Research - (2022) Volume 10, Issue 8
Treatment Modalities in the Management of Oral Lichen Planus-An Institutional Experience
Vaishnavi Devi B and Santhosh Kumar MP*
*Correspondence: Santhosh Kumar MP, Department of Oral and Maxillofacial Surgery, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, India, Email:
Abstract
Introduction: Oral lichen planus is an autoimmune mucosal disorder affecting the skin and mucosa. It has high chances of malignant transformation which affects one to two percent of the general population. The main symptoms of oral lichen planus include burning sensation, pain, white patches, irritation, and blisters. Assessment of oral lichen planus is essential prior to any specialized procedure to avert any further oral and systemic complications. Screening of lichen planus condition has become a necessity in developing countries and study of this nature will have enormous public health impact. The aim of this study is to assess the treatment modalities in the management of oral lichen planus in patients visiting different outpatient departments of private dental college hospital, Chennai. Methods: All the cases referred from the month of September 2020 to February 2021 for oral lichen planus treatment were chosen for the study. Data was collected from the dental hospital record system. Result data was tabulated in excels and imported to SPSS for correlation and association. P<0.05 was considered to be the level of statistical significance in this study. Results: Oral lichen planus has been reported with higher female incidence (58%) and more prevalent among the age group of 41 to 60 years (51%). The treatment modalities preferred in its management includes triamcinolone (50%), chlorpheniramine maleate (65%), azathioprine (45%), and vitamin c (37%). Conclusion: Within the limits of the present study, oral lichen planus has a female preponderance occurring more commonly in the age group of 41 to 60 years. It is mainly treated with triamcinolone, azathioprine, chlorpheniramine maleate and vitamin c supplements. Knowledge about the treatment modalities in the management of oral lichen planus in the patients visiting dental hospital will be helpful to the clinician to prevent any further complications before any specialized procedure.
Keywords
Autoimmune, Corticosteroids, Innovative technique, Oral lichen planus, Treatment
Introduction
Lichen planus is a chronic, immunological, common mucocutaneous disorder of the stratified squamous epithelia. Oral lichen planus usually occurs of unknown etiology [1]. It is one of the most common non-infectious oral mucosal diseases in patients. The condition can affect either the skin, oral mucosa or sometimes both [2]. About half of the patients show skin as well as oral lesions whereas some patients show only oral mucosal lesions. The oral mucosa is commonly involved and may be the only site of involvement [3]. It may also have co-incident cutaneous skin lesions typically present as small pruritic, white to violaceous flat-topped papules, predominantly on the flexor aspects of the wrists or ankles, extensor aspects of the lower legs, the skin of the lower central back and the natal cleft, genital involvement is also reported [4].
It affects one to two percent of the general adult population and is the most common noninfectious oral mucosal disease [5]. Lichen planus is a disease of elderly people and children are rarely affected. It is more common in the age group of 40 to 70 years and sometimes noticed in young adults also. Earlier studies on the prevalence of oral lichen planus have shown that it is more common among females [6,7]. It affects women more often than men in a ratio 2:3. It is considered as a premalignant condition with malignant transformation rate of 0–2% [8]. Oral lichen planus is a chronic disease that can persist in some patients for a long time, it may persist even up to 25 years.
Oral lichen planus can occur anywhere in the oral cavity. The buccal mucosa, tongue and gingiva are the most common sites in the oral cavity, whereas palatal lesions, labial mucosa are uncommon. They are usually symmetrical and bilateral lesions or multiple lesions in the mouth [9]. Gingival lesions appear as red erythematous lesions affecting the entire attached gingiva and free gingiva [10]. The dorsal surface of the tongue may have striae or annular pattern in which the keratotic lines form circles of varying size. It can be present as white striations, papules, plaques, erythema, erosions or blisters [11]. It often appears as a mixture of clinical subtypes. It is said to occur in six clinical variants as reticular, papular, plaque-like, erosive, atrophic and bullous. It resembles leukoplakia as homogenous white patches [12]. A proper case history and observation of the clinical features of the disease are usually sufficient to establish the diagnosis.
Most of the patients with oral lichen planus show no symptoms. In such cases, there will be no need for active treatment except for reassurance and to ensure that the patient is reviewed regularly. Corticosteroids are the mainstay of OLP therapy because of their activity in dampening cell mediated immune activity and are administered topically, intralesionally or systemically [13]. Treatment of OLP with cyclosporine, azathioprine, levamisole, griseofulvin, retinoids, Hydroxycholoroquine sulphate, dapsone has also been reported [14]. Our team has extensive knowledge and research experience that has translated into high quality publications in the recent years [15-34].
The main concerns with these and other current therapies are local and systemic side effects and lesion recurrence following withdrawal of treatment. There are limited studies reporting the various non-surgical treatment modalities of oral lichen planus and hence this study was planned to evaluate the various treatment modalities in the management of oral lichen planus. This study will help in raising awareness of the condition and in achieving precise diagnosis followed by treatment. Certain recommendations to the dental clinicians can be given for some specialised or high risk procedures based on the inference of this study. The aim of this study is to determine the various treatment modalities in the management of oral lichen planus in patients visiting different Outpatient departments (OPDs) of a private dental college hospital, Chennai.
Materials and Methods
Study setting and study design
The present study was conducted as a retrospective cross sectional study on the treatment modalities used in the management of oral lichen planus in the patients visiting the dental hospital. A randomized sample of patients who had reported for oral lichen planus were chosen for the study. The study took place in a private college hospital setting within the university. The retrospective data obtained from the institution was being ethically approved (ethical approval number: SDC/ SIHEC/2021/DIASDATA/0920-0221) and the number of people involved in the study includes 3 members - Guide, researcher, reviewing expert.
Sampling
All patient case records were reviewed and analysed for the study. All the cases referred for oral lichen planus from the month of September 2020 to February 2021 were included for the study. The records of all patient data were reviewed from initial to last and were arranged in chronological order. Sample size n = 166 patients. The data collected includes the demographic details, treatment undertaken for the patient. All the treatment report data were properly reviewed and cross verified by another examiner. Sampling bias was minimised by simple random sampling. Internal validity is applicable to the study.
Inclusion and exclusion criteria
The data collected includes patients in the age group of 10 to 60 years of age of both sexes with oral lichen planus symptoms. Patients with other oral lesions and systemic conditions were excluded from the study.
Data analysis
The collected data includes patients who reported with oral lichen planus and the treatment modalities preferred. Gross incomplete data was excluded as it affects the study. Excel tabulation of all the verified data and importing to the Statistical Package for Social Sciences (SPSS) software, version 1.0.0.1327 64 bit edition(IBM corp., NY, USA) for the statistical tests was done. The data was assessed by being subjected to descriptive analysis with the help of frequencies, percentage and analysed by running descriptive statistics in the form of crosstabs. Independent variables in the study include ethnicity, age, frequency and gender and the dependent variables include oral lichen planus, treatment measures. Non parametric test - Pearson’s Chi square statistical test was done and the results were obtained.
Results and Discussion
The present study was to determine the various treatment modalities in the management of oral lichen planus. The sample size of this study includes 166 case reports. The statistical software SPSS was used for the descriptive and inferential analysis. Results on categorical measurement were presented in percentage (%). Level of significance was predetermined at the probability value of P ≤ 0.05.
The age prevalence in patients reported with oral lichen planus shows that 2% reported in the age group of less than 20 years, 32% in the age group of 21 to 40 years, 51% in the age group of 41 to 60 years and 13% in the age group of more than 60 years (Figure 1). The gender prevalence in patients reporting with oral lichen planus shows that 41% males have reported and 58% female patients have reported (Figure 2).
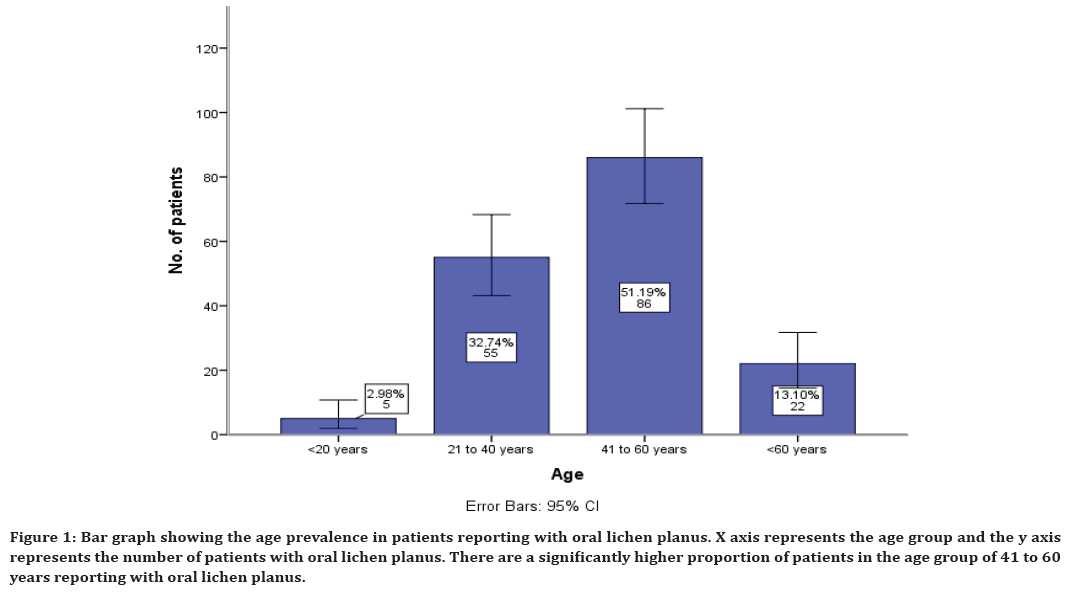
Figure 1. Bar graph showing the age prevalence in patients reporting with oral lichen planus. X axis represents the age group and the y axis represents the number of patients with oral lichen planus. There are a significantly higher proportion of patients in the age group of 41 to 60 years reporting with oral lichen planus.
Figure 2. Bar graph showing the gender prevalence in patients reporting with oral lichen planus. X axis represents the gender and the y axis represents the number of patients with oral lichen planus. There is a significantly higher proportion of female patients reporting with oral lichen planus than males.
The various treatment modalities preferred in the management of oral lichen planus shows that medications have been preferred with corticosteroids being the mainstay of treatment. This study shows the various corticosteroids preferred in the management of oral lichen planus which includes triamcinolone in 50% of cases, betamethasone 3%, clobetasol 2%, prednisolone 23% of cases. Combination of triamcinolone and betamethasone was also preferred in 19% of cases, combination of prednisolone and triamcinolone in 0.6% of cases, prednisolone and betamethasone in combination in 0.6% of cases is also preferred (Figure 3).
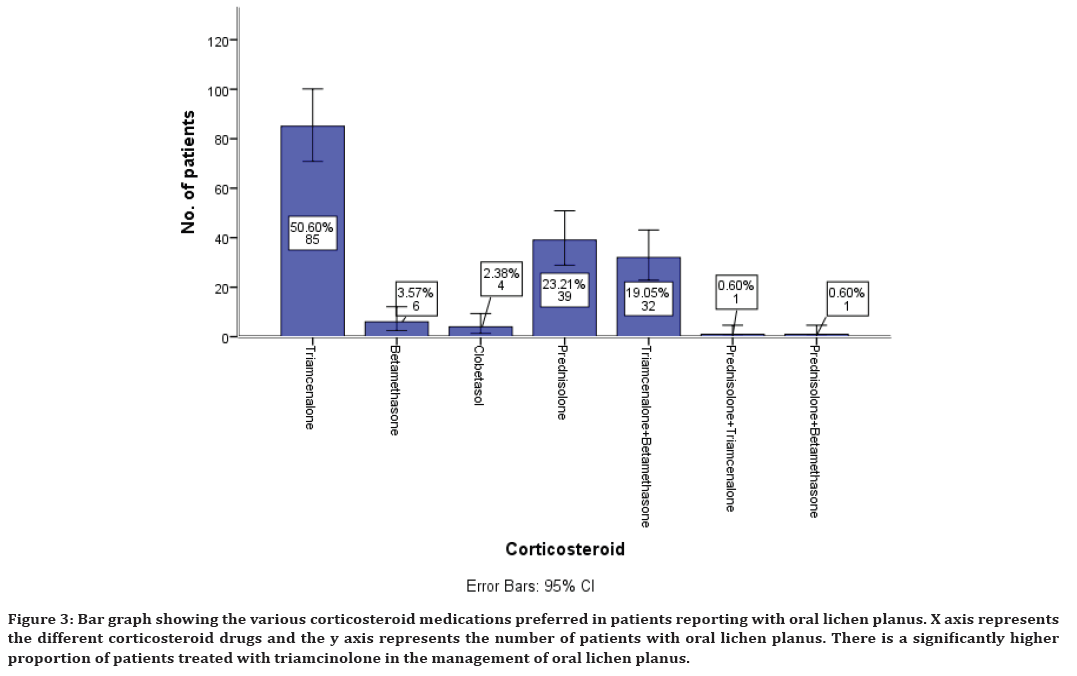
Figure 3. Bar graph showing the various corticosteroid medications preferred in patients reporting with oral lichen planus. X axis represents the different corticosteroid drugs and the y axis represents the number of patients with oral lichen planus. There is a significantly higher proportion of patients treated with triamcinolone in the management of oral lichen planus.
Similarly along with corticosteroids, antihistamines are also prescribed. The present study has shown that antihistamines used in the management of oral lichen planus include chlorpheniramine maleate in 65% of cases, diphenhydramine maleate in 22% of cases and it was also noticed that in 12% cases antihistamine is not prescribed (Figure 4).
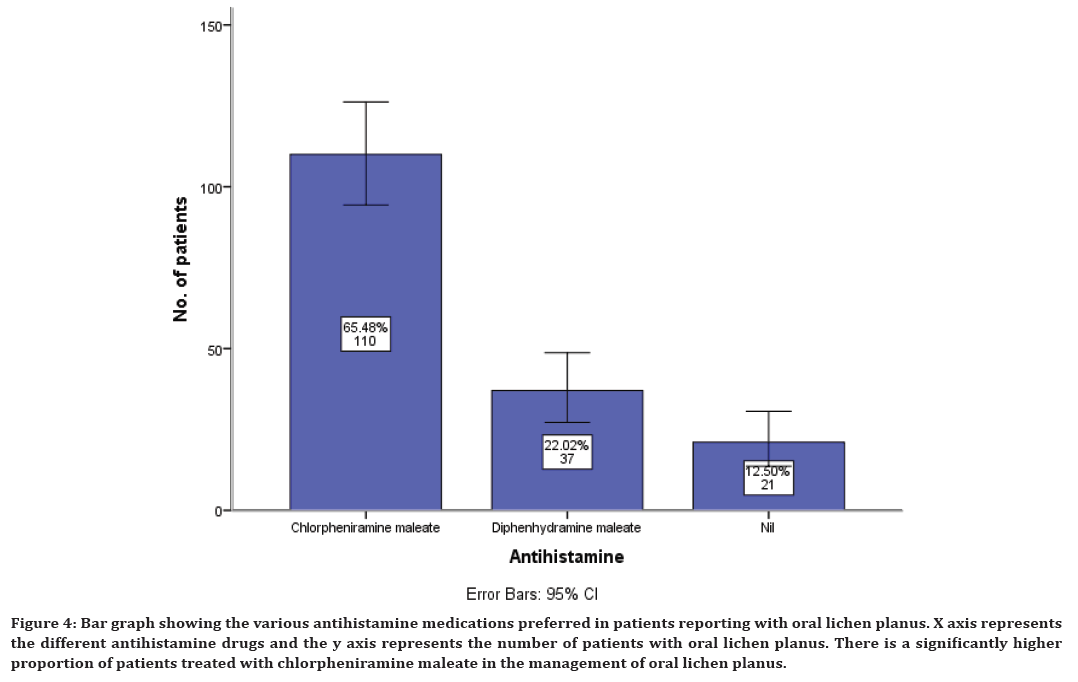
Figure 4. Bar graph showing the various antihistamine medications preferred in patients reporting with oral lichen planus. X axis represents the different antihistamine drugs and the y axis represents the number of patients with oral lichen planus. There is a significantly higher proportion of patients treated with chlorpheniramine maleate in the management of oral lichen planus.
Along with antihistamine, immunomodulatory and supplementary drugs are also prescribed. The immunomodulatory prescribed include azathioprine in 45% cases, dapsone in 29% cases and tacrolimus in 25% cases (Figure 5).
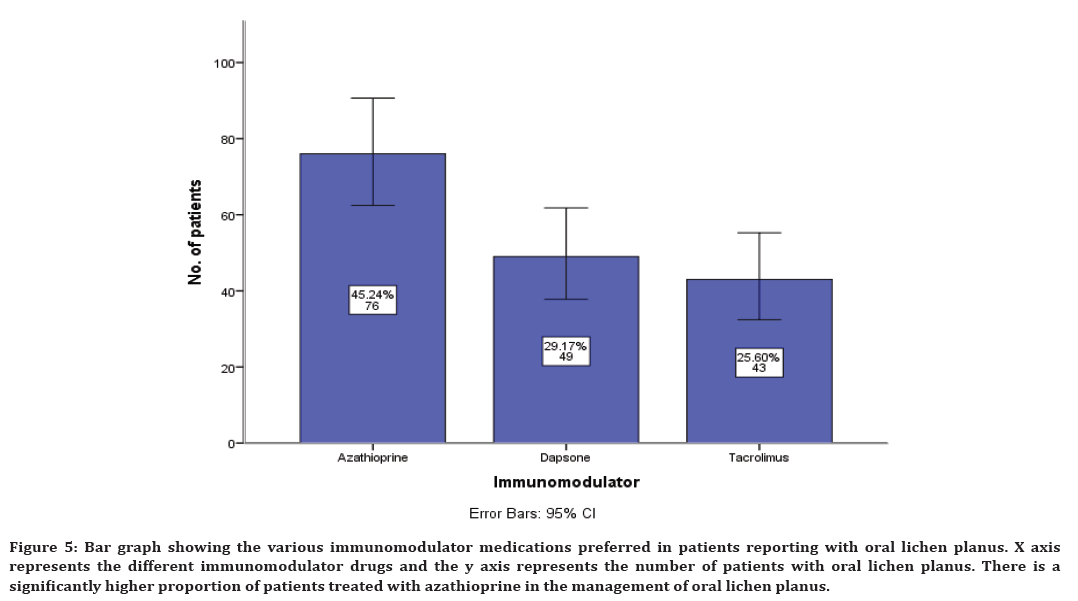
Figure 5. Bar graph showing the various immunomodulator medications preferred in patients reporting with oral lichen planus. X axis represents the different immunomodulator drugs and the y axis represents the number of patients with oral lichen planus. There is a significantly higher proportion of patients treated with azathioprine in the management of oral lichen planus.
The supplements prescribed along with other medications include B complex in 11% of cases, Vitamin C in 37% of cases, Vitamin E in 3% of cases, Alphalypoic acid in 29% of cases and Zinc in 17% of cases (Figure 6).
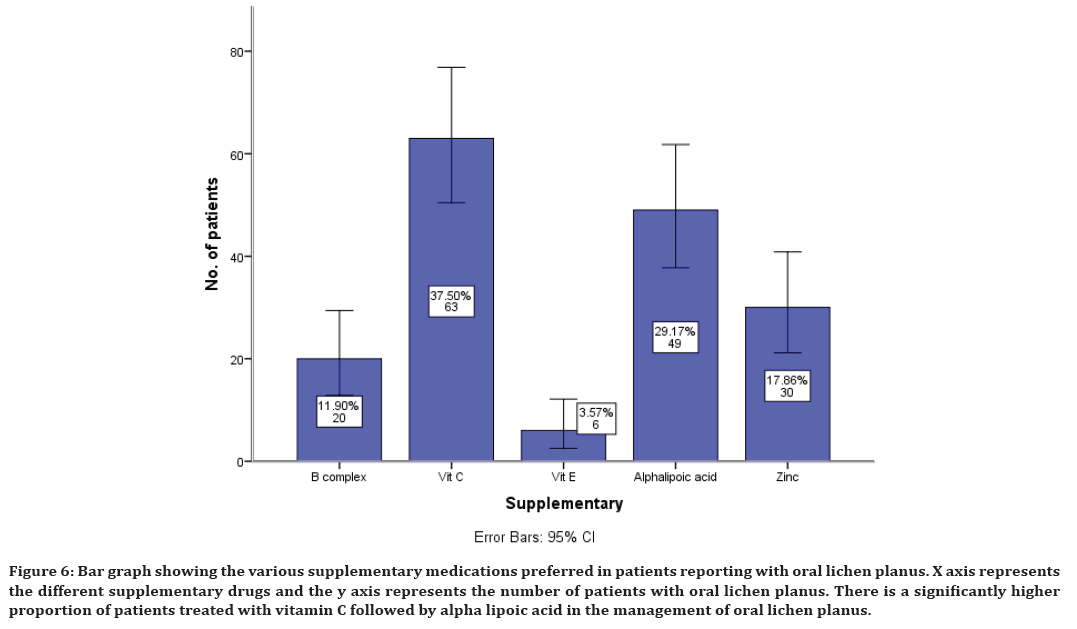
Figure 6. Bar graph showing the various supplementary medications preferred in patients reporting with oral lichen planus. X axis represents the different supplementary drugs and the y axis represents the number of patients with oral lichen planus. There is a significantly higher proportion of patients treated with vitamin C followed by alpha lipoic acid in the management of oral lichen planus.
Association between corticosteroids, antihistamines, immunomodulatory, and supplementary drugs with gender of the patients was evaluated and all the results were statistically significant with p<0.05. A higher proportion of females have been prescribed with all these drugs compared to the male patients (Figures 7 to Figure 10). There is a significantly higher proportion of female patients treated with triamcinolone in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05) (Figure 7). There is a significantly higher proportion of female patients treated with chlorpheniramine maleate in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05) (Figure 8).
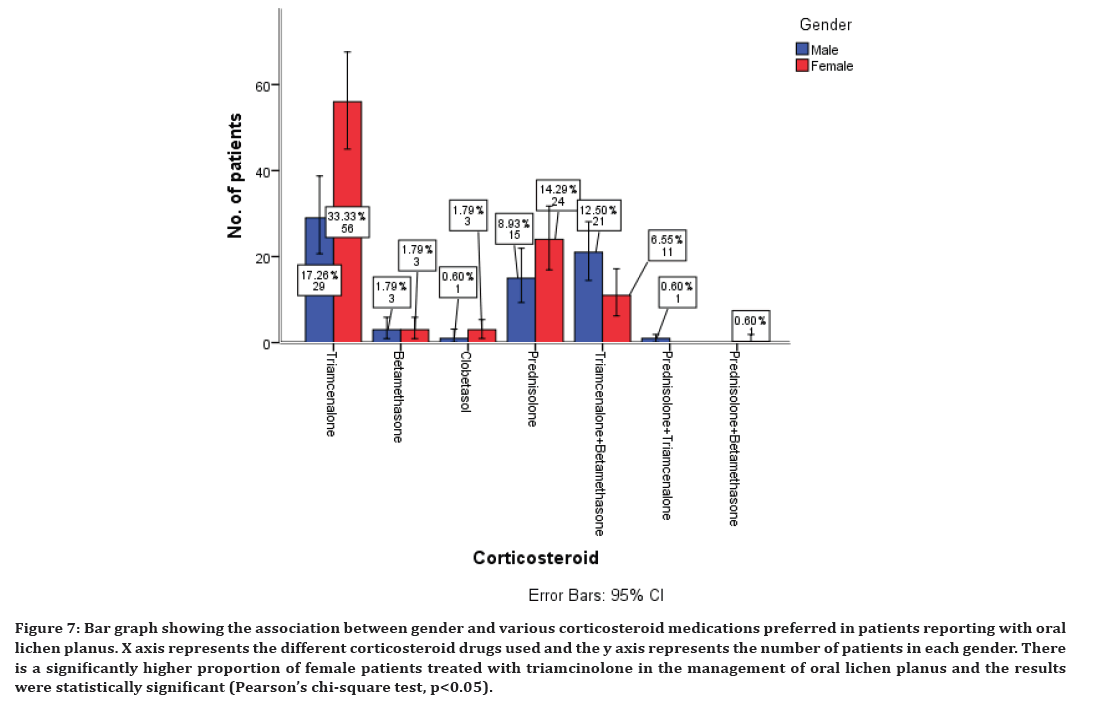
Figure 7. Bar graph showing the association between gender and various corticosteroid medications preferred in patients reporting with oral lichen planus. X axis represents the different corticosteroid drugs used and the y axis represents the number of patients in each gender. There is a significantly higher proportion of female patients treated with triamcinolone in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05).
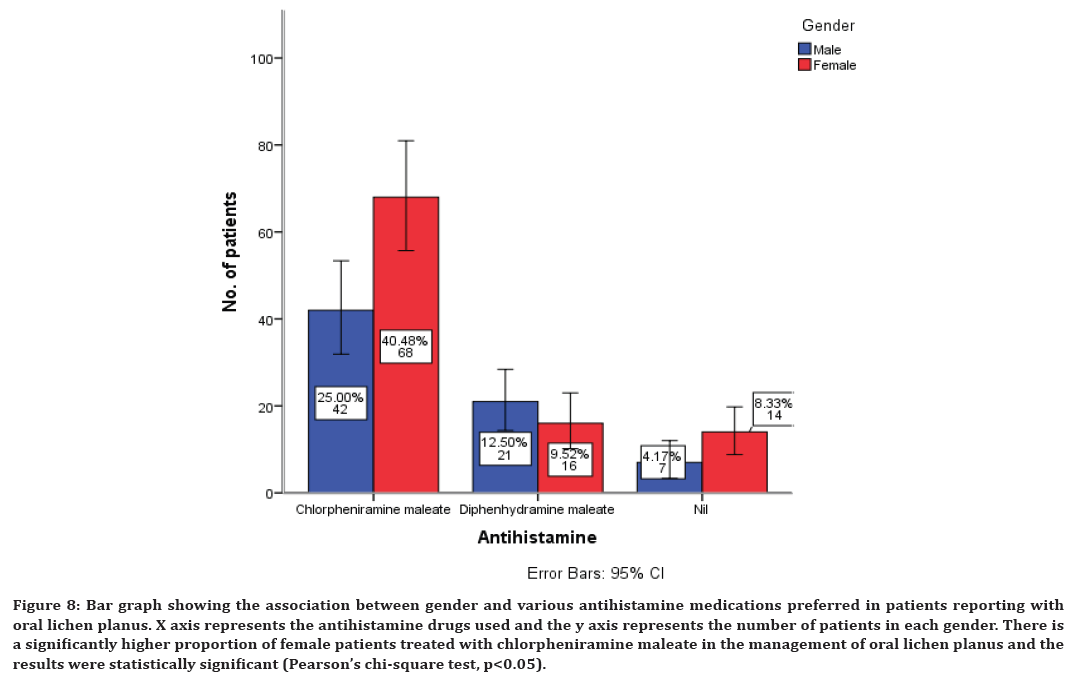
Figure 8. Bar graph showing the association between gender and various antihistamine medications preferred in patients reporting with oral lichen planus. X axis represents the antihistamine drugs used and the y axis represents the number of patients in each gender. There is a significantly higher proportion of female patients treated with chlorpheniramine maleate in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05).
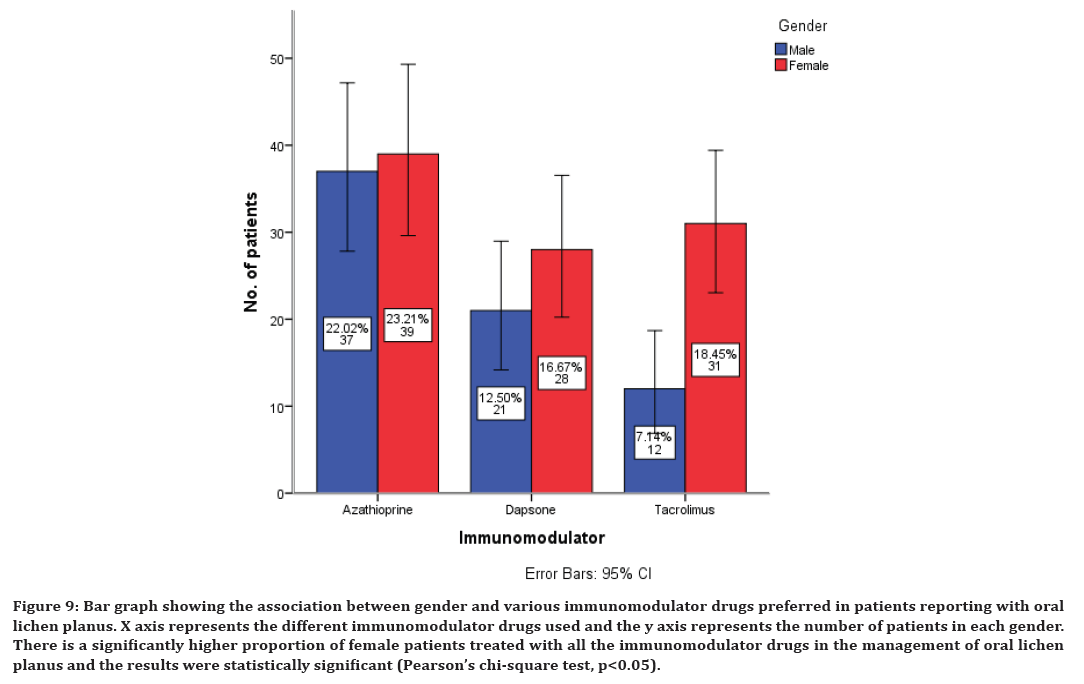
Figure 9. Bar graph showing the association between gender and various immunomodulator drugs preferred in patients reporting with oral lichen planus. X axis represents the different immunomodulator drugs used and the y axis represents the number of patients in each gender. There is a significantly higher proportion of female patients treated with all the immunomodulator drugs in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05).
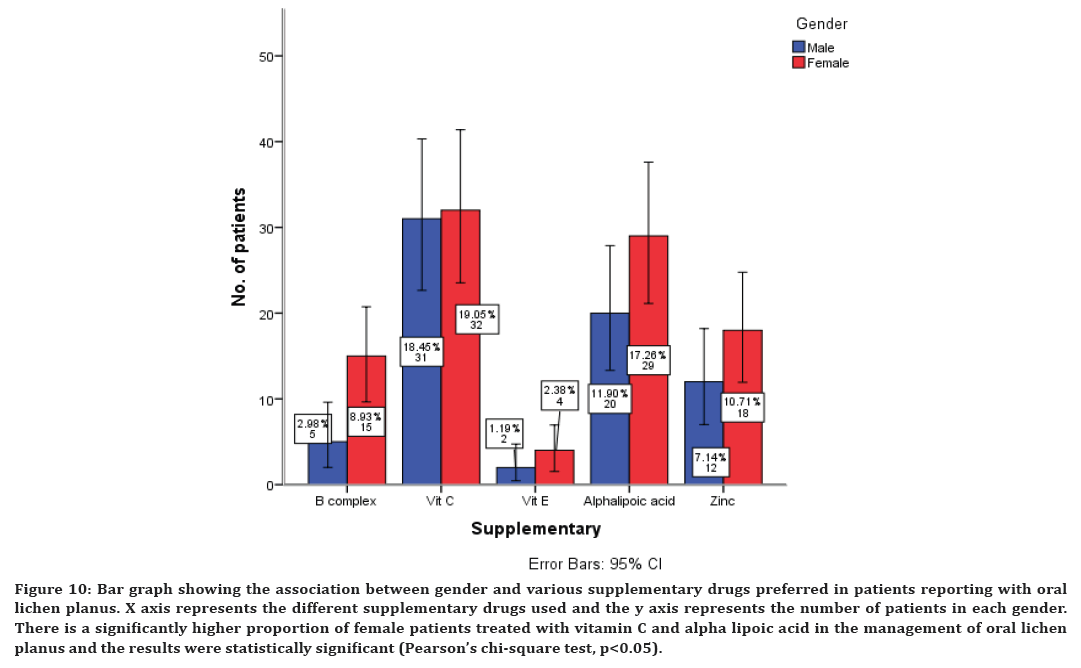
Figure 10. Bar graph showing the association between gender and various supplementary drugs preferred in patients reporting with oral lichen planus. X axis represents the different supplementary drugs used and the y axis represents the number of patients in each gender. There is a significantly higher proportion of female patients treated with vitamin C and alpha lipoic acid in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05).
There is a significantly higher proportion of female patients treated with all the immunomodulatory drugs in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05) (Figure 9). There is a significantly higher proportion of female patients treated with vitamin C and alpha lipoic acid in the management of oral lichen planus and the results were statistically significant (Pearson’s chi-square test, p<0.05) (Figure 10).
Lichen planus is a chronic autoimmune disease affecting the skin, hair, nail, and oral mucosa. The prevalence of oral lichen planus in the general adult population is 0.5–2% [35,36]. Systemic medications like NSAIDs, antihypertensive, and oral hypoglycemic can contribute to the development of oral lichenoid reactions. Amalgam, gold, and nickel from dental restorative material may also be related in some patients [37]. It includes 6 subtypes. The reticular, papular and plaque-like forms are usually painless and appear clinically as white or grey streaks keratotic lesions and may form a linear or reticular pattern on an erythematous background [38]. Characteristically, it presents as a series of fine, radiant, white striae known as ‘Wickham striae’, which may be surrounded by a discrete erythematous border. The erosive, atrophic and bullous forms are associated with a burning sensation and cause severe pain [39,40]. The atrophic and erosive forms of lichen planus have high chances to undergo malignant change.
It is a T-cell mediated autoimmune disease in which the cytotoxic CD8+ T cells trigger apoptosis of the basal cells of the oral epithelium [41]. Microscopically the disease is characterized by dense sub epithelial, intraepithelial lymphocytic infiltrate, and degeneration of basal keratinocytes. Basal keratinocytes form colloid bodies that appear as homogenous eosinophilic globules [42]. Parakeratosis, acanthosis and saw-tooth’ rete peg formation are also commonly noticed. Direct immunofluorescence studies show that these bodies stain for immunoglobulins IgA, IgG, and IgM [43,44]. These features are not specific or diagnostic of oral lichen planus.
Most cases of oral lichen planus are asymptomatic which are incidentally identified during dental visits. Patients who are not given active treatment are advised to return regularly for review, or sooner if they get symptoms [45]. All cases of oral mucosal disease should be examined carefully for fine striae both peripherally around atrophic and erosive sites and on the buccal mucosa, ventral tongue, lateral tongue and gingiva [46]. Treatment of symptomatic lichen planus ranges from the elimination of local triggering factors, systemic interventions and long-term pharmacological therapies. Oral lichen planus is associated with increased risk of malignant transformation which adds even more complexity in the management of oral lichen planus [47].
In our study, the pattern of age distribution in the prevalence of oral lichen planus showed that people of all groups are affected but the peak incidence was however observed in the age group of 41 to 60 years. This finding is in concordance with a number of previous studies in India and other parts of the world [48–50]. According to a study among the Chinese population, the mean age group of the patients reporting with oral lichen planus is in the 5th and 6th decade [51]. As the normal physiological functioning of the body decreases and the medicine intake increases with age there are high chances of occurrence in the old age groups. As it is an autoimmune disorder it rarely affects young children also [52].
More recently, it has become apparent that middle-aged women are at significantly higher risk of oral lichen planus than men in several different populations. The gender distribution of LP is balanced. An exception to the rule of equal gender distribution is oral mucosal lichen planus, where a female predominance of 1.4: 1 or 2: 1 has been found [53,54]. One possible explanation for this observation is that due to hormonal changes and stress, the sympathetic nervous system gets activated and it leads to heavy influx of inflammatory cytokines like IL-6, IL-1 and TNF by the sympathetic nervous system. The excess secretion of cortisol originates as immunosuppression through multiple mechanisms (NFκB, AP1, Raf, MAPK mediated signaling pathways). This leads to the autoimmune changes in the system in females more than males making them more susceptible to lichen planus [55,56]. In our study, the gender distribution reveals female preponderance in the occurrence of oral lichen planus. This finding is in accordance with few other studies who have shown that prevalence of oral lichen planus is more common in females than in males [57,58]. But some studies have also shown that there is a male predominance in the occurrence of oral lichen planus [59–61].
The management of the oral lichen planus is not definite and in most of the cases is symptoms based. The aim of current oral lichen planus therapy is to eliminate mucosal erythema and ulceration and reduce the risk of oral cancer. Corticosteroids are said to be the most important agents in the treatment of oral lichen planus. They can be prescribed topically into the lesion and systemically. In our study, triamcinolone is the most commonly prescribed corticosteroid when compared to the other steroid drugs. This finding is in accordance with earlier studies [62–64]. The short term systemic corticosteroid prednisolone was the second highly preferred corticosteroid for the management of oral lichen planus in our study. The rationale behind their usage is their ability to modulate inflammation and immune response. They act by reducing the lymphocytic infiltration and stabilizing the lysosomal membrane [65]. Candidiasis was commonly found and in addition, bad taste, nausea, dry mouth, sore throat may occur as minor side-effects [66].
The second line of treatment for oral lichen planus includes antihistamine. In our study, chlorpheniramine maleate was the highly preferred antihistamine drug in the management of oral lichen planus. It acts as an effective agent that can restore the normal phagocytic activity of macrophages and neutrophils, it modulates T-cell mediated immunity and it potentiates the activity of human interferon [67]. It is used as an intermittent drug along with the corticosteroids. The next line of drug therapy is the immunomodulators. It inhibits T-cell activation by stopping the synthesis and release of cytokines from the T-cells. They also prevent the release of inflammatory cytokines and mediators from mast cells. The immunomodulator preferred in our study includes azathioprine. This finding is also in agreement with a few earlier studies where they have preferred azathioprine in the management of oral lichen planus [68,69].
Sometimes, supplementary drugs are also prescribed along with these main drugs for the betterment of immunity. The present study has shown that vitamin c is the highly prescribed medication as supplement for the oral lichen planus management. This finding is in accordance with a few earlier clinical studies [70,71]. The possible protective effects of vitamins in reducing the risk of oral lichen planus may be related to antioxidant role in eliminating free radical damage. The fact that antioxidant vitamins are effective against oxidative stress may explain the significant difference between healthy individuals and patients with oral lichen planus [72,73].
Oral lichen planus is a very common condition and most frequent mucosal pathosis encountered by dental practitioners. It is imperative to identify it precisely and proper treatment be administered at the earliest. The present study has shown the age and gender prevalence of oral lichen planus and the various non-surgical treatment modalities preferred in its management. This study will have a huge impact in public health and will be helpful in creating awareness by knowing the prevalence among different gender and age groups. The findings of this study will assist the clinician to be prepared for treatment in the oral lichen planus patients by knowing its prevalence. There is a geographic limitation to the study as it predominantly covers the South Indian population. This can be modified by performing longitudinal and periodic studies to evaluate the surgical aspects in management of lichen planus. In the future, a larger population with different ethnicities can be included to provide better results for precise diagnosis. This study gives valuable information to oral health planners in proposing strategies for the development of oral health management.
Conclusion
Within the limits of the present study, oral lichen planus has a female preponderance occuring more commonly in the age group of 41 to 60 years. It is mainly treated with triamcinolone, azathioprine, chlorpheniramine maleate and vitamin c supplements. Further future studies are required to explore the surgical aspects in the management of oral lichen planus.
Acknowledgements
The authors would like to acknowledge the help and support rendered by Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India.
Funding
The present study is funded by
• Saveetha Institute of Medical and Technical Sciences, Saveetha Dental College and Hospitals, Saveetha University, India.
• Arunagiri Automobiles Private Ltd., Chennai, India.
Conflict of Interest
The authors declare no potential conflict of interest.
References
- Strassburg M, Knolle G. Diseases of the oral mucosa: A color atlas. Quintessence Publishing 1994.
- Scully C, el-Kom M. Lichen planus: review and update on pathogenesis. J Oral Pathol 1985; 14:431–58.
- Bermejo A, Bermejo MD, Román P, et al. Lichen planus with simultaneous involvement of the oral cavity and genitalia. Oral Surg Oral Med Oral Pathol 1990; 69:209-16.
- Silverman S, Griffith M. Studies on oral lichen planus. II. Follow-up on 200 patients, clinical characteristics, and associated malignancy. Oral Surg Oral Med Oral Pathol 1974; 37:705-10.
- Chainani-Wu N, Silverman S, Lozada-Nur F, et al. Oral lichen planus: patient profile, disease progression and treatment responses. J Am Dent Assoc 2001; 132:901-29.
- Sugerman PB, Savage NW, Walsh LJ, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med 2002; 13:350-65.
- Ismail SB, Kumar SKS, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci 2007; 49:89-96.
- Scully C, Beyli M, Ferreiro MC, et al. Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med 1998; 9:86-22.
- Alrashdan MS, Angel C, Cirillo N, et al. Smoking habits and clinical patterns can alter the inflammatory infiltrate in oral lichenoid lesions. Oral Surg Oral Med Oral Pathol Oral Radiol 2016 ;121:49-57.
- Vincent SD, Fotos PG, Baker KA, et al. Oral lichen planus: the clinical, historical, and therapeutic features of 100 cases. Oral Surg Oral Med Oral Pathol 1990; 70:165-71.
- Thorn JJ, Holmstrup P, Rindum J, et al. Course of various clinical forms of oral lichen planus. A prospective follow-up study of 611 patients. J Oral Pathol Med. 1988; 17:213–8.
- Sloberg K, Jonsson R, Jontell M. Assessment of Langerhans’ cells in oral lichen planus using monoclonal antibodies. J Oral Pathol 1984; 13:516-24.
- Gonzalez-Moles MA, Ruiz-Avila I, Rodriguez-Archilla A, et al. Treatment of severe erosive gingival lesions by topical application of clobetasol propionate in custom trays. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95:688-92.
- López-Jornet P, Camacho-Alonso F, Salazar-Sanchez N. Topical tacrolimus and pimecrolimus in the treatment of oral lichen planus: an update. J Oral Pathol Med 2010; 39:201-25.
- J PC, Pradeep CJ, Marimuthu T, et al. Prevalence and measurement of anterior loop of the mandibular canal using CBCT: A cross sectional study. Clin Implant Dent Related Res 2018; 20:531-34.
- Wahab PUA, Abdul Wahab PU, Madhulaxmi M, et al. Scalpel versus diathermy in wound healing after mucosal incisions: A split-mouth study. J Oral Maxillofac Surg 2018; 76:1160-644.
- Mudigonda SK, Murugan S, Velavan K, et al. Non-suturing microvascular anastomosis in maxillofacial reconstruction- a comparative study. J Cranio-Maxillofac Surg 2020; 48:599-06.
- Narayanasamy RK, Muthusekar RM, Nagalingam SP, et al. Lower pretreatment hemoglobin status and treatment breaks in locally advanced head and neck squamous cell carcinoma during concurrent chemoradiation. Indian J Cancer 2021; 58:62-68.
- Wang H, Chinnathambi A, Alahmadi TA, et al. Phyllanthin inhibits MOLT-4 leukemic cancer cell growth and induces apoptosis through the inhibition of AKT and JNK signaling pathway. J Biochem Mol Toxicol 2021; 35:1-10.
- Li S, Zhang Y, Veeraraghavan VP, et al. Restorative effect of fucoxanthin in an ovalbumin-induced allergic rhinitis animal model through NF-κB p65 and STAT3 signaling. J Environ Pathol Toxicol Oncol 2019; 38:365-75.
- Ma Y, Karunakaran T, Veeraraghavan VP, et al. Sesame inhibits cell proliferation and induces apoptosis through inhibition of STAT-3 translocation in thyroid cancer cell lines (FTC-133). Biotechnol Bioprocess Eng 2019; 24:646-52.
- Bishir M, Bhat A, Essa MM, et al. Sleep deprivation and neurological disorders. Biomed Res Int 2020; 2020:5764017.
- Fan Y, Maghimaa M, Chinnathambi A, et al. Tomentosin reduces behavior deficits and neuroinflammatory response in mptp-induced parkinson’s disease in mice. J Environ Pathol Toxicol Oncol 2021; 40:75–84.
- Zhang C, Chen Y, Zhang M, et al. Vicenin-2 treatment attenuated the diethylnitrosamine-induced liver carcinoma and oxidative stress through increased apoptotic protein expression in experimental rats. J Environ Pathol Toxicol Oncol 2020; 39:113-23.
- Gan H, Zhang Y, Zhou Q, et al. Zingerone induced caspase-dependent apoptosis in MCF-7 cells and prevents 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in experimental rats. J Biochem Mol Toxicol 2019; 33:e22387.
- Saravanakumar K, Park S, Mariadoss AVA, et al. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem Toxicol 2021; 155:112374.
- Veeraraghavan VP, Hussain S, Papayya Balakrishna J, et al. A comprehensive and critical review on ethnopharmacological importance of desert truffles: Terfezia claveryi, Terfezia boudieri, and Tirmania nivea. Food Rev Int 2021; 1-20.
- Wei W, Li R, Liu Q, et al. Amelioration of oxidative stress, inflammation and tumor promotion by Tin oxide-Sodium alginate-Polyethylene glycol-Allyl isothiocyanate nanocomposites on the 1,2-Dimethylhydrazine induced colon carcinogenesis in rats. Arab J Chem 2021; 14:103238.
- Sathya S, Ragul V, Veeraraghavan VP, et al. An in vitro study on hexavalent chromium [Cr(VI)] remediation using iron oxide nanoparticles based beads. Environ Nanotechnol Monit Manag 2020; 14:100333.
- Chandrasekar R, Chandrasekhar S, Sundari KKS, et al. Development and validation of a formula for objective assessment of cervical vertebral bone age. Prog Orthod 2020; 21:38.
- Ramakrishnan M, Dhanalakshmi R, Subramanian EMG. Survival rate of different fixed posterior space maintainers used in Paediatric Dentistry – A systematic review. Saudi Dental J 2019; 31:165-72.
- Felicita AS, Sumathi Felicita A. Orthodontic extrusion of Ellis Class VIII fracture of maxillary lateral incisor – The sling shot method. Saudi Dental J 2018; 30:265-59.
- Su P, Veeraraghavan VP, Krishna Mohan S, et al. A ginger derivative, zingerone-a phenolic compound-induces ROS-mediated apoptosis in colon cancer cells (HCT-116). J Biochem Mol Toxicol 2019; 33:e22403.
- Wan J, Feng Y, Du L, Veeraraghavan VP, et al. Antiatherosclerotic activity of eriocitrin in high-fat-diet-induced atherosclerosis model rats. J Environ Pathol Toxicol Oncol 2020; 39:61-75.
- Baccaglini L, Thongprasom K, Carrozzo M, et al. Urban legends series: Lichen planus. Oral Dis 2013; 19:128-43.
- Bermejo-Fenoll A, López-Jornet P. Familial oral lichen planus: Presentation of six families. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 102:e12-5.
- Bornstein MM, Kalas L, Lemp S, et al. Oral lichen planus and malignant transformation: a retrospective follow-up study of clinical and histopathologic data. Quintessence Int 2006; 37:261-71.
- Cooke BED. The oral manifestations of lichen planus: 50 cases. Br Dent J. 1954; 96:1–9.
- Montgomery DS, Culver GD. Lichen planus of the mouth alone. Br J Dermatol 1929; 41:45-50.
- Krutchkoff DJ, Eisenberg E. Lichenoid dysplasia: a distinct histopathologic entity. Oral Surg Oral Med Oral Pathol 1985; 60:308-15.
- Eversole LR. Immunopathogenesis of oral lichen planus and recurrent aphthous stomatitis. Semin Cutan Med Surg 1997; 16:284-94.
- Zhao ZZ, Savage NW, Pujic Z, et al. Immunohistochemical localization of mast cells and mast cell-nerve interactions in oral lichen planus. Oral Dis 1997; 3:71-76.
- Juneja M, Mahajan S, Rao NN, et al. Histochemical analysis of pathological alterations in oral lichen planus and oral lichenoid lesions. J Oral Sci 2006; 48:185-93.
- Walsh LJ, Savage NW, Ishii T, et al. Immunopathogenesis of oral lichen planus. J Oral Pathol Med 1990; 19:389-96.
- Jungell P. Oral lichen planus. A review. Int J Oral Maxillofac Surg 1991; 20:129-35.
- Eisenberg E, Krutchkoff DJ. Lichenoid lesions of oral mucosa. Diagnostic criteria and their importance in the alleged relationship to oral cancer. Oral Surg Oral Med Oral Pathol 1992; 73:699-04.
- Marder MZ, Deesen KC. Transformation of oral lichen planus to squamous cell carcinoma: a literature review and report of case. J Am Dent Assoc 1982; 105:55-60.
- Shekar C, Ganesan S. Oral lichen planus: Review. Chin J Dent Res 2011; 2:62–87.
- Boorghani M, Gholizadeh N, Taghavi Zenouz A, et al. Oral lichen planus: Clinical features, etiology, treatment and management: A review of literature. J Dent Res Dent Clin Dent Prospects. 2010; 14: 4:3-9.
- Sachdev R, Mukerjee S, Garg K, et al. Demographic prevalence of oral lichen planus in males: A retrospective study. Acta Sci Dent Sci 2019; 3:111–14.
- Xue JL, Fan MW, Wang SZ, et al. A clinical study of 674 patients with oral lichen planus in China. J Oral Pathol Med 2005; 34:467-72.
- Sousa FA, Rosa LE. Oral lichen planus: clinical and histopathological considerations. Bra J Otorhinolaryngol 2008; 74:284-92.
- Bhattacharya M, Kaur I, Kumar B. Lichen planus: A clinical and epidemiological study. J Dermatol 2000; 27:576-82.
- Lavanya N, Jayanthi P, Rao UK, et al. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofaci Pathol 2011; 15:127.
- Halonen P, Jakobsson M, Heikinheimo O, et al. Cancer risk of Lichen planus: A cohort study of 13,100 women in Finland. Int J Cancer 2018;142:18-22.
- Idrees M, Kujan O, Shearston K, et al. Oral lichen planus has a very low malignant transformation rate: A systematic review and meta-analysis using strict diagnostic and inclusion criteria. J Oral Pathol Med 2021; 50:287-98.
- Mostafa B, Ahmed E. Prevalence of oral lichen planus among a sample of the Egyptian population. J Clin Exp Dent 2015;7:e7-12.
- Shen ZY, Liu W, Zhu LK, et al. A retrospective clinicopathological study on oral lichen planus and malignant transformation: analysis of 518 cases. Med Oral Patol Oral Cir Bucal 2012; 17:e943.
- Murti PR, Daftary DK, Bhonsle RB, et al. Malignant potential of oral lichen planus: observations in 722 patients from India. J Oral Pathol 1986; 15:71-77.
- Maize JC, Fitzpatrick TB. Dermatology in general medicine. Am J Dermatopathol 1979; 1:378.
- Kanwar AJ, Ghosh S, Dhar S, et al. Oral lesions of lichen planus. Int J Dermatol 1993; 32:76.
- González-Moles M-A. The use of topical corticoids in oral pathology. Med Oral Patol Oral Cir Bucal 2010; 15:e827-31.
- Thongprasom K, Luangjarmekorn L, Sererat T, et al. Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. J Oral Pathol Med 1992; 21:456-58.
- Kini R, Nagaratna DV, Saha A, et al. Therapeutic management of oral lichen planus: A review for the clinicians. World J Dent 2011; 2:249-53.
- Kiran MS, Vidya S, Aswal GS, et al. Systemic and topical steroids in the management of oral mucosal lesions. J Pharm Bioallied Sci 2017; 9(Suppl 1):1–3.
- Lozada F, Silverman S Jr, Migliorati C. Adverse side effects associated with prednisone in the treatment of patients with oral inflammatory ulcerative diseases. J Am Dent Assoc 1984; 109:269-70.
- Chopra A, Mittal RR, Kaur B. Dapsone versus corticosteroids in lichen planus. Indian J Dermatol Venereol Leprol 1999; 65:66-68.
- Lear JT, English JS. Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol 1996; 21:56-67.
- Verma KK, Mittal R, Manchanda Y. Azathioprine for the treatment of severe erosive oral and generalized lichen planus. Acta Derm Venereol 2001; 81:378-89.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol 1993; 71:725-31.
- Hassan I, Keen A, Majid S, et al. Evaluation of the antioxidant status in patients of lichen planus in Kashmir valley–A hospital based study. J Saudi Soc Dermatol Dermatologic Surg 2013; 17:13-16.
- Jacob RA, Burri BJ. Oxidative damage and defense. Am J Clin Nutr 1996; 63:985S-90S.
- Barikbin B, Yousefi M, Rahimi H, et al. Antioxidant status in patients with lichen planus. Clin Exp Dermatol 2011; 36:851-54.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Vaishnavi Devi B and Santhosh Kumar MP*
Department of Oral and Maxillofacial Surgery, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, IndiaReceived: 23-Jul-2022, Manuscript No. jrmds-22-70103; , Pre QC No. jrmds-22-70103(PQ); Editor assigned: 25-Jul-2022, Pre QC No. jrmds-22-70103(PQ); Reviewed: 29-Aug-2022, QC No. jrmds-22-70103(Q); Revised: 13-Aug-2022, Manuscript No. jrmds-22-70103(R); Published: 22-Aug-2022
