Research - (2019) Volume 7, Issue 3
In vitro Activity of New Azole Luliconazole Compared to Fluconazole against Candida Strains Isolated from Oral Lesions of Cancer Patients
Mehrnoush Maheronnaghsh1, Parvin Dehghan2, Mahnaz Fatahinia1,3* and Ali Rezaei-Matehkolaei4
*Correspondence: Mahnaz Fatahinia, Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Iran, Email:
Abstract
Introduction: Oral candidiasis is the most common fungal infection in patient's undergone chemotherapy. The aim of this study was to investigate the incidence and causative agents of oral candidiasis along with in vitro activity of new azole luliconazole compared with fluconazole against agents of oral candidiasis in a population of cancer patients.
Materials and Methods: A total of 385 oral samples from patients with various types of cancer and undergone chemotherapy were subjected to fungal culture. The yeast isolates were then identified by using PCR-RFLP method. The MIC values for fluconazole and luliconazole were determined using broth microdilution according to the M27-S3 protocol of the CLSI. The MICs, MIC50, MIC90 and geometric mean (GM) values were evaluated for all the isolates.
Results: Totally, 36 yeast strains were isolated which were found to be as Candida albicans (n=26; 72.2%), C. glabrata (n=5; 13.8%), C. kefyr (n=3; 8.3%), Pichia kudriavzevii (C. krusei) (n=1; 2.8%) and C. stellatoidea 1 (n=1; 2.8%) species. The MICs for luliconazole against all Candida isolates ranged from 0.007 μg/mL to 2 μg/mL; compared to 0.25 μg/mL to 128 μg/mL for fluconazole. The lowest GM values were 0.85 for C. glabrata and 1.14 μg/mL for C. kefyr isolates. The GM values of both antifungal drugs showed no significant differences between the C. albicans isolates.
Conclusion: C. albicans remains the most common agent of oral candidiasis in patients with different forms of cancers. Compared to fluconazole, Luliconazole showed more activity against all Candida species and potentially can be considered as an effective antifungal agent alternative to fluconazole.
Keywords
Candida, Luliconazole, Fluconazole, Chemotherapy
Introduction
Oral candidiasis (known as oral thrush) is the most frequent fungal infection of the oral cavity caused by the genus Candida [1]. Under normal conditions, members of the genus Candida, as the most frequent fungi resident in the oral cavity, coexist with the other flora and do not cause disease but, any alteration in the oral microenvironment and/or systemic environment may led to an overgrowth of mycotic flora of the mouth and subsequently to oral fungal infections. Such alterations can especially be seen in babies less than one month, pregnant women, HIV-infected patients, and individuals with diabetes mellitus, renal failure, and immune disorders [2]. Finally, cancer patients under chemotherapy have an increased risk for developing oral candidiasis up to 40% [3,4].
Despite some surveillance health cares and the advances in medical interventions and prolonged life expectancy, the incidence of invasive systemic mycoses has increased markedly. These patients should be regularly monitored in term of oral candidiasis [1,5]. However in patients, with systemic candidiasis mortality rate is from 71% to 79% [6]. Among the healthier population, the carrier rates have been reported 20% to 75% with no sign of infection [7]. In patients with leukemia who, receiving immunosuppressive or broad-spectrum antibiotic therapy and those with AIDS the carrier rates increased to 90%, and 95%, respectively [8-11]. The diagnosis of oral candidiasis is fundamentally on the basis of clinical findings and recognition of the lesions; however it should be confirmed by mycological detection of yeast in the oral samples and/or isolation in culture. Oral candidiasis can be treated with either topical or systemic antifungal agents. Polyene antifungals drugs such as nystatin and amphotericin B were the most drugs used topically, in the treatment of oral candidiasis. Currently one of the most important medicines, particularly for patients with extreme immune weakness, is azole compounds. Among the azoles, Fluconazole was found the drug of choice in systemic treatment of oral candidiasis [12,13]. Recently, fluconazole-resistant Candida isolates such as C. albicans, C. glabrata and Pichia kudriavzevii (C. krusei) has been reported in emerging immunocompromised patients who had treated for therapy or prophylaxis [14,15].
Luliconazole is a new imidazole antifungal agent; it was originally developed in Japan as a topical antifungal drug and received marketing approval in 2005. It has broadspectrum activities against medically important fungi such as Candida, Malassezia, Aspergillus, and Trichophyton species [16].
Candidiasis in cancer patients and antifungal susceptibility profiles of Candida species in such group was rarely a matter of investigation in developing countries such as Iran. Regarding to markedly increase in the isolation rate of fluconazole-resistant Candida strains from immunocompromised patients on one hand, and broad-spectrum activity of luliconazole on the other hand, in current study, we aimed to both characterize the spectrum of Candida species causing oral infections in patients with different cancers, and to compare the in vitro activity of luliconazole (LUL) with fluconazol against these strains.
Materials and Methods
Patients, samples and isolates
The ethics permission for the study was granted by Isfahan University of Medical Sciences/Ethics Committee (IR.MUI.REC.1394.3.754). 385 patients undergoing chemotherapy who were hospitalized in some diagnostic and therapeutic centers in Isfahan and were suspected to oral candidiasis were included in the study. The patients were clinically investigated and those with pseudomembranous, erythematous, and hyperplastic lesions were selected for sampling. Two specimens were collected from each accessible and defined oral lesion by gently rubbing a sterile plain swab over the lesion. The specimens were examined by using direct microscopy and culture of the samples. A direct wet slide using 10% KOH was prepared from each sample and another swab was cultured on the chromogenic CHROMagar™ Candida medium (CHROMagar, Paris, France) and incubated at 35°C for 48h. The Diagnostic criteria for oral candidiasis were based on the clinical recognition of the lesions confirmed by mycological demonstration of yeasts, pseudohyphae or hyphae in direct smear and culture (≥ 10 CFU yeasts) [17].
Identification of the isolates
Identification of grown yeasts in the study was primarily made following the color of colonies grown on the CHROMagar™ Candida medium as per the protocol of the manufacturer. The definitive identification of the isolates to the species level performed by the previous established PCR based tests. Firstly, the genomic DNA was extracted from each isolate with boiling method [18]. The nuclear 5.8S rRNA gene and its flanking internal transcribed spacer regions (ITS1 and ITS2) were amplified through the PCR in a 25 μl reaction mixture volume using the fungal universal ITS1/ITS4 primer pair [19], and thereafter digested by restriction endonuclease MspI (Thermo Fisher Scientific, Waltham, MA, USA). The fragmented products were then separated through electrophoresis on 2% gel agarose. Final identification of each isolate was accomplished by comparison of the banding patterns with those specific banding profiles demonstrated in the previous report for Candida species [18]. Discrimination of C. dubliniensis which shares the same RFLP pattern with C. albicans was performed in a duplex-PCR approach using two species-specific PCR primer pairs, targeting sequences in ITS-1 and ITS-2 regions [20]. The sequences of the primers were as follows: CDUF (5′-AA ACTTGTCACGAGATTATTTTT) and CDUR (5′-AAA GTTTGAAGAATAAAATGGC-3′) for specific identification of C. dubliniensis and CALF (5 ′ - TGGTAAGGCGGGATCGCTT-3 ′ ) and CALR (5 ′ -GGT CAAAGTTTGAAGATATAC) for detection of C. albicans. The duplex-PCR was conducted in a reaction mixture and thermal condition as previously described [20]. Identification of isolates was achieved by comparing the size of amplified products in a 1% gel electrophoresis.
Antifungal susceptibility testing
Antifungal susceptibility testing was carried out following the microdilution method outlined in CLSI document M27-S3 [21]. Briefly, the stocks of fluconazole (Serva, USA) and luliconazole (Nihon Nohyaku Co., Osaka, Japan) were prepared in water and dimethyl sulfoxide (DMSO), respectively. Serial two-fold dilutions of the antifungals were prepared in RPMI 1640 medium buffered with MOPs buffer to pH 7.0-7.2, and 100 μl of each dilution was dispensed into 96 well round bottom microdilution plates (SPL, South Korea). The final concentrations of drugs ranged from 0.125 μg/mL-16 μg/mL for fluconazole and 0.001 μg/mL to 1 μg/mL for luliconazole. To prepare the fungal inocula, all identified isolates were sub-cultured on Sabouraud dextrose agar (SDA) and after overnight growth, the yeasts suspensions were diluted in RPMI 1640 medium and adjusted spectrophotometrically at 530 nm wavelength to yield final inoculum of 1 × 103 CFU/ml to 5 × 103 CFU/ml. 100 μl of this suspension was distributed in each well of the microplate for testing. The micro-plates were incubated at 35°C for 48 h. Turbidity was typically determined by subjective observation [17].
Results
Out of 385 oral samples, in 36 cases a yeast strains arose from the culture, which were identified by PCR method. C. albicans was the most frequently isolated species (72.2%) in the oral cavities of patients using both CHOROM agar culture medium and molecular method. The other detected Candida, were C. glabrata (n=5; 13.8%), C. kefyr (n=3; 8.3%), and each of Pichia kudriavzevii (C. krusei) and C. stellatoidea (n=1; 2.8%).
The band patterns obtained after treating MspI restriction digestion of the PCR products of Candida isolates are shown in (Figures 1 and 2). Duplex PCR with specific primers on 26 samples with RFLP profile shared for C. albicans and C. dubliniensis yielded an around 100 bp product indicative for C. albicans (Figure 3).
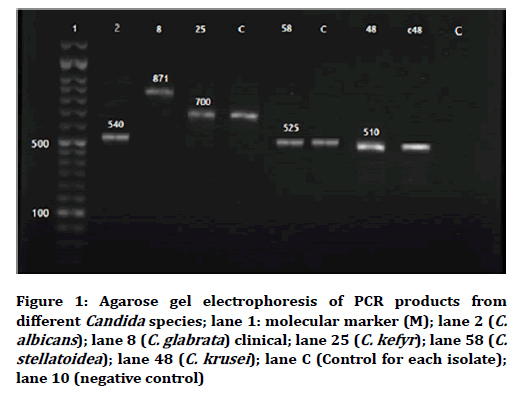
Figure 1. Agarose gel electrophoresis of PCR products from different Candida species; lane 1: molecular marker (M); lane 2 (C. albicans); lane 8 (C. glabrata) clinical; lane 25 (C. kefyr); lane 58 (C. stellatoidea); lane 48 (C. krusei); lane C (Control for each isolate); lane 10 (negative control)
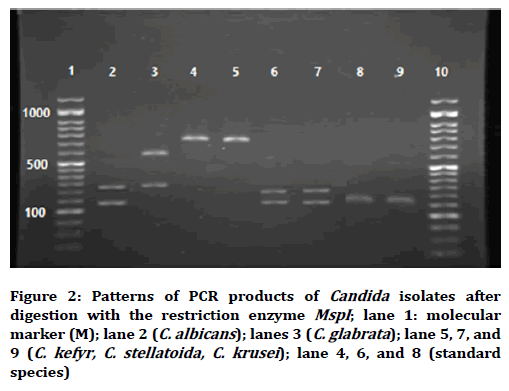
Figure 2. Patterns of PCR products of Candida isolates after digestion with the restriction enzyme MspI; lane 1: molecular marker (M); lane 2 (C. albicans); lanes 3 (C. glabrata); lane 5, 7, and 9 (C. kefyr, C. stellatoida, C. krusei); lane 4, 6, and 8 (standard species)

Figure 3. Results of duplex PCR products from Candida albicans on the 1% agarose gel electrophoresis; lane 1: 100 bp ladder; lane 2-6 (C. albicans); lane 7 C (negative control)
The ranges of luliconazole and Fluconazole minimum inhibitory concentrations (MICs) were ≥ 2-0.007 μg/ml and ≥ 128-0.5 μg/ml, respectively. The data obtained after doing broth microdilution method and also the geometric mean (GM), MIC50, MIC90 and MIC ranges of antifungal drugs against 36 clinical isolates are shown in Tables 1 and 2.
| Isolates no. | Isolate designation | Site of isolation | FLCa(µg/ml) | LuLb (µg/ml) |
|---|---|---|---|---|
| 1 | C. alb2 | Lukemia | 2 | 0.5 |
| 2 | C. alb3 | Bladder | 2 | 0.5 |
| 3 | C. alb4 | Gastrointestinal | 2 | 0.5 |
| 4 | C. alb5 | Lymphoma | >128 | 0.06 |
| 5 | C. alb9 | Lukemia | 2 | 2 |
| 6 | C. alb11 | Gastrointestinal | 4 | 0.5 |
| 7 | C. alb13 | Lukemia | 2 | 1 |
| 8 | C. alb14 | Lukemia | >128 | 0.25 |
| 9 | C. alb18 | Gastrointestinal | >128 | 0.5 |
| 10 | C. alb19 | Lukemia | 4 | 1 |
| 11 | C. alb21 | Gastrointestinal | >128 | 1 |
| 12 | C. alb22 | Lung | 2 | 0.25 |
| 13 | C. alb23 | Bone | >128 | 0.5 |
| 14 | C. alb24 | Lymphoma | 2 | 0.007 |
| 15 | C. alb27 | Lymphoma | >128 | 0.25 |
| 16 | C. alb35 | Bladder | 2 | 0.007 |
| 17 | C. alb36 | Gastrointestinal | >128 | 0.06 |
| 18 | C. alb37 | Liver | >128 | 0.06 |
| 19 | C. alb38 | Lukemia | 4 | 0.5 |
| 20 | C. alb40 | Lymphoma | >128 | 0.06 |
| 21 | C. alb42 | Lymphoma | 0.5 | 0.12 |
| 22 | C. alb44 | Liver | 0.5 | 0.03 |
| 23 | C. alb46 | Gastrointestinal | >128 | 1 |
| 24 | C. alb55 | Breast | 16 | 1 |
| 25 | C. alb49 | Gastrointestinal | >128 | 0.007 |
| 26 | C. alb50 | Gastrointestinal | >128 | 1 |
| 27 | C. glabrata1 | Lymphoma | 0.5 | 0.007 |
| 28 | C. glabrata7 | Breast | 0.5 | 0.5 |
| 29 | C. glabrata28 | Breast | 2 | 0.007 |
| 30 | C. glabrata30 | Gastrointestinal | 8 | 0.007 |
| 31 | C. glabrata47 | Liver | >128 | 0.015 |
| 32 | C. kefyr6 | Lung | 0.25 | 0.25 |
| 33 | C. kefyr25 | Bladder | 16 | 0.5 |
| 34 | C. kefyr41 | Lukemia | >128 | 0.5 |
| 35 | C. krusei48 | Gastrointestinal | >128 | 0.5 |
| 36 | C. stelatoida58 | Gastrointestinal | >128 | 1 |
Table 1: ln vitro antifungal susceptibilities of 36 clinical isolates against fluconazole and luliconazol agents
| Isolates | Antifungal | MICrange | MIC50a | MIC90b | MIC gmc | R | S | Total |
|---|---|---|---|---|---|---|---|---|
| C. albicans | FLU | >128-0.5 | >128 | >128 | 1.32 | 12 | 14 | 26 |
| LUL | 2-0.007 | 0.5 | 1 | 1.12 | 1 | 25 | ||
| C. glabrata | FLU | >128-o.5 | 0.5 | 8 | 2.6 | 1 | 4 | 5 |
| LUL | 0.007 | 0.007 | 0.5 | 0.85 | - | 5 | ||
| C. kefyr | FLU | >128-0.5 | 16 | >128 | 5.1 | 1 | 2 | 3 |
| LUL | 0.5-0.5 | 0.5 | 0.5 | 1.14 | - | 3 | ||
| C. krusei | FLU | >128 | - | - | - | 1 | - | 1 |
| LUL | o.5 | - | - | - | - | 1 | ||
| C. stelatoida | FLU | >128 | - | - | - | 1 | - | 1 |
| LUL | 1 | - | - | - | - | 1 |
a=MIC which inhibits 50% of Candida species isolates in test
b=MIC which inhibits 90% of Candida species isolates in test
c=Geometric mean MIC
Table 2: MIC data for all clinical isolates
Considering the limited number detection of Pichia kudriavzevii (C. krusei) and C. stellatoidea isolates C. stellatoidea isolate was confirmed by sucrose sugar absorption test; MIC50, MIC90, and GM MIC were not calculated for these species. In addition C. stellatoidea possess the same size band pattern of C. albicans by this method and showed creamy color on CHOROMagar. So identification of C. stellatoidea isolate was confirmed by sucrose sugar absorption test.
Discussion
The main cause of candidiasis is still C. albicans although the other non- albicans species such as C. glabrata and Pichia kudriavzevii (C. krusei) which are less susceptible to azole antifungal drugs are increasing [22,23]. Nowadays the number of immunosuppressive patients has significantly increased due to malignant disease and despite some surveillance health cares and the advances in medical interventions and prolonged life expectancy, the various opportunistic fungal infections such as candidiasis, are also increased. From more than 150 known species of Candida only 15 species detected from the patients with candidiasis. It has been documented that in 95% of infections, the pathogens involved are C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and Pichia kudriavzevii (C. krusei) [24]. At the present study from the total of 36 detected isolates, 26 (72.2%) cases were confirmed to be C. albicans and 10 (13.8%) of the isolates were identified as non-albicans species using both phenotypic and molecular methods. There may be some factors such as previous azole therapy, underlying disease, geographical location, nutrition and age of the patients that cause variation in the kind of species and etiologic agents of candidiasis [25,26].
In a study done by Mohammadi et al. on diabetic patient in Iran, the most frequent Candida species in the oral cavities of diabetic patients were C. albicans (36.2%) followed by Pichia kudriavzevii (C. krusei) (10.4%) and C. glabrata (5.1%) [27]. The later study also has done in the same country so the different frequency of isolates should be due to the different underlying diseases in patients, however at the present study we found more C. albicans and less C. glabrata.
In a prospective study done in cancer center of New York, to investigate Candida colonization and infection in cancer patients, C. albicans was the predominant species (67.3%) followed by C. glabrata (45.6%). They found the overall resistance among all isolated Candida was 9.4% to fluconazole [28]. In the present study, out of the 26 C. albicans isolates, 12 species were resistant to Fluconazole using microdilution method (more than 128 μg/ml). It should be noted that only 5 species of C. glabrata detected from the mouth of the patients and 4 (80%) isolates were sensitive to fluconazole. In this case, it can be concluded that 20% of the C. glabrata species and about half of the isolates of C. albicans (50%) were resistant to fluconazole that this result is opposed to the previous reports, therefore correct identification and alternative fungal sensitivity drugs should be considered. In agreement with findings of DiNubile et al. results C. albicans and C. glabrata were predominant yeasts isolated from the oral cavity of patients with periodontitis. They reported C. albicans and C. glabrata 75% and 12.5% respectively the same as the results of the present study C. albicans 72.2% and C. glabrata 13.8%. This finding may guide our empiric treatment to shift for old azole in high-risk patients with known predisposing factors from developing serious candida infection particular with C. albicans. The MIC of luliconazole against Candida species has been reported to be higher than that against filamentous fungi that the increasing and extensive use of fluconazole prophylaxis and triazole treatment are very much related [29]. At the present study PCR-RFLP showed fast and easy recognition alongside using CHOROMagar-Candida pheno-typing method and for the early detection of yeasts. In between, these PCR-based techniques for many species it is reliable [30,31]. PCR-RFLP method is able to detect very small amounts of DNA [32]. In this study, identification of C. albicans and Pichia kudriavzevii (C. krusei) by CHROMagar Candida and PCR-RFLP method showed the same results but C. glabrata and C. kefyr were identified only by molecular methods. In addition C. stellatoidea possess the same size band pattern of C. albicans by this method and showed creamy color on CHOROMagar. So identification of C. stellatoidea isolate was confirmed by sucrose sugar absorption test.
Luliconazole is an imidazole antifungal agent with a unique structure, as the imidazole moiety is incorporated into the ketene dithioacetate structure. Luliconazole, a new imidazole agent exhibited in vitro antifungal activity against several molds and yeasts. That is used in the form of a topical cream of 1%. The purpose this part of the study was to compare the in vitro antifungal activities of luliconazole with fluconazole for therapy of candidiasis against clinical Candida isolates of patients undergoing chemotherapy, since the majority resistant clinical Candida strains to fluconazole have been isolated from gastric cancer and lymphoma. Luliconazole with MIC range: 0.007 μg/ml-1 μg/ml demonstrated greater potency against detected isolates than the Fluconazole antifungal drug. In a study conducted by Clarkson et al. to determine the susceptibility of Candida species isolated from chemotherapy patients, it was found that the sensitivity of them to ketoconazole and clotrimazole is more than fluconazole [3]. Previous studies have shown that luliconazole had low MICs against black fungi, Aspergillus fumigatus and dermatophyte species. Uchida et al. also showed that the GM MICs of luliconazole for Malassezia furfur, Malassezia sympodialis, and Malassezia slooffiae were approximately 1.4 μg/ml, 0.1 μg/ml, and 1 μg/ml, respectively [33]. Luliconazole showed the best activity with the lowest geometric mean 0.85 for C. glabrata isolates in comparison with 1.14 μg/ml, against C. kefyr isolates. The geometric mean of luliconazole and fluconazole for C. albicans was not significantly different. In a luliconazole drug sensitivity research conducted in 2017 on candida isolates detected from different patients, the MIC range and MIC90 of vaginal isolates (HIV-) were 1-0.063 and 1μg/mL [34].
Conclusion
In conclusion, there are no clinical trials evaluating the efficacy of luliconazole in candidal infections, preclinical studies have supported such a role. In this study, given that in vitro activities of luliconazole against azoleresistant candida isolates were superior to fluconazole. The evaluation of antifungal susceptibility patterns can provide useful information on the resistance patterns of the isolates. It should be consider that luliconazole may emerge as an effective and broad-spectrum antifungal agent in the future.
Acknowledgments
Authors are thankful to the Health Research Institute, Infectious and Tropical Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences for their cooperation in this study.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- Mirhendi H, Makimura K, Zomorodian K, et al. Differentiation of Candida albicans and Candida dubliniensis using a single-enzyme PCR-RFLP method. Jpn J Infect Dis 2005; 58:235.
- Lalla RV, Latortue MC, Hong CH, et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer 2010; 18:985-92.
- Clarkson JE, Worthington HV, Eden TO. Interventions for preventing oral candidiasis for patients with cancer receiving treatment. Cochrane Database Sys Rev 2007.
- Jones JA, Avritscher EB, Cooksley CD, et al. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 2006; 14:505-15.
- Neppelenbroek K, Campanha N, Spolidório DMP, et al. Molecular fingerprinting methods for the discrimination between C. albicans and C. dubliniensis. Oral Dis 2006; 12:242-53.
- Fraser VJ, Jones M, Dunkel J, et al. Candidemia in a tertiary care hospital: Epidemiology, risk factors, and predictors of mortality. Clin Infect Dis 1992; 15:414-21.
- Ghannoum MA. Candida adherence to epithelial cells: 0. CRC Press 2017.
- Abu-Elteen K, Abu-Alteen R. The prevalence of Candida albicans populations in the mouths of complete denture wearers. New Microbiol 1998; 21:41-8.
- Safdar A, Armstrong D. Infectious morbidity in critically ill patients with cancer. Crit Care Clin 2001; 17:531-70.
- Jackson BE, Wilhelmus KR, Mitchell BM. Genetically regulated filamentation contributes to Candida albicans virulence during corneal infection. Microb Pathog 2007; 42:88-93.
- Naglik JR, Moyes DL, Wächtler B, et al. Candida albicans interactions with epithelial cells and mucosal immunity. Microb Infect 2011; 13:963-76.
- Kontoyiannis D, Mantadakis E, Samonis G. Systemic mycoses in the immunocompromised host: an update in antifungal therapy. J Hosp Infect 2003; 53:243-58.
- Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev 2011; 24:141-73.
- Case CP, Macgowan A, Brown N, et al. Prophylactic oral fluconazole and candida fungaemia. Lancet 1991; 337:790.
- Casalinuovo I, Di Francesco P, Garaci E. Fluconazole resistance in Candida albicans: A review of mechanisms. Eur Rev Med Pharmacol Sci 2004; 8:69-78.
- Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: An evidence-based review. Core Evid 2014; 9:113.
- Maheronnaghsh M, Tolouei S, Dehghan P, et al. Identification of Candida species in patients with oral lesion undergoing chemotherapy along with minimum inhibitory concentration to fluconazole. Adv Biomed Res 2016; 5.
- Mohammadi R, Mirhendi H, Rezaei-Matehkolaei A, et al. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med Mycol 2013; 51:657-63.
- White TJ, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: A guide to methods and applications 1990; 18:315-22.
- Ahmad S, Khan Z, Asadzadeh M, et al. Performance comparison of phenotypic and molecular methods for detection and differentiation of Candida albicans and Candida dubliniensis. BMC Infect Dis 2012; 12:230.
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard-Third Edition M27-A3. CLSI 2008; 28.
- Fatahi M, Shokohi T, Sooteh H, et al. Molecular identification of Candida albicans isolated from the oncology patients at four university hospitals in Mazandaran province (2005-6). J Mazandaran Univ Med Sci 2007; 17:1-11.
- Whaley SG, Berkow EL, Rybak JM, et al. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol 2017; 7:2173.
- Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 2014; 10:95.
- Pfaller M, Diekema D. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev 2007; 20:133-63.
- Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 2008; 21:606-25.
- Mohammadi F, Javaheri MR, Nekoeian S, et al. Identification of Candida species in the oral cavity of diabetic patients. Curr Med Mycol 2016; 2:1.
- Safdar A, Chaturvedi V, Cross EW, et al. Prospective study of Candida species in patients at a comprehensive cancer center. Antimicrob Agents Chemother 2001; 45:2129-33.
- DiNubile MJ, Hille D, Sable CA, et al. Invasive candidiasis in cancer patients: Observations from a randomized clinical trial. J Infect 2005; 50:443-9.
- Williams DW, Wilson MJ, Lewis M, et al. Identification of Candida species by PCR and restriction fragment length polymorphism analysis of intergenic spacer regions of ribosomal DNA. J Clin Microbiol 1995; 33:2476-9.
- McCullough MJ, Clemons KV, Stevens DA. Molecular and phenotypic characterization of genotypic Candida albicans subgroups and comparison with Candida dubliniensis and Candida stellatoidea. J Clin Microbiol 1999; 37:417-21.
- Mirhendi H, Makimura K, Khoramizadeh M, et al. A one-enzyme PCR-RFLP assay for identification of six medically important Candida species. Nihon Kokyuki Gakkai Zasshi 2006; 47:225-9.
- Uchida K, Nishiyama Y, Tanaka T, et al. In vitro activity of novel imidazole antifungal agent NND-502 against Malassezia species. Int J Antimicrob Agents 2003; 21:234-8.
- Taghipour S, Kiasat N, Shafiei S, et al. Luliconazole, a new antifungal against Candida species isolated from different sources. J Mycol Med 2017.
Author Info
Mehrnoush Maheronnaghsh1, Parvin Dehghan2, Mahnaz Fatahinia1,3* and Ali Rezaei-Matehkolaei4
1Department of Medical Mycology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran2Department of Parasitology and Mycology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
3Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
4Department of Medical Mycology, School of Medicine and Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Citation: Mehrnoush Maheronnaghsh, Parvin Dehghan, Mahnaz Fatahinia, Ali Rezaei-Matehkolaei, In vitro Activity of New Azole Luliconazole Compared to Fluconazole against Candida Strains Isolated from Oral Lesions of Cancer Patients, J Res Med Dent Sci, 2019, 7(3): 132-138.
Received: 04-Apr-2019 Accepted: 20-May-2019
