Research Article - (2021) Volume 9, Issue 12
The Impact of Combined Application of Cilantro White Varnish and ER,CR:YSGG Laser on Enamel Resistance to Caries: An In Vitro Study
Hussein Sami Abdullah1* and Aisha Aram Qasim2
*Correspondence: Hussein Sami Abdullah, Researcher (Master student) College of Dentistry,, University of Mosul, Mosul, Iraq, Email:
Abstract
Background: This study aims to investigate the efficacy of ER, CR: YSGG laser combined with functionalized tricalcium
phosphate (fetch) exclusively from Cilantro White Varnish in preventing of enamel caries.
Materials and Methods: Freshly extracted fifty sound upper first premolars teeth were divided into five groups, each group
of ten samples. Group (C): only washed with deionized water. Group (V): functionalized tricalcium phosphate (fTCP)
exclusively from Clinpro White Varnish. Group (L1): Er,Cr:YSGG laser (0.25watt, 2.8 J/cm2, 20 Hz, 11% air, 0% water) was
irradiated. Group (V/L1): First Cilantro White varnish was applied then Er,Cr:YSGG laser. Group (L1/V): Er,Cr:YSGG laser
was irradiated followed by Clinpro White varnish. Then, the pH cycling model was used to induce artificial caries. The effect
was studied using Vickers microhardness test and the obtained results were statistically analyzed. The surface morphology
was also studied by scanning electron microscopy.
Results: There was decreasing in surface microhardness in all groups due to the demineralization. Statistically there was no
significant difference between groups of combinations and group of varnish while all of these groups had a highly
significant resistance against microhardness loss. The least value of surface microhardness belonged to the control group
and laser group. Morphological changes of enamel surfaces were in relation with increasing of enamel resistance to
demineralization.
Conclusions: The impacts on enamel resistance to demineralization and reducing its solubility seem to indicate that there is
no benefit in using Er,Cr:YSGG laser at 0.25watt alone or as a pretreatment and post treatment for fTCP varnish over using
fTCP varnish alone.
Keywords
Er,Cr:YSGG Laser, Enamel demineralization, Functionalized tri-calcium phosphate, Micro hardness
Introduction
Dentistry goes back to 5000 BC, when dental caries had been thought to have been a "tooth worm," and the term "dental caries" was first mentioned around 1634 in the literature and was derived from the Latin word caries, which describes decay. The term was first used to describe teeth's holes. Dental caries is one of the oldest and most common human diseases. It is a dominant infectious and transmissible disease caused by particular tooth-adherent bacteria, primarily streptococcus mutants that metabolize sugars and release acid, which over time demineralizes the structure of the tooth [1].
Demineralization of tooth tissues leads to the diffusion of calcium, phosphate, and carbonate out of the tooth is allowed to continue, cavitation will eventually take place [2,3]. Demineralization can be reversed in its early stages through uptake of calcium, phosphate, and fluoride. Fluoride acts as a catalyst for the diffusion of calcium and phosphate into the tooth. The rebuilt crystalline surfaces, composed of fluoridated hydroxyapatite and Fluor apatite, are much more resistant to acid attack than is the original structure. modifications have been made on fluoride varnishes to include calcium and phosphate ions within varnish structure in an attempt to further enhance its effectiveness.
Functionalized tri-calcium phosphate, or fetch, is a “smart” calcium phosphate device that permits calcium and phosphate ions delivery to the teeth and acts synergistically with the addition of fluoride to improve efficacy.
Although dental caries is a preventable disease, it is still common and remains a public health problem, especially in developing countries, and certain populations in economically developed countries [4]. Therefore, there is still a need to prevent dental caries and search for alternative methods for disease prevention, or new ways of augmenting current preventive programs. One of the potentially effective preventive measures is the use of lasers As early as 1966, Stern and Sonnies, showed that irradiated enamel specimens were resistant to acid demineralization. Another study belongs to caries prevention dates back to 1971 by a rubbery laser. Erbium lasers (Er:YAG and Err, Cr:YSGG) being hard tissue lasers are developed more recently than other laser types, Erbium, chromium: yttrium-scandium-gallium-garnet (Er,Cr:YSGG) laser ,It has a wavelength of 2.79 am Erbium lasers have been proposed to prevent enamel and dentin demineralization by ‘‘Laser-Induced Prevention of Demineralization’’ (LIPD), a mechanism by which erbium laser irradiation causes thermal changes in enamel, resulting in chemical and/or morphological structure alterations without ablation.
Lasers have also been used in combination with fluoride for caries prevention and this technique is termed as laser-activated fluoride (LAF) therapy [5]. The association of laser irradiation with fluoridated products can be a most promissory alternative to increase the enamel resistance to demineralization.
Some controversies still exist in the literature regarding the appropriate Er,Cr:YSGG laser parameters, when used alone or in combination with fluoride component containing calcium and phosphate, and their efficacy according to the order of application in decreasing the solubility of enamel.
The significance of the study is to achieve additional prevention of enamel demineralization focusing on Er, Cr:YSGG laser irradiation combined with fetch containing varnish which are hoped to give promising synergistic results.
The aim of the present study was to evaluate the effect of the Er,Cr:YSGG laser, with or without use of tri-calcium phosphate containing varnish, on enamel resistance to demineralization using artificial demineralization taking in our consideration the order of treatment. The effect was studied using Vickers micro hardness test and scanning electron microscopy.
Material and Method
Ethics Statement
This study protocol was performed submitted and approved by the Local Ethics Committee (UoM.Dent/ H.L.3/ 21) Research Ethics Committee of Collage of Dentistry, University of Mosul, Nineveh, Iraq
Teeth samples preparation
A randomized controlled study design was used. The sample size estimate was calculated using G*Power version 3.1.9.2 program. The sample size of 10 specimens for each group was calculated according to study considering α=0.05, statistical power=80% and effect size=0.52. A total of 50 premolars was needed.
Freshly extracted fifty Sound upper first premolars teeth with intact buckle enamel surface were collected from individuals (16-25) years old who were received orthodontic treatment in private orthodontic clinics in Basra. The teeth were examined visually. Sound teeth (no visible evidence of a carious lesion) were involved in the study. The teeth with restorations, caries, discoloration, fractures, enamel developmental malformations, cracks, wears, fluorosis, white spot lesions, hypoplasia and exposed to bleaching agent, hydrogen peroxide or acid etching were excluded from the study [6]. The extracted teeth were cleaned, all remnants were removed , then rinsed with deionized water and maintained in thyme solution 0.1% to avoid dehydration and inhibit the growth of bacteria and fungi on the teeth surfaces until being used in a period of less than 7 days Following polishing the teeth with non-fluoridated pumice, the remaining roots were cut 2 mm below the cemento-enamel junctions with a straight diamond bur using sufficient irrigation to avoid injuring or harming the enamel, then the crown pulp was removed with an excavator. All specimens were checked under a stereomicroscope (×10) for any cracks or enamel defects and then inserted in chemical cured resin in plastic rings (Figure-1).
Figure 1:Intact upper human upper first premolars were collected and cleaned.
Figure 2:The tooth had been excluded because of crack and remnant of composite filling on the crown of the first premolar had been discovered under stereomicroscope.
The plastic rings were cut and constructed so that their upper and lower surfaces were parallel. Each ring included a single tooth that was fixed in the center of the ring's upper surface, exposing the tooth's buccal surface. The middle 1/3 of the crown's buccal surface was estimated using a caliper by measuring the distance between the cemento-enamel junction and the cusp tip, as well as the mesio-distal dimension. An adhesive tape of (4×2mm2) was fixed on the middle 1/3 of the crowns (Figure 3). Then, the crowns were painted with acid-resistant varnish (Rimmel, London, UK) except for a (4×2mm2) window of exposed enamel. The samples were dried, and tapes were removed. As in (Figure-3).

Figure 3a: (a) specimen surface was covered with adhesive tape with size of 2 × 4 millimes.

Figure 3b: 2 × 4 mm window was exposed on the buccal enamel surface.
Materials and Equipments
Clinpro White Varnish that contain 5% Sodium fluoride and functionalized tricalcium phosphate (fTCP) exclusively from 3M ESPE, made in USA, expire date 28/7/2022. As in (Figure-4).

Figure 4: Clinpro White varnish that contain 5% Sodium fluoride (2.26% or 22.600 ppm of the fluoride ion) and functionalized tricalcium phosphate (fTCP) exclusively from 3M ESPE, made in USA.
Er,Cr:YSGG laser system (Waterlase i-Plus, Biolase Technologies Inc., San Clemente, CA USA) that emits photons with 2.78 μm wavelength With parameter of (0.25watt, 2.8 J/cm2, 20 Hz, 11% air, 0% water) For the handpiece, the MZ6 tip was chosen at the focal area with a beam diameter of 600 μm in order to avoid overheating the pulp of the teeth as in figure (5). To maintain a constant spot size during the irradiation process, an endodontic file with a rubber stop was attached to the fixed handpiece with the distance of 1 mm ,as in figure (6), from the enamel surface. Also, water cooling is not always necessary [7]. The repetition rate was set to 20 Hz according to The handpiece was fixed, in its handle, perpendicular to the enamel surface. The specimens were irradiated once for 10 seconds through slowly rotating the specimens horizontally by the hand to ensure uniform irradiation and coverage of the entire exposed sample area. Table (1) were clearly shown the irradiation parameters for laser category (Table 1).
Table (1): ER, CR:YSGG laser irradiation parameters used in the present study.
| Laser group | Power (W) | Energy density (J/cm2) | Repetition rate (Hz) | Irradiation time (s) | Water | Air |
|---|---|---|---|---|---|---|
| ER,CR:YSGG (L2) | 0.25 | 2.8 | 20 | 10 | NO | 0.11 |

Figure 5:Waterlase MZ6 tip from Biolase with diameter of 600 μm.

Figure 6: Endodontic file with a rubber stopper was attached to the fixed handpiece. The distance of 1 mm exposed from rubber stopper
Experimental design of the study
To facilitate identification, each tooth was assigned a unique number between 1 and 50, and these numbers were used to randomly assign samples to one of five study groups. Each group of ten teeth was stored at room temperature in its own beaker labeled with the group name and containing 200 ml of deionized water solution. Following that, each tooth in each group was separately placed in 10 ml deionized water in a plastic jar labeled with the tooth group's name and number. and then exposed to the following caries prevention method:
- Group (C) (control group): no agent was applied, only washed with deionized water.
- Group (V) (functionalized tricalcium phosphate (fTCP) containing varnish): exclusively from Clinpro White Varnish was applied on the window of specimens’ surface for 4 minutes according to the manufacturer’s instructions. The specimens then were stored in deionized water for 4 minutes, and then the varnish layer was removed by rubber cap and low-speed handpiece.
- Group (L1): (Er,Cr:YSGG 0.25 watt, 2.8 J/cm2, 20 Hz, 11% air, 0% water) was irradiated as explained previously on the window of specimens’ surface in a scanning style.
- Group (V/L1): First Clinpro White containing functionalized tricalcium phosphate (fTCP) varnish was applied as described for group (V). After 24 hours, the varnish layer was removed [22]. Then, Er,Cr:YSGG laser laser was irradiated as explained previously.
- Group (L1/V): Er,Cr:YSGG laser was irradiated similarly to group (L1) followed by application of Clinpro White containing functionalized tricalcium phosphate (fTCP) varnish with the same detail of group (V).
Then, the pH cycling model was used to induce artificial caries in all treatment groups through a cycle of demineralization and remineralization. Also, all treatments were performed by skillful operator and the evaluation was done blindly.
PH-Cycling
Artificial caries was produced by exposing teeth samples to PH cycling. The teeth samples were immersed in a Demineralizing solution (CaCl2 2.2mM, NaH2PO4 2.2mM, and acetic acid 0.05M, PH of 4.5, adjusted with KOH 1M) for three hours and then in a Remineralizing solution (CaCl2 1.5mM, NaHPO4 0.9mM, and KCl 0.15mM, PH of 7.0) for twenty hours. Teeth samples were briefly rinsed with deionized water between solutions and placed in artificial saliva composed of NaCl 0.40, KCl 0.40, CaCL2.2H2O 0.79, NaH2PO4.2H2O 0.78, NaS9.H2O 0.005, CO(NH2)2 Urea 0.1, in 1000 ml Distilled water, PH of 7 (concentration G \ L) for 30 minutes at the end of the demineralization process and 30 minutes at the end of the remineralization process The teeth were subjected to a total of ten cycles, each cycle was lasting for one day (24 hours). Every day, the demineralizing and remineralizing solutions were changed, and the artificial saliva was replaced after each treatment. Preparation of the chemical solutions was done by the researcher at the Marine Science Centre in University of Basrah.
Microhardness assessment
All specimens were tested for their microhardness on two occasions: first before the treatments (baseline), and then at the end of the pH cycling stage (post-treatment). A Vickers hardness tester with the load of 500 gm for 15 seconds was used (according to the instruction of the machine) for assessing the microhardness. For all samples, the load and time were constant. Three indentations were made at the exposed labial enamel surface of each sample, and the average value was recorded as the microhardness of each specimen. This test was conducted at University of Basrah, Collage of Engineering, Department of Mechanic.
VHN= (kg \mm2) = 1.854 × P \d2
P= the testing load in grams.
d= the length of the diagonal line across the indentation in microns.
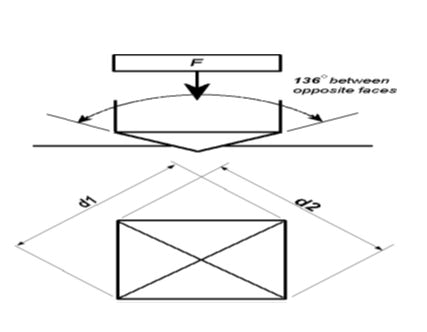
Figure 7: a) A schematic illustration of Vickers technique (Vickers microhardness machine utilizes a 136° diamond pyramid indenter that forms a square indent on the surface.

Figure 7:b) An image taken by the researcher of a tetra pyramidal indentation in Vickers technique that creates a clear, measurable indentation in the field as diagonals.
Surface morphology evaluation by Scan Electron Microscope (SEM)
Two specimens from each group that was not subjected to PH cycle were examined under SEM to observe morphological changes on enamel. teeth were mounted on the SEM holder using removable adhesive and their treated surfaces were positioned so that they would face upwards. Under vacuum conditions, each tooth was coated with gold–palladium layer using a sputter coater (Leice EM ce 600 High Vacuum Sputter Coater) and adjusted to be observed under 8 kV accelerating voltage using a SEM (Nova Nano SEM 450) at University of Basrah, Collage of Sciences, Department of Physics. The surface morphology of the teeth was examined, and representative photomicrographs were captured and digitally stored [8].
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences software program (IBM SPSS Statistics 26). Shapiro–Wilks test was applied to find out the distribution type of experimental measurement data. As the data were normally distributed, the one-way ANOVA test and Duncana test enabled us to decide whether there are any statistically significant differences between the groups or not. A p-value of less than 0.01 was considered statistically significant.
Results
Indentation surface microhardness test results
According to the obtained measurements of this study, table (2) showed the descriptive statistics including means, standard deviations, minimum and maximum values in addition to the numbers of the samples of tested groups before and after PH cycle. Based on the means values for tested groups after PH cycle, the groups of lasers then fTCP varnish, fTCP varnish then laser and fTCP varnish alone had the least reduction in the surface microhardness mean value.
Table (2): Descriptive statistics of microhardness measurements among tested groups before and after PH cycle.
| Groups Variables | Baseline | After PH Cycle | |
|---|---|---|---|
| Deionized water (C) | Mean | 310.427 | 190.115 |
| Std. Deviation | 11.77794 | 7.80915 | |
| Min. | 295.12 | 175.32 | |
| Max. | 328 | 199.75 | |
| N | 10 | 10 | |
| functionalized tri-calcium phosphate varnish (V) | Mean | 307.429 | 251.022 |
| Std. Deviation | 7.75936 | 11.98158 | |
| Min. | 294.16 | 225.96 | |
| Max. | 316.18 | 264.9 | |
| N | 10 | 10 | |
| Er,Cr:YSGG Laser 0.25 watt (L1) | Mean | 308.16 | 197.637 |
| Std. Deviation | 7.50681 | 9.96987 | |
| Min. | 295.1 | 180.85 | |
| Max. | 320.01 | 210.79 | |
| N | 10 | 10 | |
| fTCP varnish + Er,Cr:YSGG Laser 0.25 watt (VL1) | Mean | 306.664 | 255.894 |
| Std. Deviation | 11.52762 | 5.81813 | |
| Min. | 289.12 | 248.98 | |
| Max. | 330 | 267.76 | |
| N | 10 | 10 | |
| Er,Cr:YSGG Laser 0.25 watt + fTCP varnish (L1V) | Mean | 306.68 | 260.309 |
| Std. Deviation | 11.31297 | 7.60311 | |
| Min. | 291 | 245.67 | |
| Max. | 329.7 | 270.32 | |
| N | 10 | 10 | |
Table (3) ANOVA test explains that there was no significant difference for the surface microhardness readings existed among the tested groups before PH cycle at p≤0.01, while after PH cycle, there was a high significant difference among tested groups at p≤ 0.01.
Table (3): ANOVA test between tested groups at baseline and after PH cycle respectively at p≤ 0.01.
Microhardness |
Sum of Squares |
Df |
Mean Square |
F |
Sig. |
|||
|---|---|---|---|---|---|---|---|---|
|
|
Between Groups |
|
96.873 |
4 |
24.218 |
0.235 |
0.917 |
Baseline |
|
Within |
|
4645.343 |
45 |
103.23 |
|
|
|
|
|||||||
|
Groups |
|||||||
|
|
Total |
|
4742.216 |
49 |
|
|
|
|
|
Between Groups |
|
46642.82 |
4 |
11660.71 |
147.381 |
0 |
After PH |
|
Within |
|
3560.376 |
45 |
79.119 |
|
|
Cycle |
|
|||||||
|
Groups |
|||||||
|
|
Total |
|
50203.2 |
49 |
|
|
|
Duncana multiple analysis range test was done to further explain that there was no significant difference existed before PH cycle at p≤ 0.01, as all groups were arranged in a homogenous subset of data representing the surface microhardness means values for each group before PH cycle, (Table-4).
Table (4): Duncana Multiple Analysis Range test for tested groups before PH cycle.
| Groups | N | Subset for alpha = 0.01 |
|---|---|---|
| 1 | ||
| VL1 | 10 | 306.664 |
| L1V | 10 | 306.68 |
| V | 10 | 307.429 |
| L1 | 10 | 308.16 |
| C | 10 | 310.427 |
Sig. |
0.469 |
Duncana multiple analysis range test was done to further explain that there was a high significant difference of microhardness values for groups after PH cycle existed at p≤ 0.01. All groups were arranged in nonhomogeneous subsets of data representing the surface microhardness means values of each group after PH cycle at which there were no significant difference between L1V group , VL1 group and V group and all of these groups had a highly significant resistance against microhardness loss, while the least value of surface microhardness belonged to the control group of deionized water and in L1 group where ER,CR:YSGG laser at 0.25watt was used as demonstrated in table (5).
Table (5): Duncana Multiple Analysis Range test for tested groups after PH cycle.
| Groups | N | Subset for alpha = 0.01 | |
|---|---|---|---|
| 1 | 2 | ||
| C | 10 | 190.115 | |
| L1 | 10 | 197.637 | |
| V | 10 | 251.022 | |
| VL1 | 10 | 255.894 | |
| L1V | 10 | 260.309 | |
| Sig. | 0.065 | 0.031 | |
Table (6) showed that the percentage of surface microhardness loss (SML%) for all groups was calculated according to equation: SML%=SMH2 –SMH1 / SMH1 × 100 (SML%: the percentage of microhardness loss, SMH1: surface microhardness at baseline, SMH2 : surface microhardness after PH cycle.)
Table (6): The percentage of surface microhardness loss of all tested groups.
| Groups | SML% |
|---|---|
| De-ionized water | 0.3875 |
| fTCP varnish | 0.1834 |
| Er,Cr:YSGG Laser (0.25 watt) | 0.3586 |
| fTCP varnish + Er,Cr:YSGG Laser | 0.1655 |
| Er,Cr:YSGG Laser + fTCP varnish | 0.1512 |
Table (6) was further explained in figure (8) indicated that after PH cycle, Er,Cr:YSGG Laser + fTCP varnish group had the minimum values of surface microhardness reduction followed by fTCP varnish + Er,Cr:YSGG Laser group then fTCP varnish group while the control group of De-ionized water and Er,Cr:YSGG Laser group had the maximum value of surface microhardness reduction.
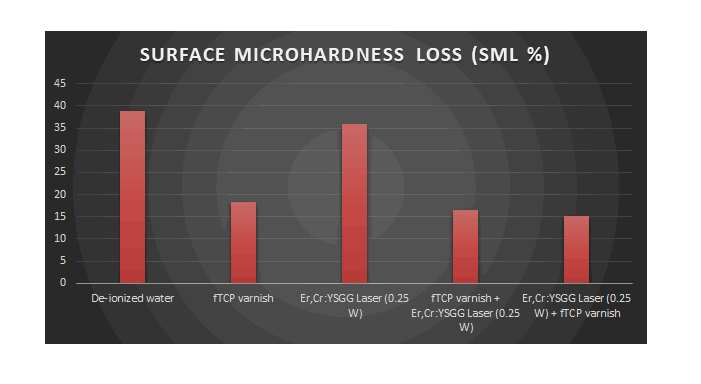
Figure 8:The percentage of surface microhardness loss among tested groups.
Surface morphology by SEM evaluation:
SEM examination of the enamel surface treated with deionized water of control group (group C) reveals a flattened smooth surface with numerous enamel prism ends and absence of cracks and surface deposits in the polished untreated enamel. As in (figures 9).

Figure 9:SEM images of control tooth untreated polished enamel surface. It can be evidenced the flatten enamel rods in higher magnification. Original magnification: 200000 x.
However, the SEM picture of the enamel surface after treatment with functionalized tri-calcium phosphate varnish (group V) reveals the formation of a dense surface coating of numerous granular particles and amorphous crystals, implying the formation of CaF2-like products.
The SEM image of the enamel surface treated with ER,CR:YSGG laser irradiation 0.25 watt (group L1) reveals a slightly rough surface promoted by the laser irradiation which can form and retain the CaF2 -like crystals and globules with evidence of fissures, cracks, conical craters and sharp enamel projections leading to the surface which lacked the typical enamel prism ends and has irregular undulated surface.
The SEM image of the enamel surface treated with functionalized tricalcium phosphate varnish followed by ER,CR:YSGG laser irradiation at 0.25 watt (group VL1) reveals a relatively smooth, more homogeneous surface in comparison to the group treated with laser alone. Less cracks are observed on the glazed enamel surface, most likely due to the application of fTCP varnish prior to laser irradiation. This is consistent with the widespread presence of granular and globular particles, as well as amorphous homogeneous crystals, on the enamel surface. As in (Figure- 10).

Figure 10: SEM images of enamel surface of fTCP varnish followed with of ER,CR:YSGG laser 0.25W sample (VL1). It can be observed the presence of some globules in the irregularities promoted by laser irradiation. Original magnification: 10000 x
The SEM image of the enamel surface after ER,CR:YSGG laser irradiation 0.25 watt followed by functionalized tri-calcium phosphate varnish (L1V) group demonstrates the formation of particle agglomerates in the irregularities caused by laser irradiation. However, a greater number and concentration of globules and crystals produce primarily in ablated areas. As in (figure-11).

Figure 11:SEM images of enamel surface of ER,CR:YSGG laser 0.25 watt combined with fTCP varnish sample (L1V group). craters and formation of ablation areas with sharp enamel projections are monitored with crystals like formation. It can be observed the formation of globules in the ablated areas. Original magnification: 12000 x
Finally, figure (13) shows the result of the EDS microanalysis along with the morphological evaluation using SEM of enamel surface of ER,CR:YSGG laser (0.25 w) followed by functionalized tri-calcium phosphate varnish (L1V) specimen. The distributions of the Ca, P, and F mass percentages on enamel are shown on the enamel surface. The evidence of the fluoride signal (red line) may suggest that these globules could be globules of CaF2 -like material (Figure -12).
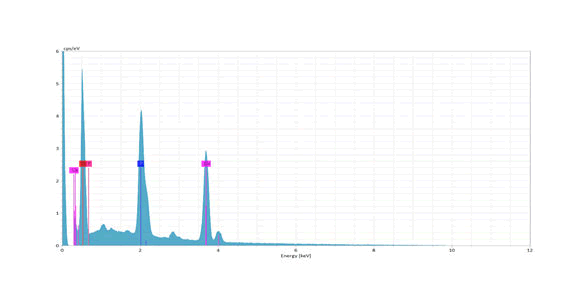
Figure 12: EDS analysis graph of a specimen in ER,CR:YSGG laser (0.25W) followed by functionalized tri-calcium phosphate varnish (L1V) group. The distributions of the Ca, P, and F mass percentages on enamel are shown on the enamel surface. The evidencing of the fluoride signal (red line) may suggest that these globules could be globules of CaF2 -like material. EDS, energy-dispersive X-ray.
Discussion
Dental caries is a chronic infectious and communicable disease caused by specific bacteria,
The effectiveness of topical fluorides in general and fluoride varnishes in particular regarding the reduction of dental caries has been reported widely.
The cavities prevention effect of fluoride is owing to the development of acid-resistant fluorapatite crystals, which inhibit demineralization and enhance tooth structure remineralization. Various approaches incorporating the topical use of calcium and phosphate in order to increase effectiveness, including varnish-containing functionalized tri-calcium phosphate (fTCP).
Laser induced enamel resistance is a novel technique for caries prevention. Er,Cr:YSGG (wavelength: 2.78 μm) has a large hydroxyapatite absorption. Controversial topics are present considering the optimum energy range for caries prevention application of erbium lasers. Laser photothermic effect will melt and fuse crystals from hydroxyapatite so that more acid resistant enamel will be generated. Studies claimed that caries prevention should include the sub-ablative energy density.
In the current study, Er,Cr:YSGG laser beams were applied at energy density in experimental groups of 2.8 J/cm2 and power of 2.5 watt Also, we used 600 μm diameter laser tips to achieve greater energy density with low laser power The quantity of pulses should be adequate, but the total energy transferred must be held to a minimum to prevent pulpal damage While the frequency value for erbium lasers is not agreed, 20 Hz in this study was used as in the previous studies. In some previous studies, the low energy intensity of laser as well as the use without water would avoid the ablation of the target tissue. Much from there, excess water in the region through laser therapy has been shown to increase the potential for ablation and indeed induce the porosity of the tooth surface and hence the diffusion of acids into deep layers.Therefore, we chose laser irradiation without cooling of water in our research. As of now, the findings have shown that caries inhibition has been reduced whereas exposure period has increased, after 10 seconds, enamel irradiation could result in an enamel surface being heated up to a temperature higher than 650˚C, resulting in chemical changes leading to more soluble, crystal with less resistance to acid. That's why in this study we use the 10 second period.
However, it is necessary to investigate the clinical efficacy of professional functionalized tri-calcium phosphate containing varnish combined with ER, CR: YSGG laser in preventing dental caries. In addition, a comparison between the preventive effects of these techniques has not been clearly identified before on the teeth of people of Iraq. Therefore, evaluating the effectiveness of Er,Cr:YSGG laser’s alone and its combination with functionalized tri-calcium phosphate containing varnish (fTCP) as a pretreatment and post treatment strategy in increasing the caries resistance to demineralization is the aim of our study.
The effect of Er,Cr:YSGG laser alone and in combined with fTCP varnish on human permanent teeth enamel’s resistance to demineralization was studied using Vickers microhardness test and scanning electron microscopy (SEM).
As enamel softening is a clinical characteristic of caries, laser induced caries prevention can also be assessed by means of a microhardness measurement. Therefore, microhardness test has been used in this analysis because it is known as a straightforward, more precise and less exhausting tool. Demineralization and remineralization studies believe that SMH indentations can be regarded as non-destructive and reasonably fast process. A strong association between enamel microhardness and the loss of minerals was estimated in carious lesions.
According to the current study, the descriptive statistics and ANOVA Test demonstrated the difference among the tested groups at baseline and after PH cycle and noticed that there was no significant difference before PH cycle, while after the addition of the test materials and introduction in to the PH cycle, there was a high significant difference among tested groups as further noticed at Duncana, a Multiple Analysis Range Test for test groups after PH cycle, which illustrated that the groups of L1V, VL1 and V had the least reduction in the surface microhardness mean value without any significant difference between these three groups, indicating that these groups will effectively protect the enamel of permanent dentition. In fTCP varnish Group, there was 18.34 % loss in the percentage of microhardness as compared to the control group of deionized water which had 38.75 % loss in the percentage of microhardness after ph. cycle. The findings of present study have shown that topical application of fTCP varnish was effective in the prevention of demineralization process. Calcium fluoride (CaF2) has been developed on enamel surfaces and fluoride was released to fluid levels after a professional application of fluoride with calcium and phosphate has been performed. This effect contributed to decrease the demineralization of enamel. The research of revealed that sodium fluoride varnishes with fTCP had significantly higher SMH than unprotected teeth, and hence the protective effect was substantially higher. The current study favored the use of fTCP varnish to enhance the time of contact between fluoride with tri-calcium phosphate and tooth surface to make the tooth more caries resistant.
Regarding laser irradiation alone, there was 35.86% loss in the percentage of microhardness in L1 group as compared to the control group of deionized water which had 38.75 % loss in the percentage of microhardness after ph. Cycle. Er,Cr:YSGG (0.25watt) laser provided least effect on reducing surface microhardness loss and statistically had no significant difference compared with control group. This decrease in enamel SMH in laser group could be due to the laser radiation effect formed in the production of fine irregularities and cracks in enamel surfaces that favored enamel brittleness as seen in SEM laser group images, which revealed that the enamel surface was melted and fused with the formation of cracks. Enamel surface cracks were not seen in the fTCP varnish group micrograms and were covered with amorphous crystals and globular deposits. These cracks serve as the base for acid attacks and cause enamel to be fragile, resulting in a reduction of SMH. These findings were in agreement with results which indicated that the advantages achieved with laser radiation alone are not higher than those with topically treated fluoride treatment. On the other hand, when comparing the untreated group with similar but separate laser experiment (2004) indicated that laser was not able to cause changes in surface hardness following an in-situ cariogenic challenge. On contrast, researchers have attributed improvements in surface resistance to the photo-chemical effect of a laser by lowering the carbonate contents or partial decomposition of the organic matrix.The photothermic effect of laser will melt and fuse crystals from hydroxyapatite so that more acid resistant enamel will be generated. The difference may be related to small volunteer specimen that subjected to a short period of time and for a low cariogenic challenge. Sample differences including total mass, hardness, thickness, hydration and other characteristics can lead to different results when the specimen interacts with lasers. Pulse duration, laser fluence (efficient beam diameter on sample) and repetition rate are other significant factors. Laser equipment/manufacturer and irradiation requirements must also be taken into consideration in predicting laser effects of enamel in addition to the sample characteristics. This may confirm that higher temperatures may be required to modify the enamel's chemical properties and to extract carbonate and hydroxyl groups.
A few studies have tested the effects of combination of fluoride application and Er,Cr:YSGG laser irradiation on demineralization prevention of permanent teeth enamel. In addition, the use of fluoride therapy and various types of lasers together could lead, according to previously mentioned studies, to a higher resistance of enamel compared with the use of fluoride or laser alone. In the literature, some inconsistencies regarding the order of fluoride application and laser irradiation can be found. Tagomori and Morioka (1989) found that laser irradiation with a subsequent application of fluoride can lead to greater intake of fluoride in enamel than when reversing the order. Alternatively, laser irradiation of the fluoride covered enamel could cause the enamel’s outer layer to melt and fluoride ions to bind to the hydroxyapatite crystals with the formation of fluorapatite crystals that are more resistant to acid than hydroxyapatite. Also examined the effectiveness of this combination by applying fluoride compounds prior to irradiation. He found that the mechanical properties of the calcium fluoride-like deposits have improved. have the same findings and found that it was possible for hydroxyapatite crystals to convert directly into fluorapatite crystals using laser irradiation in the presence of fluoride. In contrast, in the present study there was no significant difference in the microhardness according to the order of treatment when Er,Cr:YSGG 0.25watt (2.8 J/cm2) laser irradiation with functionalized tri-calcium phosphate varnish was used. Statistically, Er,Cr:YSGG (0.25watt) combined with functionalized tri-calcium phosphate varnish either before or after treatment could not provide additional benefit to the fTCP varnish effect against demineralization resistance of enamel. There was 15.12% loss in the percentage of microhardness in L1V group, 16.55% loss in the percentage of microhardness in VL1 group and 18.34% loss in the percentage of microhardness in V group as compared to the control group of deionized water which had 38.75% loss in the percentage of microhardness after ph. Cycle. reached to the same results, he stated that laser irradiation of the enamel combined with fluoride (fluoride varnish and/or APF) did not show better results than in case of control groups, and caries activity that resulted in the formation of cavities occurred after just 6 months, indicating no synergistic impact. In another trial, evaluated the effect of combined application of topical APF gel and Er,Cr:YSGG laser (Power= 0.25watt) in comparison with either laser alone or fluoride. Atomic absorption spectrometry was used to identify calcium ions. No significant differences were found between the three treatment groups. In the groups treated with fluoride and in combinations of acidulated phosphorous fluoride gel (APF) and Er,Cr:YSGG laser, with the control group, Fekrazad and Ebrahimpour, (2014) showed significant difference in calcium content among these groups. They observed that combination of laser Er,Cr:YSGG with fluoride or fluoride by itself resulted in significantly less solubility of the enamel than laser application alone. However, there was no substantial difference between fluoride use and combined treatment of laser and fluoride. In addition, no important differences were observed between laser-only treatment and no treatment group Similar findings were obtained by who showed no significant difference in decreasing groups microhardness. Also by who found that although the combination of Er,Cr:YSGG laser and APF gel appeared more efficient than their isolated use, differences were not significant.
Overall, the diversity of results from different studies is likely due to variations in irradiation parameters, laser settings, demineralization solutions, fluoride agent type and demineralization measurement methods used in various studies. The form of laser and laser parameters like power and fluency appeared to be more significant than the order of the application of fluoride and laser irradiation.
The surface morphology of enamel was viewed using Scanning electron microscope examination which was in accordance with a study done by Hicks Puneet Banda.
The scanning electron micrographs was obtained in the control study showed the typical design of the regular enamel that is fairly smooth with shallow depression and enamel prisms. The smooth and rough eroded surfaces were observed on Er,Cr:YSGG laser group. At greater magnification, the eroded surface, which was occurred as irregular areas with rough surfaces. There was a thermal degeneration of the surface, giving lava molten like appearance and an irregular structure. Similar detrimental effects were found in the SEM examinations of studied made by Olivi. The overall effect of the Er,Cr:YSGG laser was not homogeneous; the effect was found only in regions that were in direct contact with the laser. Molten lava-like regions were found in previous laser-induced demineralization prevention (LIPD) studies due to thermal degeneration and lack of cooling. Previous studies have shown that very high surface temperatures are needed to achieve significant enamel melting zones, and that Er,Cr:YSGG laser irradiation of 13.74 J/cm2 energy density cannot produce that change in temperature.
The SEM micrographs of both the lasing + varnish and that of varnish +lasing group were in accordance with the Hick. The addition of fTCP varnish treatment alone or prior to and after the ER:CR,YSGG laser irradiation of the enamel resulted in surface coating granular and globular material. The granular and globular surface coating would provide mineral deposition during the cariogenic attack so that the surface layer had fluoride in the form of calcium fluoride obtained by the reaction of the fluoride in the fTCP varnish with enamel. This was confirmed in the present study by EDS analysis that showed fluoride red signal in the L1V group sample and in accordance to increase in the surface microhardness measurements in groups of combinations.
Additional studies are needed to completely characterize the surface and chemical changes in enamel following laser and/or fTCP varnish treatment. Consequently, further studies are needed on the impact of various demineralization solutions and various methods to assess the resulted demineralization. Furthermore, additional studies on the impact of the Er,Cr:YSGG laser on caries prevention may be beneficial.
Conclusion
The impacts on enamel resistance to demineralization and reducing its solubility seem to indicate that there is no benefit in using Er,Cr:YSGG laser at power of 0.25 watt alone or as a pretreatment and post treatment for fTCP varnish over using fTCP varnish alone.
Acknowledgements
Not applicable.
References
- Souza-Gabriel AE, Colucci V, Turssi CP et al. Microhardness and SEM after CO2 laser irradiation or fluoride treatment in human and bovine enamel. Microsc Res Tech. 2010; 73(4):1030–1035
- Petzold M. The influence of different fluoride compounds and treatment conditions on dental enamel: A descriptive invitro study of Caf2 precipitation and microstructure. Caries Res. 2001; 35(1): 45–51
- Ogaard B, Caf2 formation. Cariostatic properties and factors of enhancing the effect. Caries Res. 2001; 35(1): 40–4.
- Rabelo JS, Ana PA, Benetti C, et al. Changes in dental enamel oven heated or irradiated with Er,Cr:YSGG laser. Analysis by FTIR. Laser Phys. 2010;20: 871–875.
- Bedini R, Manzon L, Fratto G et al. Micro hardness and morphological changes induced by Nd:Yag laser on dental enamel: an in vitro study. Ann Ist Super Sanita. 2010; 46:168-72
- Vieira KA, Steiner-Oliveira C, Soares LE, et al. In vitro evaluation of enamel demineralization after several overlapping CO2 laser applications. Lasers Med Sci. 2015;30(2):901–7.
- Tagomori S, Morioka T. Combined effects of laser and fluoride on acid resistance of human dental enamel. Caries Res. 1989;23(4):225-31
- Malik A, Parmar G, Bansal P, et al. Effect of laser and fluoride application for prevention of dental caries: A polarized microscope analysis. J Dent Lasers. 2015;9(1):11-5
Author Info
Hussein Sami Abdullah1* and Aisha Aram Qasim2
1Researcher (Master student) College of Dentistry,, University of Mosul, Mosul, Iraq2Department of Pedi. Ortho. Preventive Dentistry, College of Dentistry,University of Mosul, Mosul, Iraq
Citation: Hussein Sami Abdullah*, Aisha Aram Passim, The Impact of Combined Application of Cilantro White Varnish and ER, CR: YSGG Laser on Enamel Resistance to Caries: An Study, J Res Med Dent Sic, 2021, 9(12): 1-13.The Impact of Combined Application of Cilantro White Varnish and ER,CR:YSGG Laser on Enamel Resistance to Caries: An In Vitro Study , J Res Med Dent Sci, 2021, 9(12): 534-544
Received: 01-Dec-2021 Accepted: 15-Dec-2021 Published: 22-Dec-2021
