 2010; 33:235-243.
- Bordoni V, Bolgan I, Brendolan A, et al. Caspase-3 and -8 activation and cytokine removal with a novel cellulose triacetate super-permeable membrane in an in vitro sepsis model. Int J Artif Organs 2003; 26:897-905.
- Omu AE, Al-Azemi MK, Al-Maghrebi M, et al. Molecular basis for the effects of zinc deficiency on spermatogenesis: An experimental study in the Sprague-dawley rat model. Indian J Urol 2015; 31:57-64.
- Liu XR, Wang YY, Dan XG, et al. Anti-inflammatory potential of β-cryptoxanthin against LPS-induced inflammation in mouse Sertoli cells. Reprod Toxicol 2016; 60:148-55.
- Zhou XL, Yang QS, Ni SZ, et al. Protective effects of lipoxin A4 in testis injury following testicular torsion and detorsion in rats. Mediators Inflamm 2014; 898056.
- Mukasa A, Yoshida H, Kobayashi N, et al. Gamma delta T cells in infection-induced and aut oimmune-induced testicular inflammation. Immunology 1998; 95:395-401.
- Yang R. Effect of toxoplasma gondii infection on cytokines and spermatogenic cells in rats. Chinese J Parasitol Parasitic Dis 2011; 29:274-278.
- Rodriguez-Dorantes M, Lopez-Griego L, Zarazua-Cruz CM, et al. Altered expression of cytokines and sex steroid receptors in the reproductive tract of cysticercotic male mice. Parasite Immunol 2010; 32:91-100.
- Al-Bader A, Omu AE, Dashti H. Chronic cadmium toxicity to sperm of heavy cigarette smokers: Immunomodulation by zinc. Arch Androl 1999; 43:135-140.
- Ball JJ, Kegley EB, Lawrence TE, et al. Zinc injection as a novel castration method in beef bulls: effects on performance, behavior, and testosterone and haptoglobin concentration. J Anim Sci 2018; 96:890-901.
- Marti S, Velarde A, de la Torre JL, et al. Effects of ring castration with local anesthesia and analgesia in Holstein calves at 3 months of age on welfare indicators. J Anim Sci 2010; 88:2789-2796.
- Liu H, Zhang S, Liu C, et al. Resveratrol ameliorates microcystin-LR-induced testis germ cell apoptosis in rats via SIRT1 signaling pathway activation. Toxins 2018; 10:e235.
- Mu Y, Yin TL, Yin L, et al. CCTRP3 attenuates high-fat diet-induced male reproductive dysfunction in mice. Lin Sci 2018; 132:883-899.
- Kopalli SR, Cha KM, Ryu JH, et al. Korean red ginseng improves testicular ineffectiveness in aging rats by modulating spermatogenesis-related molecules. Exp Gerontol 2017; 90:26-33.
- Aly HA, El-Beshbishy HA, Banjar ZM. Mitochondrial dysfunction induced impairment of spermatogenesis in LPS-treated rats: Modulatory role of lycopene. Eur J Pharmacol 2012; 677:31-38.
- Kalkman HO, Feuerbach D. Antidepressant therapies inhibit inflammation and microglial M1-polarization. Pharmacol Ther 2016; 163:82-93.
- Rossetti AC, Paladini MS, Racagni G, et al. Genome-wide analysis of LPS-induced inflammatory response in the rat ventral hippocampus: Modulatory activity of the antidepressant agomelatine. World J Biol Psychiatr 2018; 19:390-401.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Arzu Yalcin1*, Oğuzhan Kavrık1, Ulker Tunca1, Mustafa Saygin1 and Ozlem Ozmen2
1Suleyman Demirel University, Faculty of Medicine, Department of Physiology, Isparta, Turkey2Mehmet Akif Ersoy University, Faculty of Veterinary Medicine, Department of Pathology, Burdur,, Turkey
Citation: Arzu Yalcin, O?uzhan Kavr?k, Ulker Tunca, Mustafa Saygin, Ozlem Ozmen, The Role of Agomelatine against Testicular Tissue Damage Due To Lipopolysaccharide-Induced Sepsis in Rats, J Res Med Dent Sci, 2022, 10 (8):65-82.
Received: 03-Aug-2022, Manuscript No. jrmds-22-71085; , Pre QC No. jrmds-22-71085(PQ); Editor assigned: 04-Aug-2022, Pre QC No. jrmds-22-71085(PQ); Reviewed: 18-Aug-2022, QC No. jrmds-22-71085; Revised: 23-Aug-2022, Manuscript No. jrmds-22-71085(R); Published: 30-Aug-2022
