Research - (2022) Volume 10, Issue 5
Effect of Disinfection on Some Mechanical Properties of Polycarbonate Base Material
Ali Saleh Hatem* and Zainab Saleh Abdullah
*Correspondence: Ali Saleh Hatem, Department of Prosthodontics, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Dentists face a variety of serious challenges, including the need for ideal material in order to deliver successful dental care to patients. To produce an effective prosthesis with satisfactory appearances these materials should be biologically compatible, readily usable, inexpensive, and easy to manipulate with regulated technological procedures. Removable prosthodontics entails the use of a variety of tools and multipurpose products that are difficult to sterilize or clean, such as prostheses, impressions, and stone casts, which increases the risk of cross-contamination between both the dental clinic and the laboratory.
Materials and Methods: Three NAOCL concentrations (0.25%,0.5% and 0.75 %) were used as determined by the pilot study, which yielded the most suitable results. A total of 200 sample were prepare and divided into 3 experimental group and a control group (2%chlorhexidine,0.25% and 0.5% NAOCL). Each group was subdivided into five identical subgroups. For each subgroup, ten specimens were utilized for each test (elongation percentage, shore D hardness, roughness, impact, color change).
Results: The results of the tests showed significant decrease of Impact strength of the polycarbonate after immersion in (2% chlorhexidine) and it continuous decrease in (0.25% NAOCL) while the lowest mean value was for the (NAOCL 0.5%). The results of surface hardness showed significant slightly increase with (2% chlorhexidine) and decreased with (0.25% NAOCL), (NAOCL 0.5%) respectively. The results of surface roughness showed slightly increased values in (2% chlorhexidine) when compared to control group while increase when immersion in (0.25% NAOCL), (0.5% NAOCL) concentration respectively. The percent of elongation test showed the polycarbonate non-effected when immersed in (2% chlorhexidine) and decrease when immersed in (0.25% NAOCL), (0.5% NAOCL) concentration respectively. The results of color stability showed increased values in (2% chlorhexidine) when compared to control group and continuous increase when immersion in (0.25%NAOCL), (0.5% NAOCL) concentration respectively.
Conclusion: The polycarbonate resin material can be safely immersed in (2% chlorhexidine) disinfectant solution without any damaging effect on surface hardness, surface roughness, percent of elongation and color stability. As well, immersion in (2% chlorhexidine) was preferable than NAOCL disinfectant solution in comparison to the control group.
Keywords
Properties of polycarbonate, Disinfection, Chlorhexidine, NAOCL
Introduction
Dentists face a variety of serious challenges, including the need for ideal material in order to deliver successful dental care to patients. To produce an effective prosthesis with satisfactory appearances these materials should be biologically compatible, readily usable, inexpensive, and easy to manipulate with regulated technological procedures [1].
The denture should have been aesthetically attractive, perform well, and be biocompatible with the surrounding oral tissues that sustain it. A successful denture should be dimensionally compatible to improve chewing performance, be convenient for patients, and avoid inflammation of the oral tissues [2].
Polycarbonate PC (poly (Bisphenol A) carbonate) is an amorphous polymer with signs of crystallinity in certain places [3]. It's made of high-quality plastic that's low in weight and translucent. Furthermore, PC has exceptional mechanical properties such as structural stability, excellent impact resistance [4]. high plastic deformation without fracture or crack [5]. and high thermal resistance, retaining its properties over a broad temperature range of 140°C to –20°C [6]. PC, has certain defects in terms of properties, such as low chemical tolerance, limited scratch resistance, and sensitivity to UV rays, which causes color changes. PC may be improved by adding the right additives or mixing it with other polymers [4].
Disinfection is the use of chemical substances to destroy or remove organisms that might cause infection, although physical procedures such as heat treatments are also included [7]. Many mechanical and chemical approaches have been employed to clean and disinfect the denture surfaces from the bacteria that had accumulated on them. Chemicals such as 2% sodium hypochlorite, chlorhexidine and denture brushing have been utilized, however these efforts have had detrimental effects on the acrylic resin foundation of the denture [8].
Materials and Method
In this study, Polycarbonate Extra rigid polymer M10 XR, Deflex, Argentina, Hyposol 3%sodium hypochlorite and Chlorhexidine 2% (Poland) were used.
Pilot Study
polycarbonate specimens were immersed in NAOCL suspensions at various concentrations (0.25%, 0.5% and 0.75% for each concentration) (20 Mins, 3 times daily for 15 days). For each party, three specimens were prepared. By measuring percent of elongation and surface roughness, the concentrations of 0.25% and 0.5 % were chosen because they offered the most suitable results.
Specimen Grouping
A total of 200 specimens were made and grouped into control group (normal saline referred to as group A) and three experimental groups (2% chlorhexidine referred to as group B, 0.25% NAOCL referred to as group C and0.5% NAOCL referred to a group D). Finally, every group was further subdivided into five subgroups according to the intentionally performed test.
Mold Fabrication
Plastic and metal sheets (1-4 mm) in thickness according to each test were cut by laser engraving machine (JL- 1612, Jinan Link Manufacture and Trading Co., Ltd., China). The cutting was performed in accordance with predetermined requirements for every test as determined by a computer software Auto CAD 2019 (Autodesk Inc., San Rafael, CA, USA) (Figure 1).

Figure 1: A, plastic mold for impact test. B, plastic mold for elongation test. C, metal mold for hardness and roughness test. D, metal mold for color stability test.
Injecting Thermoplastic Material Specimens
Polycarbonate cartridges were loaded into the DEFLEX MAD automated programmable device and injected into the flask as recommended by the manufacturer: as shown in figure 2. Polycarbonate injection under pressure (5-7 Bar) heat (305oC+/- 10C) for (15 min). as in (Figure 2).

Figure 2: Automatic programmable device DEFLEX MAD.
The proper cartridge of injecting material was selected. At its closed end, a vaseline base lubricant was applied, and the cartridge was inserted into one of the two heating cylinders, After the flask's two halves were assembled, screws used to fasten them, and then the flask was placed and secured in the injecting unit, the pressure was kept constant for 1 minute to compensate for the setting contraction. The cylinder was then pulled away from the flask by around 3 to 4 mm, allowing the cartridge to be disengaged. The flask was then removed and the used cartridge was automatically discharged by pressing the evacuation key, After the flask was cooled, the screws were loosened and the flask halves were slowly opened. The specimens were removed from the molds.
Preparation, Finishing and Storage of Specimens
After de-flasking of the test specimens, every specimen was finished and polished Except for the surface roughness test, they were finished and any flashes of materials were removed. The polycarbonate specimens were separated and the sprue were removed by using metal disk to cut it then each specimen was finished by using special finishing plastic burs used to finish them, then all the specimens were finished by using a sand paper with (120) grain size and to avoid over heating the specimens were cooled by immersing them in rubber bowel filled with water (finishing the specimens for 15 second then immersion in the water for 15 second).
Polishing of the specimens was done in lathe polishing machine by using ruge disc and soft brush with pumice as in (figure 3). To achieve a high-gloss finish, a dental lathe was utilized at a low speed (1500rpm) with continuous water cooling to avoid overheating the specimens.
Figure 3: finished and polished equipment.
Testing Procedures
Impact strength test
Bar shaped specimens with length, width, and thickness of (80 mm x 10 mm x 4 mm) (ISO.179-1, 2000 for unnotched specimens) were constructed, Charpy type impact testing instrument was used. Horizontally the specimen was supported at its ends and hit by a free swinging pendulum. 30 joules testing capacity were used, the impact strength is expressed in joules by the scale reading on the side of Charpy type impact testing instrument. The Charpy impact strength of un-notched specimen was calculated in KJ/m2 by the following equation [9]:
Impact strength = E/B.D x 103 (KJ/m2)
E: is the impact absorbed energy in joules.
E: is the impact absorbed energy in joules.
D: is the thickness Test samples
Shore D hardness test
The test was done according to (ASTM D2240-03 standard). The dimensions are 25mm in length, 25mm in width and 6mm in thickness. Five areas were marked on the surface, six millimeters separated from each other. Shore D durometer (Laizhou Laihua Co., China) with a blunt end and a diameter 1.40 diameter with 30o cone mm used for testing. The specimen is placed on a flat table, which is raised up by a lever until the indenter contacts the specimen for 3 second. The hardness at each point was measured, then the mean of the three measurements was considered as the hardness of the specimen.
Surface roughness test
Specimens for roughness test were fabricated and tested according to ADA specification No.12, 1999. A Portable digital device with 0.001μm accuracy was used. After placing the specimen on a stable and hard surface, the stylus touches the specimen surface at three areas and travels (11mm) along the surface after touching the initial point. The mean of these three readings was measured and considered as the roughness of the specimen.
Percent of elongation test
Dumbbell shaped specimens with dimensions of (60±2mm overall length, 16±1mm length of narrow parallel side portion, 12±1mm width at the ends, 3±0.2mm width of narrow parallel side portion, 2±0.2mm thickness and 12±1mm large radius) (ISO 527: 1993plastic).
The test was measured using Tinins Olsen testing machine as in (figure 4) for measuring elongation strength at a cross head speed of 5mm / min and with a 50 mm grip – to – grip displacement at the failure was recorded in millimeter (mm) and the percent of elongation values were calculated from the following equation:
Figure 4: Tinins Olsen testing machine.
Percent of elongation = displacement (final gauge length – initial gauge length) / initial gauge length * 100%
Elongation = displacement /16 mm (initial gauge length) * 100%
Color stability
Rectangular-shaped samples with diameter of 20mm length, 10mm width and thickness of 0.8mm were fabricated for color change test. The amount of light transmission of the sample was measured by using a spectrophotometer as a function of wavelength. The sample was located over the light opening of the device and then subjected to light. The percentage and the reading of transmitted light, was then acquired from the screen of the computer attached with the device.
The Statistical Analysis
The statistical analysis was done using one-way ANOVA (Analysis of variance) and post hoc tests (Tukey HSD) by statistical analysis software (IBM SPSS Statistics 26). Levene’s test was also conducted to determine the homogeneity of variances.
The probability (P) value of > 0.05 was considered nonsignificant statistically (NS), P values of ≤ 0.05 were considered statistically significant (S), and P values of ≤ 0.01 were considered highly significant (HS).
Results
Impact Test
The experimental group A (control) showed highest mean values followed by the chlorhexidine group (B) and NAOCL 0.25% (group C) while the lowest mean value was for the NAOCL0.5% (Group D). Tukey HSD test was conducted to compare means of each two groups of all four impact strength test groups. There were significant differences between group A and D, and non-significant between all other each two groups of all study groups. as seen in (Table 1).
| Impact Strength | ANOVA | Tukey HSD | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Min | Max | Mean | ±SD | F | P value | Groups | P value |
| (A) | 230.23 | 242.79 | 235.258 | 4.39429 | 4.867 | (S).006 | A B | 0.978 |
| A C | 0.052 | |||||||
| (B) | 228.42 | 239.68 | 234.567 | 3.63863 | A D | 0.02 | ||
| B C | 0.122 | |||||||
| (C) | 225.17 | 236.91 | 230.601 | 4.37565 | B D | 0.053 | ||
| (D) | 224.19 | 233.1 | 229.924 | 2.96958 | C D | 0.98 | ||
| Levene statistics=1.177 , P value=.332(NS) | ||||||||
Table 1: Minimum values, maximum values, means, standard deviation, ANOVA (one way), and post-hoc test of impact strength.
Shore D Hardness Tests
The experimental group (NAOCL 0.5%) showed lowest mean values followed by the (NAOCL 0.25%) and (control) while the highest mean value was for the (chlorhexidine 2%), Tukey HSD test was conducted to compare means of each two groups of all four surface hardness test groups. There were significant differences between group B and D, and Non-significant between all other each two groups of all study groups as seen in (table 2).
| Shore D Hardness | ANOVA | Tukey HSD | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Min | Max | Mean | ±SD | F | P value | Groups | P value |
| (A) | 80 | 86.34 | 82.551 | 2.41663 | 3.508 | .025(S) | A B | 0.966 |
| A C | 0.482 | |||||||
| (B) | 80.79 | 84.89 | 82.958 | 1.49633 | A D | 0.092 | ||
| B C | 0.243 | |||||||
| (C) | 78.78 | 83.89 | 81.286 | 1.83235 | B D | 0.032 | ||
| (D) | 77.36 | 82.98 | 80.43 | 1.98555 | C D | 0.764 | ||
| Levene statistics=2.130, P value=.113 | ||||||||
Table 2: Minimum values, maximum values, means, standard deviation, ANOVA (one way), and post-hoc test of hardness.
Surface Roughness Tests
The experimental group (control) showed lowest mean values followed by the (chlorhexidine 2%), (NAOCL 0.25%) respectively while the highest mean value was for the (NAOCL 0.5%), Tukey HSD test was conducted to compare means of each two groups of all for surface roughness test groups. There were non-significant differences between group A and B, and significant between all other each two groups of all study groups (Table 3).
| Surface roughness | ANOVA | Tukey HSD | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Min | Max | Mean | ±SD | F | P value | Groups | P value |
| (A) | 3.271 | 3.583 | 3.4235 | 0.1183 | 36.207 | .000 (H.S) | A B | 0.883 |
| A C | 0 | |||||||
| (B) | 3.335 | 3.624 | 3.4699 | 0.09512 | A D | 0 | ||
| B C | 0.006 | |||||||
| (C) | 3.527 | 3.976 | 3.7936 | 0.15838 | B D | 0 | ||
| (D) | 3.717 | 4.236 | 3.9892 | 0.17927 | C D | 0.019 | ||
| Levene statistics=2.182, p value=.107 | ||||||||
Table 3: Minimum values, maximum values, means, standard deviation, ANOVA (one way), and post-hoc test of Surface roughness.
Percent of Elongation
The experimental group (2% chlorhexidine) showed highest mean values followed by (control group and 0.25%NAOCL) group, while the lowest mean value was for the (NAOCL 0.5%) as seen in (Table 4).
| Percent of Elongation | ANOVA | |||||
|---|---|---|---|---|---|---|
| Group | Min | Max | Mean | ±SD | F | P value |
| (A) | 0.692 | 0.887 | 0.7823 | 0.06573 | 2.417 | .082(N.S) |
| (B) | 0.7007 | 0.836 | 0.7844 | 0.03898 | ||
| (C) | 0.69 | 0.808 | 0.7471 | 0.04319 | ||
| (D) | 0.6723 | 0.802 | 0.7348 | 0.05148 | ||
| Levene statistics= 2.252, p value=.099 | ||||||
Table 4: Minimum values, maximum values, means, standard deviation, ANOVA (one way), and post-hoc test of percent of elongation.
Color Stability
The experimental group (0.5% NAOCL) showed highest mean values followed by (0.25% NAOCL and chlorhexidine) groups, while the lowest mean value was for the control group, Tukey HSD test was conducted to compare means of each two groups of all four color stability test groups. There were non-significant differences between group A and B, and highly significant between all other each two groups of all study groups as seen in (Table 5).
| Color Stability | ANOVA | Tukey HSD | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Min | Max | Mean | ±SD | F | P value | Groups | P value |
| (A) | 2.109 | 2.271 | 2.1986 | 0.052073 | 50.605 | .000(H.S) | A B | 0.099 |
| A C | 0 | |||||||
| (B) | 2.172 | 2.317 | 2.2581 | 0.052308 | A D | 0 | ||
| B C | 0 | |||||||
| (C) | 2.254 | 2.493 | 2.3712 | 0.073116 | B D | 0 | ||
| (D) | 2.385 | 2.532 | 2.4827 | 0.041264 | C D | 0 | ||
| Levene statistics= 1.227, P value=.314 | ||||||||
Table 5: Minimum values, maximum values, means, standard deviation, ANOVA (one way), and post-hoc test of color stability.
SEM Result
The results indicate well distributed and increase degree of roughness into polycarbonate matrix with 2%chx and more roughness in 0.25% ,0.5% NAOCL respectively as show in figure (Figure 5).
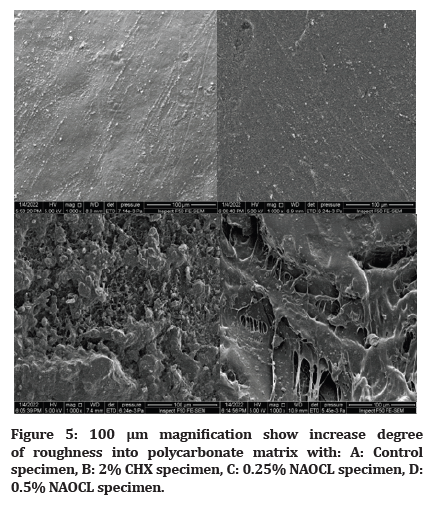
Figure 5:100 μm magnification show increase degree of roughness into polycarbonate matrix with: A: Control specimen, B: 2% CHX specimen, C: 0.25% NAOCL specimen, D: 0.5% NAOCL specimen.
Discussion
Because the denture is worn in the mouth and is exposed to a variety of chemical compounds as well as multiple stresses, the denture base materials must have specialized qualities to meet as many of the requirements as possible [10]. An at-home disinfecting process can be used to immerse prosthetic devices in solutions. Disinfectants, on the other hand, interfere with the polymer's properties [11].
Impact strength test is the ability of material to dropped on a hard surface, it broke. This was connected to the amount of energy adsorption that causes a material to fracture when subjected to a quick blow [12]. The high impact strength of polycarbonate was due to a number of factors related to its structure, including the presence of a considerable aromatic component of phenyl groups (benzene ring) in its backbone and pendant oxygen and hydrogen groups of modest size These The tangling with the neighboring polymer is enabled by the oxygen and hydrogen groups. hydrogen bonding and the creation of chains All of these elements will result in Intermolecular movement resistance should be increased [13]. The results of the study showed that impact strength for (0.5% NAOCL, 0.25% NAOCL) decreased significantly when compared to the control group. Polymer degradation may cause the material to dissolve when immersed in liquids [14]. Van der walls forces were used to achieve interfacial adhesion between inorganic fillers and organic matrix; these weak bindings may reduce the elastic modulus of modified polymer at low stress, but they tend to break down at high impact loading, making the material more brittle [15,16].
Shore D hardness is defined as a material's resistance and ability to abrade opposing dental structures [17]. After immersion, there was statistically significant decrease in surface hardness comparison to the control group, except for test group B (chlorhexidine), increase polycarbonate samples degradation with increase NAOCL concentration as shown in SEM. After the degradation, the damage in the matrix makes the water transport mechanism even more active, water absorption increases [18]. PC and water are polar polymer and polar solvent, respectively. Therefore, solvent-solute interaction, namely ‘like dissolves like” will occur. PC also contains oxygen in carbonate group, CO3 which is susceptible to water absorption. Crowding of water molecules at PC polymer chain causes the polymer structure to deform and open up, leading to higher free volume [19]. Water molecules diffuse more quickly into polymers, acting as a plasticizer and gradually relaxing the polymer chain, resulting in a decrease in hardness [20].
Surface roughness is significant factor because bacteria can accumulate on rough denture surfaces, affecting oral health [21]. The roughness of denture is affected by the material's properties, polishing techniques, and the operator's skill [22]. The findings of the ANOVA tests showed that the surface roughness of the 2%chlorhexidine group was statistically non-significant change when compared to the control groups. The surface roughness test showed that the mean surface roughness values for the (0.25% NAOCL) and (0.5% NAOCL) was a statistically highly significant increase when compared to the control group. This increase might be due to fact that when a polymer is exposed to a solution, it suffers hydrolytic breakdown as a result of the chemical interaction between the solution and the organic matrix in the open spaces between the polymer chains [23].
Elongation is a measurement of how far a material can stretch before breaking. When a material needs to tolerate bigger deformation without breaking, it needs to have a high elongation [24]. After immersion, there was statistically significant decrease in percent of elongation for 0.25%, 0.5% NAOCL respectively comparison to the control group, except for test group B (disinfectant), This decrease might be due to fact that when a polymer is exposed to NAOCL solution, increase the BPA release although higher pH values [25].
color stability: Many denture base resins have been produced that make processing easier and faster, While all these materials have appropriate mechanical properties, color stability is also important since a change in appearance indicates a decrease in longterm denture quality [26].The spectrophotometer study shows significant differences between the tested groups in both the (0.25% NAOCL) and (0.5% NAOCL) groups, One of the possible reasons for the significant color differences in the color stability test when compared to those immersed in distilled water groups is the surface porosity caused by the dissolution of a small soluble component of the material, which causes the color change, or the increased roughness caused by the immersion disinfectant method, which leads to accelerated chemical degradation [14].
Conclusion
The polycarbonate resin material can be safely immersed in (2% chlorhexidine) disinfectant solution without any damaging effect on surface hardness, surface roughness, percent of elongation and color stability. As well, immersion in (2% chlorhexidine) was preferable than NAOCL disinfectant solution in comparison to the control group.
Conflicts of Interest
All authors declare that they have no competing interests.
Funding
The project is completely self-funded.
Ethical Approval
In-vitro study.
References
- Al-Kebsi AM, Al-Motareb FL, Al-Hamzy M, et al. Multiple risk factors of Candida albicans associated denture stomatitis. On J Dent Oral Health 2018; 1:1-5.
- Raj PA, Dentino AR. Denture polymers with antimicrobial properties: a review of the development and current status of anionic poly (methyl methacrylate) polymers. Future Med Chem 2013; 5:1635-45.
- Kaneko T, Tatara T, Hirose M. Response to Muggleton et al. Acta Anaesthesiol Scand 2020; 64:866.
- Tsintzou GP, Antonakou EV, Achilias DS. Environmentally friendly chemical recycling of poly (bisphenol-A carbonate) through phase transfer-catalysed alkaline hydrolysis under microwave irradiation. J Hazard Mater 2012; 241:137-45.
- Mark JE. Physical Properties of Polymers Handbook. 2022.
- https://books.google.com/books/about/The_Chemistry_of_Polymers.html?id=5XFsT69cX_YC
- Hardy K, Stark J. Mathematical models of the balance between apoptosis and proliferation. Apoptosis. 2002; 7:373-81.
- Nazirkar RB. Effect of PBSW and Biofertilizers on the Solubilization of P from RP to Soybean in Inceptisol. Asian J Soil Sci 2014; 9:240-3.
- DeHoff PH, Barrett AA, Lee RB, et al. Thermal compatibility of dental ceramic systems using cylindrical and spherical geometries. Dent Mater 2008; 24:744-52.
- Hiroshi Takahashi, Minoru Kawaguchi, Ryo Nakashiro, et al. Effect of Water Sorption on the Ultimate Transverse Strength of a Heat-cured Denture Base Resin Relined with Direct Denture Reline Materials. Nihon Hotetsu Shika Gakkai Zasshi 42: 668-72.
- Kimoto S, Kimoto K, Murakami H, et al. Survival analysis of mandibular complete dentures with acrylic‐based resilient liners. Gerodontology. 2013; 30:187-93.
- https://www.abebooks.com/book-search/title/applied-dental-materials/
- Wang X, Brydson R, Jha A, Ellis J. Microstructural analysis of Al alloys dispersed with TiB2 particulate for MMC applications. J Microsc 1999; 196:137-45.
- McDonough WF, Arevalo RD. Crust–mantle and core–mantle recycling. Geochim Cosmochim Acta 2006; 18:A409.
- Freire M, Oliveira MC. Under the sign of crisis: rise and fall of Rubião. Machado de Assis in Line 2013; 6:66-82.
- Machado RM, Palmeira-De-Oliveira A, Martinez-De-Oliveira J, et al. Vaginal films for drug delivery. J Pharm Sci 2013; 102:2069-81.
- Rajaee S. Pure and copure submodules. Int J Algebra 2014; 8:649-53.
- Comyn J. Wood adhesives: Chemistry and Technology. Int J Adhes Adhes 1985;5(3):163.
- White N. Grasslands. The Antioch Review. 2006;64(2):323.
- Alexandre CD, Vranjac SD. Mass vaccination campaign against serogroup C meningococcal disease, municipality of Itapeva, Sao Paulo. Rev Public Health 2005; 39:139-40.
- Senouci H, Lounici M, Rahal K. Antidiphtheria immunity of the Algerian population: A seroepidemiological study. Med Mal Infect 2004; 34:316-20.
- https://www.imdb.com/title/tt0327597/
- Kimoto S, Kimoto K, Kitamura A, et al. Effect of dentist's clinical experience on treatment satisfaction of a complete denture. J Oral Rehabil 2013; 40:940-7.
- Kranke P, Apfel CC, Roewer N. Reported data on granisetron and postoperative nausea and vomiting by Fujii et al. are incredibly nice!. Anesth Analg 2000; 90:1004-6.
- Krishnan M, Sharma DG. The effect of alloy properties on the heat flow across the casting/mold interface. Metallurgica Mater 1993; 28:447-51.
- Powers ET, Powers DL, Gierasch LM. FoldEco: a model for proteostasis in E. coli. Cell Rep 2012; 1:265-76.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ali Saleh Hatem* and Zainab Saleh Abdullah
Department of Prosthodontics, College of Dentistry, University of Baghdad, IraqCitation: Ali Saleh Hatem, Zainab Saleh Abdullah, Effect of Disinfection on Some Mechanical Properties of Polycarbonate Base Material, J Res Med Dent Sci, 2022, 10 (5): 92-98.
Received: 27-Apr-2022, Manuscript No. JRMDS-22- 63045; , Pre QC No. JRMDS-22-63045 (PQ); Editor assigned: 29-Apr-2022, Pre QC No. JRMDS-22-63045 (PQ); Reviewed: 13-May-2022, QC No. JRMDS-22-63045; Revised: 18-May-2022, Manuscript No. JRMDS-22-63045 (R); Published: 25-May-2022
