Research - (2021) Volume 9, Issue 4
To Study the Effect of Dexmedetomidine Administered as Bolus Vs. as BolusPlus-Infusion on Subarachnoid Anaesthesia with Hyperbaric Bupivacaine in Lower Limb Orthopaedic Surgery
Mayuri Golhar, Manisha Yadav, Nandita Kad and Tarun Yadav*
*Correspondence: Tarun Yadav, Department of Anaesthesiology, Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, India, Email:
Abstract
Spinal anaesthesia is the regional anaesthesia obtained by blocking the spinal nerves in the subarachnoid space. It is a preferred method as it reduces the risk of vomiting and pulmonary aspiration in patients with full stomach along with that it is useful in patients with chronic airway diseases.
Keywords
Spinal anaesthesia, Orthopaedic surgery, Lower limb
Introduction
Spinal anaesthesia is the regional anaesthesia obtained by blocking the spinal nerves in the subarachnoid space. It is a preferred method as it reduces the risk of vomiting and pulmonary aspiration in patients with full stomach along with that it is useful in patients with chronic airway diseases [1].
Effective post-operative pain management is essential to facilitate proper rehabilitation and acceleration of the return to functional capability [2]. Post-operative pain relief can be achieved by various methods namely systemic opioid and non-opioid peripheral nerve blocks and local wound infiltration, each with their own merits and demerits
A number of adjuvants have been added to spinal local anaesthetics e.g. opioids like morphine, buprenorphine, fentanyl [3]. These opioids produce satisfactory analgesia for 24 hours postoperatively, but are frequently associated with side effects like respiratory depression, itching, nausea, vomiting and urinary retention. Selective Alpha2-agonist like dexmedetomidine has been used as adjuvant by intrathecal, epidural, intravenous route [4].
Dexmedetomidine is a selective α2-adrenoceptor agonist with sedative, anxiolytic, sympatholytic, and analgesic-sparing effects. It potentiates the effect of local anaesthetics and prolongs the duration of both motor, sensory spinal blockade and postoperative analgesia [5] So, we conducted this study to study the effects of intravenous dexmedetomidine administered as bolus-versus-bolus plus infusion on characteristics of intrathecal hyperbaric bupivacaine in lower limb orthopaedic surgery.
Materials and Methods
After approval of the Institutional Ethics Committee one hundred fifty consenting patients in the age group of 18-50 years, belonging to ASA physical status I and II scheduled for lower limb orthopaedic surgeries were enrolled in this study prospective, randomized, double-blind study. Patients were explained the purpose and protocol of study and informed and written consent to participate in study was obtained. A detailed preoperative evaluation was performed on the day before surgery, and standard fasting orders were advised.
All patients were monitored for automated noninvasive blood pressure, pulse oximetry and electrocardiogram. All patients received 2.5 ml of 0.5% hyperbaric bupivacaine intrathecally. Patients were randomly allocated on the basis of a sealed envelope technique into three groups with fifty patients in each group irrespective of gender.
The study drug was made to 20 ml of final volume and was administered as an intravenous infusion over 10 minutes, starting 10 min from the subarachnoid block.
Sample size was calculated based on work of previous researcher kavya [6,7] with an effect size of 0.55, a power of 80%, an α of 0.05. A minimum of 50 patients were included in each group.
Group A patients received 12.5mg intrathecal bupivacaine followed by single dose of intravenous dexmedetomidine 1μg kg-1 over 10 minutes and then intravenous normal saline. Group B patient received 12.5mg intrathecal bupivacaine followed by 1μg kg-1 of dexmedetomidine intravenously over 10 minutes and then intravenous dexmedetomidine 0.4μg kg-1hr-1 till end of surgery and group C (Control group) received an equivalent amount of normal saline.
Following administration of drugs time of administration of block, highest level of sensory block in segments, time to achieve highest level of sensory block, degree of motor block by Bromage scale prior to surgery, time of start of surgery, time to achieve 2 segment regression of sensory blockade, time of completion of surgery, time of regression of motor block Bromage score zero), time of complete regression of sensory block ( S2 dermatome), need of rescue analgesic i.e. diclofenac injections post-op, total duration of surgery, total duration of motor block , total duration of sensory block, time to void, any other complications (dizziness, fatigue, shivering, tremors, headache, hypotension, bradycardia etc.), intraoperative sedation score and other parameters such as heart rate, respiratory rate, blood pressure,SpO2 etc. were recorded.
Statistical testing was done using Statistical Package for Social Sciences (SPSS) version 21.0. Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD. Quantitative variables were compared using Independent t test between the two groups and ANOVA between three groups. Qualitative variables were compared using Chi-Square test/Fisher’s Exact test. For all statistical tests, a p value of less than 0.05 was taken to indicate a statistically significant difference.
Results
Demographic profile
Demographic parameters age, gender, height, weight, ASA grade and duration of study were comparable. No statistically significant difference was seen between group A, B and C. (p value>0.05)
Characteristics of sensory block
Onset of sensory blockade:
No significant difference was seen in onset of sensory blockade at T10 (seconds) between group A, B and C. (p value >.05) Mean ± SD of onset of sensory blockade at T10 (seconds) in A was 141.6 ± 41.32, B was 137.6 ± 42.31 and C was 148.8 ± 45.34 with no statistically significant difference between them.
Time for attaining highest level of sensory block
Significant difference was seen in time for attaining highest level of sensory block (seconds) between group A, B and C. (p value <.05) Mean ± SD of time for attaining highest level of sensory block (seconds) in A was 334.6 ± 71 and B was 338±76.5 which was significantly lower as compared to C that was 373.4 ± 78.03. No significant difference was seen in time for attaining highest level of sensory block (seconds) between A and B. Time to reach maximum sensory level is not statistically significant between the group A and group B.
Maximum height of sensory block
No statistically significant difference was seen in the distribution of maximum height of sensory block between group A as compared to group B. (p value>.05) Proportion of patients with maximum height of sensory block: T4, T6 and T8 in group A was 14.00%, 86.00% and 0.00% respectively which was comparable with group B (12.00%, 88.00% and 0.00% respectively) with no statistically significant difference in distribution between them. Statistically significant difference was seen in the distribution of maximum height of sensory block between group C as compared to group A and group B. (p value<.05) Proportion of patients in group C with maximum height of sensory block: T8 was 12.00% of patients which was significantly higher as compared to group A (0.00% of patients) and group B (0.00% of patients). Proportion of patients in group C with maximum height of sensory block: T4 was 4.00% of patients which was significantly lower as compared to group A (14.00% of patients) and group B (12.00% of patients).
Time to achieve two segment regression of sensory blockade
Statistically significant difference was seen in time to achieve two segment regression of sensory blockade (minutes) between group A, B and C. (p value <.05) Mean ± SD of time to achieve 2 segment regression of sensory blockade (min) in group B was 155.8 ± 17.65 which was significantly higher as compared to group A (137.7 ± 11.92) and group C (83.4 ± 13.79). Mean ± SD of time to achieve 2 segment regression of sensory blockade (min) in group C was 83.4 ± 13.79 which was significantly lower as compared to group B (155.8 ± 17.65) and group A (137.7 ± 11.92) (Figure 1).
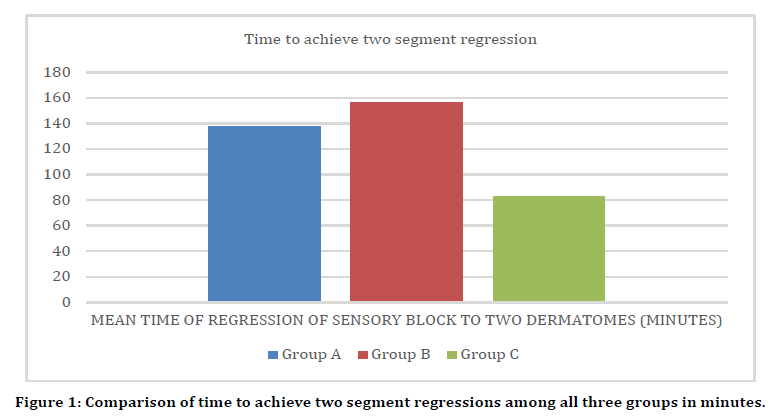
Figure 1. Comparison of time to achieve two segment regressions among all three groups in minutes.
Comparison of total duration of sensory block
Statistically significant difference was seen in total duration of sensory block (minutes) between group A, B and C. (p value <.05) Mean ± SD of total duration of sensory block (minutes) in group B was 261.2 ± 29.2 which was significantly higher as compared to group A (239.2 ± 30.02) and group C (166.9 ± 20.8) Mean ± SD of total duration of sensory block (minutes) in group C was 166.9 ± 20.8 which was significantly lower as compared to group B (261.2 ± 29.2) and group A (239.2 ± 30.02)(Figure 2).
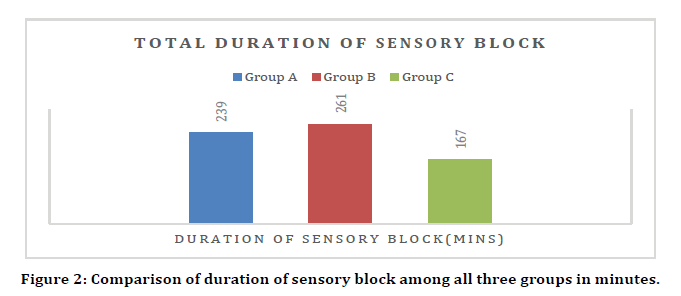
Figure 2. Comparison of duration of sensory block among all three groups in minutes.
Comparison of need of post op rescue analgesic
Statistically significant difference was seen in the distribution of need of diclofenac injection postop between group A, B and C except between group A and group B. (p value<.05) There was no need of diclofenac injection post-op in 90% of patients in group B and 90% of patients in group A which was significantly higher as compared to 20% of patients in group C. Diclofenac injection post-op was needed in 80% of patients in C which was significantly higher as compared to 10% of patients in A and 10% of patients in B (Figure 3).
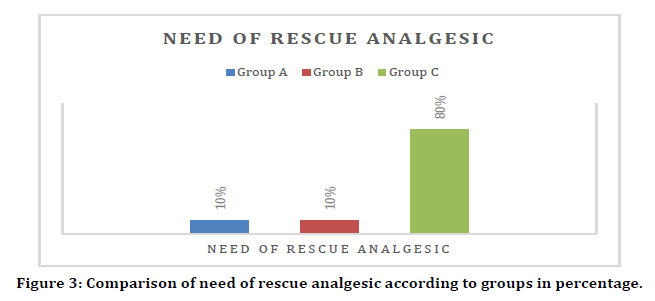
Figure 3. Comparison of need of rescue analgesic according to groups in percentage.
Characteristics of motor block
Time of onset of motor block (Bromage score 3)
Statistically significant difference was seen in time of onset of motor block (minutes) (Bromage 3) between group A, B and C. (p value <.05) Mean ± SD of time of onset of motor block (minutes) (Bromage 3) in group C was 10.56 ± 1.86 which was significantly higher as compared to group B (8.66 ± 1.64) and group A (7.82 ± 1.8) Mean ± SD of time of onset of motor block (minutes) (Bromage 3) in group A was 7.82 ± 1.8 which was significantly lower as compared to group B (8.66 ± 1.64) and group C (10.56 ± 1.86).
Time to complete regression of motor block
Statistically significant difference was seen in time of regression of motor block (minutes) (Bromage 0) between group A, B and C. (p value <.05) Mean ± SD of time of regression of motor block (minutes) (Bromage 0) in group B was 224.4 ± 31.6 which was significantly higher as compared to group A (206.2 ± 27.75) and group C (132.6 ± 19.44) Mean ± SD of C was 132.6 ± 19.44 which was significantly lower as compared to group B (224.4 ± 31.6) and group A (206.2 ± 27.75). Intravenous Dexmedetomidine bolus-plusinfusion prolongs motor blockade significantly higher than dexmedetomidine bolus and normal saline Groups. (p value <0.05). (Figure 4).
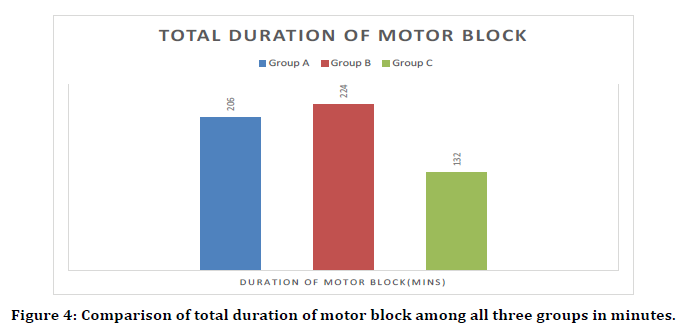
Figure 4. Comparison of total duration of motor block among all three groups in minutes.
Hemodynamic data
No statistically significant difference was seen in pulse rate (per minute), SpO2(%), respiratory rate (per minute), systolic blood pressure, diastolic blood pressure and mean blood pressure at T0, T5, T10, T15, T20, T25, T30, T45, T60, T75, T90, T105, T120, T135, T150, T165, T180, T210, T240, T270, T300, T330, T360 between group A, B and C.(p value>0.05).
Sedation score
Ramsay Sedation score [6] was statistically highly significant (p<0.001) in group B comparison to other to groups. It was significant on comparison of group A V/S group B (p<0.05) and group B V/S group C (p<0.05). While length off sedation was more in group B when compared to group A (Figure 5).
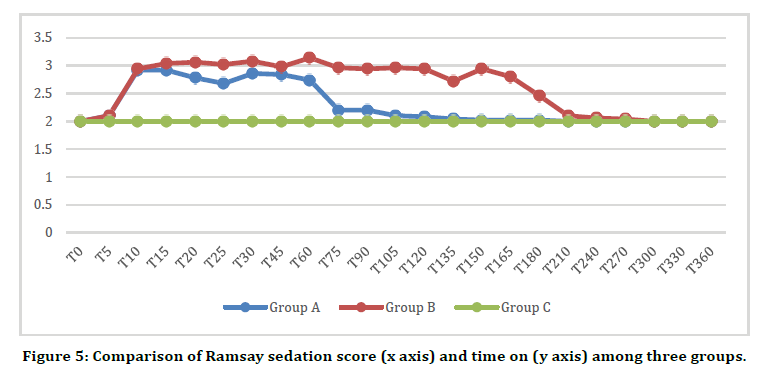
Figure 5. Comparison of Ramsay sedation score (x axis) and time on (y axis) among three groups.
Side effects
Statistically significant difference was seen in the distribution of side effects between group A and group C and between group B and group C. (p value<0.05) Side effects was nil in 88% of patients in group C which was significantly higher as compared to 48% of patients in group A and 62% of patients in group B. Bradycardia in 34% of patients in group A and 28% of patients in group B which was significantly higher as compared to 2% of patients in group C. Hypotension in 14% of patients in group A, 6% of patients in group B and 8% of patients in group C. Nausea in 4% of patients in group B, 4% of patients in group A which was significantly higher as compared to 2% of patients in group C (Table 1).
| Side effects | A (n=50) | B (n=50) | C (n=50) | Total | P value |
|---|---|---|---|---|---|
| Nil | 24(48%) | 31(62%) | 44(88%) | 99 (66%) | 0.0001 |
| Bradycardia | 17(34%) | 14(28%) | 1(2%) | 32(21.33%) | A vs |
| Hypotension | 7(14%) | 3(6%) | 4(8%) | 14 (9.33%) | B : 0.408 |
| Nausea | 2(4%) | 2(4%) | 1(2%) | 5 (3.33%) | A vs |
| Total | 50(100%) | 50(100%) | 50(100%) | 150(100%) | C:<0001 |
| B vs | |||||
| C:0001 |
Table 1: Comparison of side effects between groups A, B and C.
Discussion
Dexmedetomidine is a selective α2-A receptor agonist with sedative and analgesic effects, it has inhibitory role in transmission and perception of pain. In our study had three study groups, Group A patients received 12.5mg intrathecal bupivacaine followed by single dose of intravenous dexmedetomidine 1μg kg-1 over 10 minutes and then intravenous normal saline. Group B patient received 12.5mg intrathecal bupivacaine followed by 1μg kg-1 of dexmedetomidine intravenously over 10 minutes and then intravenous dexmedetomidine 0.4μg kg-1hr-1 till end of surgery and group C (Control group) received an equivalent amount of normal saline.
We observed Group A and B had higher total duration of sensory block when compared to control group. These results are comparable with [7]. Duration of sensory block was more in group B when compared to group A. So, Group B > Group A > Group C in terms of total duration of sensory analgesia. Similar results were achieved when total duration of motor blockage was observed. Group B>Group A>Group C. It appears that giving iv bolus dose of dexmedetomidine flowed by infusion as an adjuvant to intrathecal bupivacaine is a more effective way to increase duration of motor block and sensory analgesia.
Group A and group B both had more maximum height of sensory block when compared to control group with no difference, when group A and B were compared. When we considered time to achieve highest level of sensory block, group A and group B both had lower time compared to control group. While no difference was observed when group A and B were compared. It indicates that iv dexmedetomidine in conjugation with spinal anaesthesia not only helps in quickly achieving highest level of sensory block but also increases maximum height of block. Similar observations were made by Dinesh et al. [8].
We used inj. diclofenac 75 mg im as postoperative rescue analgesic when Visual Analog Scale (VAS) ≥ 4. In lines with Kaya [7] and Hong [9], it was observed that need of postoperative rescue analgesic was less in dexmedetomidine groups when compared to control group. Only 10 % patients required rescue analgesic in group A and group B in contrast to control group C where 80% of patients required rescue analgesic.
We observed no significant cardiovascular and respiratory variability (heart rate, blood pressure, spo2 and respiratory rate) in our study in contrast to previous researchers [7,8]. This may be due to the fact that we enrolled only health ASA I and II physical status patients.
In our study we observed Ramsay sedation score6 and it was observed that sedation score was more in dexmedetomidine bolus plus infusion group i.e. group B followed by group A and then control group C. These results validate conscious sedation with dexmedetomidine which can be of a great help in patient under spinal anaesthesia.
We observed side effects like bradycardia, hypotension, nausea were observed more in dexmedetomidine group A and group B when compared to control group C. These results were similar to results observed by Tekin et al. [10] and Whizar et al. [11].
Conclusion
From current study we observed that after subarachnoid block, single dose of intravenous dexmedetomidine 1μg kg-1 over 10 minutes (dexmedetomidine bolus) and 1μg kg-1 of dexmedetomidine intravenously over 10 minutes and then intravenous dexmedetomidine 0.4μg kg-1hr-1 till end of surgery (dexmedetomidine bolus-plus-infusion) resulted in significant prolongation of time to two segment regression, postoperative analgesia, sensory block and motor block with maintenance of haemodynamic parameters. Intravenous dexmedetomidine bolus-plus-infusion was more effective than dexmedetomidine bolus at prolongation of time to two segment regression, postoperative analgesia, sensory block and motor block of spinal anaesthesia with 0.5% hyperbaric bupivacaine. Dexmedetomidine resulted in good sedation levels in all the patients without significant respiratory depression and complications. So we conclude that intravenous dexmedetomidine bolus-plus-infusion can be preferred over intravenous dexmedetomidine bolus as an adjuvant to subarachnoid anesthesia with bupivacaine.
References
- Perlas A, Kirkham KR, Billing R, et al. The impact of analgesic modality on early ambulation following total knee arthroplasty. Reg Anesth Pain Med 2013; 38:334-339.
- Halder S, Das A, Mandal D, et al. Effect of different doses of dexmedetomidine as adjuvant in bupivacaine-induced subarachnoid block for traumatized lower limb orthopaedic surgery: A prospective, double-blinded and randomized controlled study. J Clin Diagn Res 2014; 8:GC01.
- Brown DL. Spinal, epidural and caudal anesthesia. In, Miller RD, ed. Miller’s Anesthesia, 7th edition. Philadelphia, Churchill Livingstone; 2010; 1611-1638.
- Kad N, Dhoundiyal D, Ahlawat M, et al.To study the effects of intravenous dexmedetomidine on characteristics of subarachnoid block using hyperbaric bupivacaine in lower limb orthopaedic surgery. Int J Med Res Prof 2016; 2:62-65.
- Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res 2011; 5:128-133.
- Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974; 2:656–659.
- Kaya FN, Yavascaoglu B, Turker G, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth 2010; 57:39–45.
- Dinesh CN, Sai Tej NA, Yatish B, et al. Effects of intravenous dexmedetomidine on hyperbaric bupivacaine spinal anesthesia: A randomized study. Saudi J Anaesth 2014; 8:202-208.
- Hong JY, Kim WO, Yoon Y, et al. Effects of intravenous dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients. Acta Anaesthesiol Scand 2012; 56:382–387.
- Tekin M, Kati I, Tomak Y, et al. Effect of dexmedetomidine IV on the duration of spinal anesthesia with Prilocaine: A double-blind, prospective study in adult surgical patients. Curr Ther Res 2007; 68:313–324.
- Whizar-Lugo V, Gómez-Ramírez IA, Cisneros-Corral R, et al. Intravenous dexmedetomidine vs. Intravenous clonidine to prolong bupivacaine spinal anesthesia. A doublé blind study. Anestesia en Mexico 2007; 19:143–146.
Author Info
Mayuri Golhar, Manisha Yadav, Nandita Kad and Tarun Yadav*
Department of Anaesthesiology, Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, IndiaCitation: Mayuri Golhar, Manisha Yadav, Nandita Kad, Tarun Yadav, To Study the Effect of Dexmedetomidine Administered as Bolus Vs. as Bolus-Plus-Infusion on Subarachnoid Anaesthesia with Hyperbaric Bupivacaine in Lower Limb Orthopaedic Surgery, J Res Med Dent Sci, 2021, 9 (4):117-122.
Received: 20-Feb-2021 Accepted: 31-Mar-2021
