Original Article - (2019) Volume 7, Issue 3
Validation of a New Scrub typhus Rapid Kit-Standard Q Tsutsugamushi IgM/IgG
Selvaraj Stephen1*, Jothimani Pradeep1, Shanmuga Priya T2 and Kengamuthu Sarangapani2
*Correspondence: Selvaraj Stephen, Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth (Deemed-to-be-University), India, Email:
Abstract
A new rapid kit manufactured in India, Standard Q Tsutsugamushi IgM/IgG (SD Biosensor) for Scrub typhus diagnosis targeting both IgM and IgG antibodies to Orientia tsutsugamushi, is validated for its performance. The result obtained is compared keeping ST IgM and ST IgG ELISA (Scrub Typhus Detect IgM/IgG ELISA System, InBios International, California, USA) as reference standard. Percentage sensitivity, specificity, positive predictive value and negative predictive values for SD Biosensor IgM were 97.52, 96.90, 96.72 and 97.66 respectively, with a kappa value of 0.944, which is considered as ‘very good’. However with reference IgG antibody detection, the percentage sensitivity, specificity, positive predictive value and negative predictive value were 49.15, 98.48, 47.20 and 68.42 respectively with a kappa value of 0.489, which is considered as ‘moderate’. In acute scrub typhus, IgM antibodies predominate and are consistently present during the early days of illness. Presence of ST IgG antibodies points to either a past infection or relapse/recurrence. This kit may be useful for diagnosing acute scrub typhus for IgM antibodies because of its cost-effectiveness. Inclusion of strong positive and week positive controls in the kit is recommended so as to compare with the test results. As is the case with all rapid kits, regular and frequent monitoring of the performance is mandatory and clinical correlation is essential.
Keywords
Orientia tsutsugamushi, Scrub typhus, Rapid immunochromatographic test, IgM ELISA, IgG ELISA
Introduction
Scrub typhus is an important emerging/re-emerging infectious disease across India. Once confined to the shrub vegetation of jungles, this zoonotic disease is now reported even in the urban parts of India and almost from every state and Union territories [1-13]. The mainstay of Scrub typhus (ST) diagnosis is serology, since isolation of the etiological agent Orientia tsutsugamushi is hazardous, requiring Bio-safety level III containment facilities. Serological tests include the non-specific Weil-Felix (WF) test and specific tests like Enzyme Linked Immunosorbent Assay (ELISA), Immunofluorescent Assay (IFA) etc. Many Indian as well as overseas researchers have employed serological tests like WF [1,4,5,7,9,11], ELISA [14-21] and IFA [10,16,20,21] for identification of Q tsutsugamushi in clinical specimens. Rapid kits based on lateral flow cytometry for the rapid diagnosis of ST are available in Indian market [2,6-9,12]. A new such rapid kit Standard Q tsutsugamushi IgM/IgG (SD Biosensor, Gurgoan, Haryana) is about to be marketed soon. The aim of this study was to validate this particular kit for its performance in detecting IgM and/or IgG antibodies to Q tsutsugamushi in clinically and laboratory confirmed cases of acute Scrub typhus.
Materials and Methods
This prospective laboratory based study was conducted on archived blood samples which were collected and preserved at -20ºC during the period of August 2018 to February 2019 in the Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Pondicherry. After getting waiver from our Institutional Human Ethics Committee (IHEC), this research was initiated for evaluation of Scrub typhus rapid kit-Standard Q Tsutsugamushi IgM/IgG (Lot No: C029003; Expiry Date: 07/10/2020) (SD Biosensor, Gurgoan, Haryana). The sample size was calculated based on 87.5% sensitivity of the serological test and margin of error as 6%, using the formula: “n=[t2 × p(1-p)]/m2”, Where t=confidence level at 95%, p=sensitivity of the tests, m=margin of error at 6%. Number of samples to be tested was 117 (n=117), which was rounded upto 120. ST IgM/IgG ELISA positive and negative samples of febrile cases and normal controls were included as follows:
Febrile patients: InBios ELISA IgM positive 120; Negative 60;
Febrile patients: InBios ELISA IgG positive 120; Negative 60;
Voluntary Blood Donors (IgM and IgG Negative): 70
(n=250 each for IgM and IgG categories)
The test was performed as per manufacturer ’ s instructions:
The rapid test Standard Q Tsutsugamushi is pre-coated with recombinant Tsutsugamushi antigen conjugated to gold colloid. About 10 μl of serum or whole blood is added to the specimen well of the device and 3 drops (90 μl) of buffer was added to the buffer well and the results were observed after 15 minutes. Violet band appearing in the control as well as a test line (IgM and/or IgG) indicates that the sample is positive. Absence of violet band in test line indicates negative test. The test is invalid if there is no development of violet band in the control line.
The reference tests ST IgM and IgG ELISA (InBios International, California, USA) were carried out in strict compliance with the procedures specified in the technical brochures and as reported earlier [6]. Twenty samples comprising of both positive/negative (IgM and/or IgG) were blinded and sent to Microbiology Laboratory, Indira Gandhi Government General Hospital and Post Graduate Institute, Pondicherry for Inter-laboratory validation.
Statistical analysis
Sensitivity, Specificity, Positive predictive value, and Negative predictive value for SD Biosensor Standard Q Tsutsugamushi IgM/IgG was calculated keeping ST InBios IgM and IgG ELISA as the reference by using MedCalc Diagnostic Test Calculator (Online).
Results
The cut off values (OD) for the reference test ST IgM ELISA (InBios, USA) was ≥ 0.55 [1] and for ST IgG it was ≥ 1.8 [10]. Out of 250 serum samples, 180 were among febrile patients, which include 120 IgM ELISA positive and 60 negative cases. Similarly, for IgG detection 180 febrile patients were included among which 120 were positive for IgG and 60 negative. Seventy blood donors negative for both ST IgM and IgG ELISA were included as controls in both tests.
Barring two serum samples, remaining 118 were positive for IgM by both kits (98.33%). However, regarding ST IgG detection, only 56 out of 120 ELISA positive samples (46.67%) were positive by Standard Q Tsutsugamushi IgM/IgG kit. Among 60 IgM and IgG ELISA negative samples, five (8.33%) were positive for IgM and two (3.33%) for IgG rapid kit. All 70 blood donors were negative for IgM and IgG by both InBios ELISA as well as Standard Q Tsutsugamushi IgM/IgG kits.
The result obtained is compared keeping ST IgM and ST IgG ELISA (Scrub typhus Detect IgM/IgG ELISA System, InBios International, California, USA) as reference standard. Percentage sensitivity, specificity, positive predictive value and negative predictive values for SD Biosensor (Standard Q Tsutsugamushi) kit IgM were 97.52, 96.90, 96.72 and 97.66 respectively, with a kappa value of 0.944, which is considered as ‘ very good ’ . However with reference IgG antibody detection, the percentage sensitivity, specificity, positive predictive value and negative predictive value were 49.15, 98.48, 47.20 and 68.42 respectively with a kappa value of 0.489, which is considered as ‘moderate’ (Table 1).
| Category | Kappa (95% C.I) | Sensitivity (95% C.I) | Specificity (95% C.I) | Positive Predictive Value (PPV) (95% C.I) | Negative Predictive Value (NPV) (95% C.I) |
|---|---|---|---|---|---|
| SD Biosensor Rapid IgM vs InBios IgM ELISA | 0.944 (0.903-0.985) | 97.52% (92.93% to 99.49%) | 96.90% (92.25% to 99.15%) | 96.72% (91.83% to 98.73%) | 97.66% (93.16% to 99.22%) |
| SD Biosensor Rapid IgG vs InBios IgG ELISA | 0.489 (0.392-0.586) | 49.15% (39.83% to 58.52%) | 98.48 % (94.63% to 99.82%) | 47.20% (87.87% to 99.15%) | 68.42% (64.44% to 72.15%) |
Table 1: Diagnostic test evaluation of Rapid ST (SD Biosensor) vs Conventional ELISA (InBios, USA) (n=250)
In Standard Q Tsutsugamushi IgM/IgG rapid kit (SD Biosensor) IgM and IgG were detected in the same port, whereas in Scrub typhus IgM/IgG rapid ImmuneMed Inc kit has two separate ports for IgG and IgM. The coating of recombinant antigens are different in both the rapid kits. ImmuneMed kit contains a mixture of more than two strains (cr56, kr56 and r21) of Q. tsutsugamushi, whereas Standard Q Tsutsugamushi IgM/IgG rapid kit (SD Biosensor) used a recombinant antigen.
Discussion
False positivity was observed in five cases for ST IgM and two cases in ST IgG for this SD Biosensor kit. Another rapid kit for Scrub typhus, which detects total antibodies (IgM+IgG+IgA) to Q. tsutsugamushi (SD Bioline Tsutsugamushi kit; Standard Diagnostics, Seoul, Korea), was reported as highly satisfactory in 2014 with sensitivity, specificity, PPV and NPV of 91.67%, 90.48%, 91.67% and 90.48% respectively [9].
However, over a period of five years (2011-2015) the performance of the very same kit was found to be very poor regarding its sensitivity, specificity, PPV and NPV of 23.3%, 100%, 100% and 56.60% respectively [2]. Pote et al. [8] also experienced a lower sensitivity of 38.0% but a specificity of 100% for this kit. This reiterates the necessity of subjecting the rapid kits to regular and more frequent quality control. SD Bioline rapid was used from the year 2011 to 2015. From January 2015, onwards we started getting a large number of false negative results even in strongly suspected ST cases based on clinical and laboratory findings. We examined the following two different lots of SD Bioline Tsutsugamushi Rapid Test.
The main problem with the rapid kits in general is the failure to maintain the cold chain during storage, transport to the suppliers and performing the test with patients’ blood samples by the end user. What we have observed with SD Bioline can also happen in the future with Standard Q Tsutsugamushi IgM/IgG rapid kit (SD Biosensor) if the cold chain is not adequately maintained by the kit manufacturers. Additionally, the performance of the rapid kits must be checked regularly and monitored with known positive and negative controls. ST InBios IgM Rapid kit has performed well with percentage sensitivity, specificity, PPV and NPV of 99.25, 93.02, 95.68, and 98.77 respectively [6].
However, with regard to the performance of InBios IgG ELISA, it was only moderate with percentage sensitivity, specificity, PPV and NPV of 44.12, 83.49, 46.15 and 82.33 respectively.
Another rapid ST kit (MyTest Scrub typhus Ab Test Card, Delhi) has been recently validated by Mund et al., according to whom IgM results showed sensitivity, specificity, PPV and NPV were 97.23%, 100%, 100% and 98.52% respectively [7]. However, these authors have not compared IgG results of MyTest rapid kit against InBios IgG ELISA.
ST IFA IgM/IgG is the gold standard for the serological diagnosis of Scrub typhus [10,15-21]. However, IFA has several inherent drawbacks like subjectivity of the test interpretation, need for technical expertise, exorbitant cost and the kits have to be imported. ST ELISA kits have been validated by both overseas and Indian Rickettsiologists and found to be equally sensitive and specific [1,10,11,15,16,19,21,22].
A rapid kit which is not available in India as of now has been evaluated by us earlier which detects both IgM and IgG antibodies in the same kit in two different ports (ImmuneMed Scrub typhus Rapid, Gangwon-do, South Korea) (Figure 1).
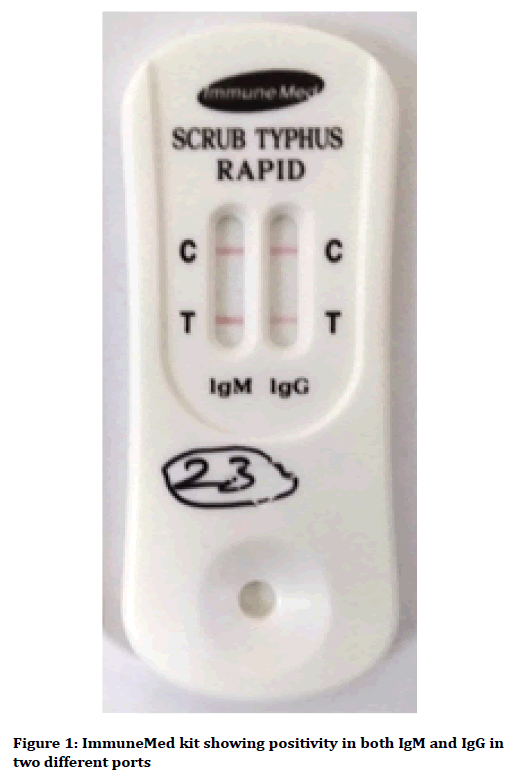
Figure 1. ImmuneMed kit showing positivity in both IgM and IgG in two different ports
For IgM this kit had percentage sensitivity, specificity, PPV and NPV of 87.00, 94.64, 96.67 and 80.30 respectively. Whereas for IgG, the sensitivity, specificity, PPV and NPV were 77.32, 86.44, 90.36 and 69.86 respectively [11].
In the inter laboratory validation of SD Biosensor Standard Q Tsutsugamushi IgM/IgG kit, a concordance of 100% between the two laboratories was observed for ST IgM detection. However, one sample was reported as IgG negative by the second laboratory, and hence the concordance was 95% (k=0.615 which is considered as ‘good’). We have observed that IgG positive lines were light violet, which could be sometimes mistaken as negative (Figure 2). In any case, clinical correlation is a must.
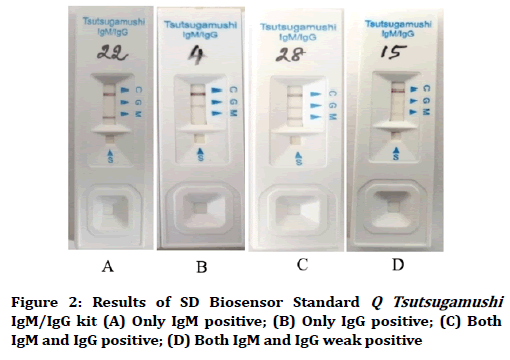
Figure 2. Results of SD Biosensor Standard Q Tsutsugamushi IgM/IgG kit (A) Only IgM positive; (B) Only IgG positive; (C) Both IgM and IgG positive; (D) Both IgM and IgG weak positive
Conclusion
This rapid SD Biosensor Standard Q Tsutsugamushi IgM/IgG kit (as well as other ST rapid kits) do not include known strong positive and weak positive serum controls. While the detection of ST IgM antibody by the rapid kit is ‘ very good ’ (k=0.944), regarding IgG antibody the sensitivity is only ‘moderate’ (k=0.489). In IgG positive samples lines are faint and sometimes take more time to appear (15-25 minutes)
Cost Comparision
InBios Scrub typhus Detect IgM Rapid Test and InBios Scrub typhus Detect IgG Rapid Test cost each 280 INR per test (thus totalling 560 INR per sample for IgM+IgG), whereas MRP for SD Biosensor kit is 200 INR only per test, which can detect not only IgM but IgG as well in a single cassette. MyTest Scrub typhus Ab Test Card, Delhi, is quite affordable with MRP of 165 INR per test, but needs to be properly validated for the performance of IgG.
Source of Support
SD Biosensor Health Care Pvt. Ltd. has provided sufficient number of Standard Q Tsutsugamushi IgM/IgG kits (Lot No: CO29003) free of cost for its validation.
Acknowledgement
The authors are grateful for the free supply of Standard Q Tsutsugamushi IgM/IgG kits by SD Biosensor Health care Pvt. Ltd., Gurugram, Haryana, India. Authors thank the Chairman, Vice-Chancellor, Dean-Academic, Dean of Faculty and Dean-Research of Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth (Deemed-to-be-University), Pondicherry, for the facilities provided.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- Rahi M, Gupte MD, Bhargava A, et al. DHRICMR guidelines for diagnosis and management of Rickettsial diseases in India. Indian J Med Res 2015; 141: 417-22.
- Stephen S, Anitharaj V, Pradeep J. Poor sensitivity of a rapid kit for diagnosing Scrub typhus: Need for continuous monitoring and regular quality check. J Pure App Microbiol 2018; 2:1583-6.
- Gupta N, Chaudhry R, Mirdha B, et al. Scrub typhus and leptospirosis: The fallacy of diagnosing with IgM enzyme linked immunosorbant assay. J Microb Biochem Technol 2016; 8:071-5.
- Sengupta M, Anandan S, Daniel D, et al. Scrub typhus seroprevalence in healthy Indian population. J Clin Diagn Res 2015; 9:DM01-02.
- Borkakoty B, Jakharia A, Biswas D, et al. Co-infection of scrub typhus and leptospirosis in patients with pyrexia of unknown origin in Longding district of Arunachal Pradesh in 2013. Indian J Med Microbiol 2016; 34:88-91.
- Anitharaj V, Stephen S, Pradeep J, et al. Serological diagnosis of acute Scrub typhus in South India: Evaluation of InBios Scrub typhus detect IgM rapid test and comparison with other serological tests. J Clin Diagn Res 2016; 10:7-10.
- Mund K, Pattnaik D, Patro S, et al. Serodiagnosis of Scrub Typhus cases by different diagnostic tests. Int J Curr Microbiol App Sci 2019; 8:2145-52.
- Pote K, Narang R, Deshmukh P. Diagnostic performance of serological tests to detect antibodies against acute scrub typhus infection in central India. Indian J Med Microbiol 2018; 36:108-12.
- Stephen S, Sangeetha B, Ambroise S, et al. Outbreak of scrub typhus in Puducherry & Tamil Nadu during cooler months. Indian J Med Res 2015; 142:591-7.
- Varghese GM, Rajagopal VM, Trowbridge P, et al. Kinetics of IgM and IgG antibodies after scrub typhus infection and the clinical implications. Int J Infect Dis 2018; 71:53-5.
- Stephen S, Kim SH, Pradeep J, et al. Evaluation of immunemed Scrub typhus rapid kit, for diagnosis Scrub typhus in South India. J Vect Borne Dis 2016; 53:283-7.
- Munilakshmi P, Krishna MV, John MS, et al. FUO cases showing prevalence of Scrub Typhus: A comparative study by ELISA and rapid test in a tertiary care hospital in Andhra Pradesh, India. Int J Curr Microbiol App Sci 2015; 4:632-40.
- Patricia KA, Hoti SL, Kanungo R, et al. Improving the diagnosis of Scrub Typhus by combining groEL based polymerase chain reaction and IgM ELISA. J Clin Diagn Res 2017; 11:DC27-31.
- Silpasakorn S, Waywa D, Hoontrakul S, et al. Performance of SD bioline Tsutsugamushi assays for the diagnosis of scrub typhus in Thailand. J Med Assoc Thai 2012; 95:S18-22.
- Blacksell SD, Jenjaroen K, Phetsouvanh R, et al. Accuracy of rapid IgM-Based immunochromatographic and immunoblot assays for diagnosis of acute Scrub typhus and Murine typhus infections in Laos. Am J Trop Med Hyg 2010; 83:365-9.
- Watthanaworawit W, Turner P, Turner C, et al. Diagnostic accuracy assessment of immunochromatographic tests for the rapid detection of antibodies against orientia tsutsugamushi using paired acute and convalescent specimens. Am J Trop Med Hyg 2015; 93:1168-71.
- Lijuan Z, Si H, Yuming J, et al. A rapid, sensitive and reliable diagnostic test for scrub typhus in China. Indian J Med Microbiol 2011; 29:368-71.
- Ching WM, Rowland D, Zhang Z, et al. Early diagnosis of scrub typhus with a rapid flow assay using recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi. Clin Diagn Lab Immunol 2001; 8:409-14.
- Kingston HW, Blacksell SD, Tanganuchitcharnchai A, et al. Comparative accuracy of the InBios Scrub typhus detect IgM rapid test for the detection of IgM antibodies by using conventional serology. Clin Vaccine Immunol 2015; 22:1130-2.
- Kim YJ, Park S, Premaratna R, et al. Clinical evaluation of rapid diagnostic test kit for scrub typhus with improved performance. J Korean Med Sci 2016; 31:1190-6.
- Coleman RE, Sangkasuwan V, Suwanabun N, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg 2002; 67:497-503.
- Blacksell SD, Paris DH, Chierakul W, et al. Prospective evaluation of commercial antibody-based rapid tests in combination with a loop-mediated isothermal amplification PCR assay for detection of Orientia tsutsugamushi during the acute phase of scrub typhus infection. Clin Vaccine Immunol 2012; 19:391-5.
Author Info
Selvaraj Stephen1*, Jothimani Pradeep1, Shanmuga Priya T2 and Kengamuthu Sarangapani2
1Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Sri Balaji Vidyapeeth (Deemed-to-be-University), Pondicherry, India2Laboratory of Microbiology, Indira Gandhi Government General Hospital and Post Graduate Institute, Pondicherry, India
Citation: Selvaraj Stephen, Jothimani Pradeep, Shanmuga Priya T, Kengamuthu Sarangapani,Validation of a New Scrub typhus Rapid Kit-Standard Q Tsutsugamushi IgM/IgG, J Res Med Dent Sci, 2019, 7(3): 159-163.
Received: 18-May-2019 Accepted: 20-Jun-2019
